Abstract
Adsorption and micellization behaviour of binary surfactant mixtures containing a nonionic surfactant, polyoxyethylene (20) oleyl ether (C18-1E20), and a cationic surfactant, cetylpyridinium chloride (CPC), was studied at the air–water interface using the Wilhelmy plate method. A pseudo-phase separation model was used to analyse mixed micellization. A Margules equation with one constant (interaction parameter, β) was fitted to the nonideal behaviour of the mixed surfactant system. This system shows synergism (β = −6.0) for micellization. The dynamic behaviour and foamability of binary mixtures at the same bulk concentration and at different mole fractions were also studied using drop volume and horizontal impinging jet methods, respectively. It was found that with an increase in the mole fraction of C18-1E20, the foamability of a mixture increases and t* value decreases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most applications of surfactants require the surface-active agent to have a wide range of interfacial properties which cannot be provided by a single surfactant alone. Hence, surfactant mixtures are usually employed. Also, naturally occurring surfactants are rarely pure; they are often found in the form of mixtures of two or more surfactants. The properties of surfactant mixtures can vary significantly from those of the constituent compounds. Hence, the study of mixed surfactant systems is of crucial importance.
The variation of properties in a mixed surfactant system is due to the various interactions amongst the surfactant molecules. Depending on the kind of interaction, the mixed system can show synergism or antagonism. Many mixtures of surfactants, especially ionic with nonionic, exhibit surface properties significantly better than those obtained with either component alone, thus showing synergism [1, 2]. Such effects greatly improve many technological applications such as emulsion formulation, emulsion polymerization, coating operation, personal care and cosmetic products, pharmaceuticals and petroleum recovery.
Before these mixtures can be employed in potential applications, their properties—both static and dynamic—need to be thoroughly understood. Since the main use of surfactants in most applications is due to their properties of altering interfaces, the rate at which a surfactant migrates to the interface is important. The equilibrium studies of surfactants do not give any idea about this rate. Hence, the study of surfactant dynamics is important for surfactant applications.
Dynamic surface tension plays a vital role in many interfacial processes like textile wetting, paper and pulp industry, foaming, surface rheology and coating of solids [3]. The dynamic surface tension can be measured using drop weight [4], oscillating jet [5], capillary wave [6] and maximum bubble pressure methods [7]. Here, we employed the drop volume method for determining dynamic surface tension. This method was preferred over the others as it can cover a broad time scale, is cheap, simple to handle, requires a small sample size, has good reproducibility and is applicable for either liquid–air or liquid–liquid systems. Another property critical in applications is the foaming ability of the surfactants. This characteristic property is useful in applications like detergency, cleaning, firefighting, cosmetics and so on. Generally, in such applications, mixtures of surfactants like ionic, nonionic and co-surfactants are employed to give better performance in the finished product.
Commercially, nonionic surfactants containing fatty alcohol ethoxylates are used in pharmaceutical and healthcare applications. Polyoxyethylene (20) oleyl ether (C18-1E20) is generally used for high-level soluble expression of different integral membrane proteins [8], whereas cetylpyridinium chloride (CPC) is used, for instance, in mouthwashes and nasal sprays. It can also be used as an antiseptic which kills microorganisms, and it also helps to reduce dental plaque and gingivitis [9].
In the present study, the mixed micellization of C18-1E20 (nonionic) and CPC (cationic) was studied. The purpose of this study was to investigate the interaction in this mixed surfactant system for the first time. Since CPC is an expensive surfactant, mixing it with a relatively cheaper surfactant like C18-1E20 can allow formulations which are not only economical but which also combine the germicidal properties of CPC with the emulsification and detergency properties of C18-1E20.
Experimental Procedures
Materials
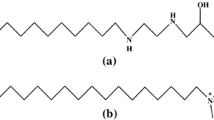
Polyoxyethylene (20) oleyl ether (C18-1E20: mixture of ethoxylates with an average ethoxylation degree of 20 mol EO) was provided by UNITOP Chemicals, Mumbai, and was used as received. Cetylpyridinium chloride (CPC) was procured from S. D. Fine Chemicals Ltd., India, and used without any further purification. Distilled water of surface tension 71.0 ± 1.0 mN/m and conductivity of 0.0002 S/m was used to prepare surfactant solutions for all experimental purposes. The chemical structures of both surfactants are shown in Fig. 1.
Methods and Instrumentation
Static Surface Tension Measurements
The surface tension of pure and mixed surfactants was measured using a KRUSS K11 tensiometer by the Wilhelmy plate method. The platinum plate used for the measurements was cleaned with distilled water and flamed before each measurement. The instrument was standardized using distilled water having a surface tension value of 71.0 ± 1.0 mN/m. All the measurements were carried out at 298 ± 0.5 K using a thermostat (Thermo Haake DC 10, Germany). Each surface tension value was the average of three readings at an interval of 30 s. Each set of experiments was replicated thrice and the repeatability of the γ values found was within ±0.7 mN/m. C18-1E20 and CPC mixtures were prepared by weighing known amounts of C18-1E20 and CPC on an analytical balance (model GR 202, AND Company Ltd.) with an accuracy of 0.1 mg and further diluted to the desired concentration using distilled water.
Dynamic Surface Tension
Dynamic surface tension (DST) measurements were carried out by the drop volume method using a syringe pump (Kopran Laboratories Ltd. KS 50). The experiment consists of measuring the volume of the drop which detaches itself under the influence of gravity alone from the tip of the needle [4]. The dynamic surface tension values were determined by varying the flow rates using the syringe pump. The correction factor is considered because only a portion of the drop volume is released from the capillary during its detachment [10]. Harkins and Brown [11] showed that the boundary (surface) tension was related to drop volume and needle radius using Eq. 1:
where γ is the boundary tension, m the drop mass, \(g\) the local acceleration of gravity, r the capillary radius, φ an empirically derived correction factor, V the drop volume and Δd the density difference between dropping liquid and surrounding medium and \(F = \frac{\varphi }{2\pi }\).
The needle diameter was chosen such that \(\frac{r}{{V^{1/3} }}\) should fall in the range 0.3–1.2. Hence, a syringe volume of 10 ml and a needle diameter of 2 mm were chosen [12, 13]. The dependence of F on \(\frac{r}{{V^{1/3} }}\) is given by the quadratic Eq. 2,
Horizontal Impinging Jet Method
The apparatus for the measurements of the foamability was recently developed in our laboratory. In this method, surfactant solution at constant flow rate is made to impinge on a flat surface covered with a thin layer of the same liquid, which generates polydispersed foam. Those bubbles which stabilize at a fine size are segregated from those bubbles which grow in size due to coalescence. The rate of fine foam generation determined at each surfactant concentration is taken as a measure of foamability. All the experiments were carried out at a temperature at 303 ± 2 K. The details of this method are given in our earlier publication [14].
Mixed Micellization Theory
The mixed micelle behaviour is studied with the pseudo-phase separation model, which considers micelles as a separate macroscopic bulk phase in equilibrium with surfactant monomers in the bulk as well as at the interface. The regular solution theory given by Rubingh [15] gives the strength of the interaction in a binary system of surfactants which can be characterized using the β parameter. Negative β implies negative deviation from ideality, i.e. an attractive interaction (synergism), whereas positive β implies positive deviation from ideality, i.e. a repulsive interaction (antagonism). The critical micelle concentration (CMC) of an ideal mixture of two surfactants is given by Clint [16]:
where C 12, C 1 and C 2 are the CMC values of the mixture, C18-1E20 and CPC, respectively. α 1 and α 2 are the mole fractions of C18-1E20 and CPC in the total mixed solution.
The CMC of nonideal mixture can be calculated using following Eq. 4 where concentrations from Eq. 3 are replaced by activities,
where f 1 and f 2 are the activity coefficients of C18-1E20 and CPC, respectively. The interaction parameter β can be evaluated by considering the least-square difference between calculated and experimental CMC values.
Results and Discussion
Static Surface Tension Studies and Mixed Micelle Formation
The surface tension measurements of C18-1E20 and CPC were conducted for a range of various surfactant concentrations from premicellar to postmicellar concentrations, and the graph of surface tension vs. concentration is shown in Figs. 2 and 3. The premicellar concentration region was fitted using the Szyszkowski equation (Eq. 8), and postmicellar concentration was fitted with linear regression.
γ 0 and γ are the surface tensions of water and surfactant solution, π is the surface pressure, Γ max is the maximum surface excess concentration, C is the concentration of surfactant, R is the universal gas constant, T is the absolute temperature and n is the number of ionizable species in the surfactant molecule. Table 1 shows the CMC of the surfactants in pure aqueous solution measured by the surface tension method at 298 K.
Similarly, for the mixed surfactant system (C18-1E20 and CPC), solutions of different compositions (0.1 < α 1 < 0.9) were prepared and surface tension was measured for a range of various concentrations. The graph of CMCmix vs. mole fraction of C18-1E20 is shown in Fig. 4.
The interaction strength between C18-1E20 and CPC was estimated by using interaction parameter β. From Fig. 4, it is clear that experimental CMC values at different mole fractions of C18-1E20 are always lower than those of the ideal curve (CMC ideal). This indicates attractive interaction between the two components which facilitates surfactant chain transfer from the monomeric to the micellar pseudo-phase, forming mixed micelles. This may be due to the coiling of the ethoxylated chains of the nonionic surfactant (C18-1E20) around the charged head group (pyridinium ion) of the cationic surfactant molecule (CPC), hence reducing the electrostatic repulsion between positively charged surfactant head groups, as well as to steric interaction between the tails which favours micelle formation [19]. As a result of the presence of a cationic head group in combination with a bulky nonionic polyoxyethylene head group in the mixed micelle, the CMC value is reduced and hence non-ideality is observed [20].
Dynamic Surface Tension and Foamability Studies
The dynamic surface tension and foamability of C18-1E20 and CPC mixed aqueous solutions were measured at various mole fractions of C18-1E20, keeping the total surfactant concentration fixed at 0.1 mM. Dynamic surface tension (DST) data were analysed using Eq. 9 [3, 21].
where γ t is the surface tension of the surfactant solution at time t, γ m is the mesoequilibrium surface tension (where γ t only shows a small change with time), and γ 0 is the surface tension of the pure solvent. Equation 9 was used to calculate n and t* values of the surfactant. n is related to the difference between the energies of adsorption and desorption of the surfactant, and t* is the time required for γ t to reach half the value between γ 0 and γ m and is related to surfactant concentration. Figure 5 shows change in surface tension as a function of time at various mole fractions of C18-1E20 at constant 0.1 mM bulk concentration.
It was observed from Fig. 6 that t* value decreases with increase in the mole fraction of C18-1E20. t* is indicative of the time required for the surfactant to reach the interface. This decrease in the t* value is because of change of composition in the mixed monolayer, i.e. more C18-1E20 molecules go to the air/water surface.
From Fig. 4, we see that the CMCmix crosses 0.1 mM at less than 0.001 mole fraction of C18-1 E20; beyond which, it continues to remain considerably below 0.1 mM. Thus, at all mole fractions beyond this, the solution being postmicellar, it shall have an abundance of surfactant available in the form of micelles, over and above that required to reduce the surface tension at the existent value. Hence, on creation of fresh surface, these micelles shall be available to provide surfactant monomers to go to the freshly formed interface. Hence, the t* values for the mixtures are intermediate between those of the pure surfactants.
Foamability is a measure of the foaming capacity of a surfactant. Formation of foam takes place through the introduction of gas bubbles in the solution. Foamability depends upon the type of the surfactant used and the concentration of the surfactant. It is the dynamic property of foam-generating power of the surfactant solution. Surfactant adsorption at the air–water surface decreases surface tension and hence less energy is required to generate foam. The rate of surface tension reduction is directly related to foamability [22]. It is observed that foaming ability of a surfactant solution mainly is a function of surface tension and viscosity [23]. Another parameter affecting foamability is micellar stability which is inversely related to foaming ability. Stable micelles are less capable of providing the flux of surfactants necessary to stabilize the new air–solution interface created during the foaming process, hence they produce less foaming [3].
Figure 7 shows, for CPC at 0.1 mM total surfactant concentration, the volume of fine foam generation plotted as a function of time. The slope of the linear portion of the curve is referred to as foamability. This slope is the rate of fine foam generation expressed in milliliters per second. Similarly, foamability of various mixtures of CPC and C18-1E20 was also analysed using the same method. Figure 8 shows the effect of surfactant composition on foamability. At this concentration, the solution of C18-1E20 shows higher foamability than the solution of CPC. Above C18-1E20 mole fraction of 0.5, an increase in the foamability is observed. This increase in the foaming is due to the change in the composition of mixed monolayer and may be due to an increase in the intermolecular cohesive forces between C18-1E20 with CPC. These hydrogen bonding, cohesive forces and van der Waal forces increase adsorption at the air–water interface and hence increase foaming.
Conclusion
It can be concluded from the experimental results that, as far as micellization is concerned, the C18-1E20–CPC binary system shows negative deviation from ideality, i.e. synergism with an interaction parameter of β = −6.0. Dynamic studies show that the t* value decreases with increase in the mole fraction of C18-1E20. Foamability was found to increase above a C18-1E20 mole fraction of 0.5.
Abbreviations
- C18-1E20 :
-
Polyoxyethylene (20) oleyl ether
- CPC:
-
Cetylpyridinium chloride
- CMC:
-
Critical micelle concentration
- DST:
-
Dynamic surface tension
- C :
-
Concentration of surfactant
- C 1, C 2 :
-
Critical micelle concentrations of surfactants 1 and 2, respectively
- C 12 :
-
Critical micelle concentration of surfactant mixture
- F :
-
Correction function
- f :
-
Activity coefficient
- \(g\) :
-
Local acceleration of gravity
- m :
-
Drop mass
- n :
-
Number of ionizable species in the surfactant molecule, exponent in dynamic surface tension equation
- R :
-
Universal gas constant
- r :
-
Capillary radius
- T :
-
Absolute temperature (K)
- t :
-
Time
- t*:
-
Time required for γ t to reach half the value between γ 0 and γ m
- V:
-
Drop volume
- X 1, X 2 :
-
Mole fractions of surfactants 1 and 2 in the mixed micelle, respectively
- α 1, α 2 :
-
Overall mole fractions of surfactants 1 and 2, respectively
- β :
-
Interaction parameter
- Γ max :
-
Maximum surface excess concentration
- γ :
-
Surface tension (mN/m)
- γ 0 :
-
Surface tension of pure solvent (mN/m)
- γ t :
-
Surface tension of the surfactant solution at time t
- γ m :
-
Meso equilibrium surface tension
- Δd :
-
Density difference between dropping liquid and surrounding fluid
- π :
-
Surface pressure
- φ :
-
Empirically derived correction factor
References
Maeda H (2004) Electrostatic contribution to the stability and the synergism of ionic/nonionic mixed micelles in salt solutions. J Phys Chem B 108:6043–6051
Hines JD (2001) Theoretical aspects of micellization in surfactant mixtures. Curr Opin Colloid Interface Sci 6:350–356
Rosen MJ, Kunjappu JT (2012) Surfactants and interfacial phenomena. Wiley, New York
Lando JL, Oakley HT (1967) Tabulated correction factors for the drop-weight-volume determination of surface and interfacial tensions. J Colloid Interface Sci 25:526–530
Thomas WDE, Hall DJ (1975) Solution/air interface, II. Adsorption of surfactants in dilute aqueous solutions. Anomalous oscillating jet phenomena. J Colloid Interface Sci 51:328
Austin M, Bright BB, Simpson EA (1967) The measurement of the dynamic surface tension of manoxol OT solutions for freshly formed surfaces. J Colloid Interface Sci 23:108–112
Mysels KJ (1986) Improvement in the maximum-bubble-pressure method of measuring surface tension. Langmuir 2:428
Spirin AS, Swartz JR (2008) Cell free protein synthesis: methods and protocols. Wiley VCH, Weinheim
Asadoorian J, Williams KB (2008) Cetylpyridinium chloride mouth rinse on gingivitis and plaque. Am Dent Hyg Assoc 82:42
Adamson AW, Gast AP (1997) Physical chemistry, 6th edn. Wiley, New York
Harkins WD, Brown FE (1919) The determination of surface tension (free surface energy), and the weight of falling drops: the surface tension of water and benzene by the capillary height method. J Amer Chem Soc 41:499–524
Harkins WD (1952) The physical chemistry of surface films. Reinhold, New York
Adam NK (1941) The physics and chemistry of surfaces. Oxford University Press, London
Powale RS, Bhagwat SS (2006) Influence of electrolytes on the foaming of sodium lauryl sulphate. J Disp Sci Technol 27:1181–1186
Rubingh DN (1979) In: Mittal KL (ed) Solution chemistry of surfactants, vol 1. Plenum, New York, pp 337–354
Clint JH (1975) Micellization of mixed nonionic surface active agents. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 71:1327–1334
Klammt C, Schwarz D, Fendler K, Haase W, Dotsch V, Bernhard F (2005) Evaluation of detergents for the soluble expression of α-helical and β-barrel type integral membrane proteins by a preparative scale individual cell-free expression system. FEBS J 272:6024–6038
Sata N, Sasaki H (1957) In: Schulman JH (ed) Proceedings of the second international congress of surface activity, vol 1: gas/liquid and liquid/liquid interface. Butterworth’s Scientific Publications, London, pp 340–348
Lainez A, Del BP, Junquera E, Aicart E (2004) Mixed micelles formed by n-octyl-β-d-glucopyranoside and tetradecyltrimethylammonium bromide in aqueous media. Langmuir 20:5745–5752
Negm NA, Sabagh AME (2011) Interaction between cationic and conventional nonionic surfactants in the mixed micelle and monolayer formed in aqueous medium. Quim Nova 34:1007–1013
Hua XY, Rosen MJ (1988) Dynamic surface tension of aqueous surfactant solutions: I. Basic parameters. J Colloid Interface Sci 124:652–659
Garrett PR, Moore PR (1993) Foam and dynamic surface properties of micellar alkyl benzene sulphonates. J Colloid Interface Sci 159:214–225
Bikerman JJ (1953) Foams,theory and industrial applications. Reinhold, New York
Acknowledgments
The authors are thankful to UGC-GREEN TECH for financial assistance and the Department of Science and Technology (DST), Government of India for their financial support under DST-FIST program (SR/FST/ETII 007-2007). The authors would also like to acknowledge UNITOP Chemicals for providing polyoxyethylene (20) oleyl ether.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Desai, S., Bhagwat, S.S. Mixed Micellization of Polyoxyethylene (20) Oleyl Ether with Cetylpyridinium Chloride at the Air–Water Interface. J Surfact Deterg 19, 1169–1174 (2016). https://doi.org/10.1007/s11743-016-1874-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1874-z