Abstract
Performance and efficiency of anionic [sodium lauryl ether sulfate (SLES) and sodium α-olefin sulfonate (AOS)] and amphoteric [cocamidopropyl betaine (CAB)] as well as nonionic [cocodiethanol amide (DEA), various ethoxylated alcohols (C12–C15–7EO, C10–7EO and C9–C11–7EO) and lauramine oxide (AO)] surfactants in various dishwashing liquid mixed micelle systems have been studied at different temperatures (17.0, 23.0 and 42.0 °C). The investigated parameters were critical micelle concentration (CMC), surface tension (γ), cleaning performance and, foaming, biodegradability and irritability of anionic (SLES/AOS) and anionic/amphoteric/nonionic (SLES/AOS/CAB/AO) as well as anionic/nonionic (SLES/AOS/DEA/AO, SLES/AOS/C12-C15-7EO/AO, SLES/AOS/C10–7EO/AO and SLES/AOS/C9–C11–7EO/AO) dishwashing surfactant mixtures. In comparison to the starting binary SLES/AOS surfactant mixture, addition of various nonionic surfactants promoted CMC and γ lowering, enhanced cleaning performance and foaming, but did not significantly affect biodegradability and irritability of dishwashing formulations. The anionic/nonionic formulation SLES/AOS/C9–C11–7EO/AO shows both the lowest CMC and γ as well as the best cleaning performance, compared to the other examined dishwashing formulations. However, the results in this study reveal that synergistic behavior of anionic/nonionic SLES/AOS/ethoxylated alcohols/AO formulations significantly improves dishwashing performance and efficiency at both low and regular dishwashing temperatures (17.0 and 42.0 °C) and lead to better application properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Different types of interfacial processes, such as interfacial tension, interfacial viscosity, rolling-up process, electric charge, emulsification and active ingredient penetration are involved in the cleaning of hard surfaces. These components in the washing process can be very different, depending on a variety of surfaces that have to be cleaned, the ingredients of the cleaners, etc. As for aqueous solution, the most popular way of cleaning, the separation of soil from surface is physical, and it is based on the adsorption of both ions and surfactants that will be cleaned [1].
Surfactants (a contraction of the term surface active agents), also known as detergents or tensides, are amphiphilic molecules that possess both hydrophobic and hydrophilic properties. A typical surfactant molecule consists of a long hydrocarbon “tail” that dissolves in hydrocarbon and other nonpolar solvents, and a hydrophilic “headgroup” that dissolves in polar solvents (typically water) [1]. Depending on the nature of the hydrophilic group, surfactants are classified as anionic, cationic, nonionic and amphoteric. Because of its dual affinity, an amphiphilic molecule in different solvents shows more or less marked tendency to spontaneous accumulation in the phase boundary surfaces by reducing surface tension of the solution and formation of aggregates of molecules (micelles). Namely, when a sufficient amount of surfactant is dissolved in water, several bulk solution properties are significantly changed, particularly surface tension (which decreases) and ability of the solution to solubilize hydrocarbons (which increases). These changes do not occur until a minimum bulk surfactant concentration is reached. This concentration is called the critical micelle concentration (CMC). Below the CMC, the surfactant exists mainly as solvated monomeric species, whereas above the CMC these monomers undergo self-assembly to form roughly spherical structures (having an overall diameter of ~5 nm) known as micelles. The CMC appears to be a fundamental micellar quantity to study self-aggregation of amphiphilic molecules in a solution. Also, for estimating detergency power and detergent concentration of use, it is very important to determine both the CMC and surface tension of surfactants used in a particular cleaning product [2].
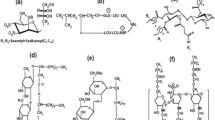
In most EU countries there are three types of products for manual dishwashing, cleaners of economic, regular and high (concentrated) classes with different contents of surfactants, about 10, 15–18 % and more than 20 %, respectively. The main task and purpose of dishwashing liquids is cleaning plate surfaces, but since consumers’ hands are also immersed in the liquid during washing and the skin is directly exposed to high concentration of cleaning agents, mildness of the surfactant formulation is of more concern than in other household cleaning products. As a mixture of different types of surfactants (anionic, cationic, nonionic and amphoteric), the formulation often shows a synergistic effect in those products, and it is the mixture that is used rather than individual surfactants. However, this mixture is responsible for the irritancy of the formulation. The most commonly used surfactants in dishwashing liquids are anionic [alkylethoxy sulfate (R(OCH2CH2) n –OSO3 −Na+), alkyl sulfate (R–OSO3 −Na+), alkylbenzene sulfonate (R–Ar–SO3 −Na+) and α-olefin sulfonate (R–CH2–CH=CH–CH2–OSO3 −Na+)] and nonionic [(alkyl dimethyl amine oxide (R–(CH3)2NO), alcohol ethoxylate (R(OCH2CH2)nOH) and alkyl amide (CH3(CH2) n C(=O)N(CH2CH2OH)2)), as well as amphoteric alkyl betaine (R–N+–(CH3)2CH2–COO−)] where R is C x H2x+1 and x typically in the range of 12–18 [3]. Anionic surfactants are an excellent choice since they have a good cleaning performance and excellent foaming, in addition to being low cost and easily available. However, as these surfactants are highly versatile, they suffer from several shortcomings, including incompatibility with cationics [3], hard water sensitivity and consumer’s skin irritation. Nonionic surfactants (amine oxides, ethoxylates, etc.) have a lower foaming performance and somewhat higher price [4], and are also highly synergistic, owing to their lack of a charged headgroup. Mixtures of anionic and nonionic surfactants can provide overall benefits and advantages such as mildness, wetting, foam volume and foam stability of a formulation. For example, amine oxide surfactants at neutral pH, like in dishwashing liquids, exhibit strong interaction with anionic surfactants and advanced performance benefits, such as improving grease emulsification, minimizing the irritancy of anionic surfactants, as well as stabilizing foam which is also long lasting [4]. Betaines and amine oxides provide performance enhancement in a mixture with anionic surfactants, mostly in foam boosting, stability, and grease removal efficiency [5]. These nonionic and amphoteric surfactants can also minimise anionic irritancy and be compatible with all other surfactants; they have little hard water susceptibility and are stable in acid and alkaline solutions.
Dishwashing liquid is applied directly on the plate or a sponge, brush or pad, or diluted in medium-temperature hot water (40–50 °C), but in developed markets, water temperature can be much lower (around 20 °C), as in case of potable water. Recommended concentrations of dishwashing liquids depend on the concentration of the surfactant and vary from 0.04 to 0.4 %. For the soils from baking, frying and roasting that are difficult to remove, it is common to let them sit for 10–20 min in medium-hot water with a concentration of dishwashing liquid ranging from 0.2 to 0.7 %, and the result is that very difficult soils can be easily removed from the surface. The surface energy of the dish material, which affects the soil persistence, ranges from high (metal and ceramic) to low (plastic) [6]. As the soils may have transformed to extremely difficult soils during a higher-temperature process of baking or cooking, good dishwashing liquid has to be capable of removing them [7].
Cleaning performance is the most important characteristic of a dishwashing liquid, but foaming and biodegradability, as well as irritability, are important for estimating both dishwashing quality and consumer perception of products. In addition to this scientific approach, detergent formulations are subject to performance and consumer testing. A number of test methods have been developed to make a total evaluation of a dishwashing liquid's efficiency and to establish performance standards of dishwashing formulations. Efficiency is generally understood as the cleaning quality or capability of a detergent (liquid or powder) to remove soil or stains. For determination of a dishwashing detergent's cleaning performance as well as foaming, there are “Recommendation for the Quality Assessment of the Cleaning performance of Hand Dishwashing Detergent” [8], and “Methods for Ascertaining the Cleaning Performance of Dishwasher Detergents” [9], both testing the washing of soiled plates as the number of ceramic test plates washed (plate count test). Both types of tests, low-fat soil and normal soil, for performance testing by plate count test are made by mixing beef tallow, palm oil, margarine, butter, lard, sunflower oil, olive oil, skim milk powder, flour and water with red color (Reactive red 180). A low-fat mixture has 20 % fat, 60 % carbohydrates and 20 % protein, while normal has 60 % fat, 30 % carbohydrates and 10 % of protein, all calculated as dry matter. The primary conditions for testing a dishwashing liquid are water temperature as well as water hardness for the market where the product is sold. Performance can be divided into two areas: grease cleaning and foaming, while grease cleaning can be divided into dishwashing at recommended dilution and soaking, reserved for cleaning very stubborn stains. In addition to the plate count test, both a Baumgartner test [10] and the test developed by Procter and Gamble [11] are based on grease removal from a polypropylene surface, while a third test is based on ceramic or plastic test plates soiled with pure grease or mixed, colored, greasy soil [12].
Detergent foam is a mass of gas cells separated by thin films of liquid formed on the surface of the liquid, creating a dispersion where a large proportion of gas volume is dispersed in a liquid. Testing foam is not connected with dishwashing cleaning performance, but it is important because consumers assume that if the foam is low, cleaning performance is also low; so, all dishwashing liquid producers always try to have good and stable foaming. Foam is mainly evaluated by standard methods for evaluating the foaming ability (foam volume) of diluted liquid detergents, the Ross–Miles foam test [13–15], and can be used for evaluation of liquid detergents or mixtures of pure surfactants. Standard adaptations of this test have been designed, like the shake foam cylinder test [16], miniplate test [17], or a perforated disc method [18].
Biodegradability of a detergent formulation is one of the primary requirements of modern cleaning products. Biodegradability of surfactants and surfactant formulations means their degradation can proceed through metabolic activity of microorganisms. There are two types of biodegradability. Primary degradation can be defined as when the molecule structure has changed sufficiently to lose its surfactant properties. Ultimate degradation is when a surfactant molecule has been decomposed to carbon dioxide, methane, water, mineral salts and biomass. Testing surfactants and detergent biodegradability is mainly done by a standard method [19] known as the "Closed Bottle Test". By analysing biochemical oxygen demand (BOD), this standard method specifies the means for evaluating “ultimate biodegradability” of organic compounds in an aqueous medium, at a given concentration, by using aerobic microorganisms. The method applies to all organic compounds which are sufficiently water soluble by measuring BOD during 28 days [20].
Hand dishwashing is an activity that exposes a person to a potential irritant [21]. The Zein test is based on solubilization of water-insoluble protein (Zea mays) by surfactants; irritation of skin and solubility of Zein protein is related. This test for evaluation of relative mildness of surfactants or detergents is one of the nine recommended methods for testing skin irritability, and it is done according to Invittox Protocol No. 26 [22]. Values of Zein test results should be below 200 for liquid detergents, and below 165 for hair and body care products like shampoos or body showers. The achieved results are expressed as mg of nitrogen in 100 mL of detergent solution, and that is called the Zein number.
In order to appropriately select individual surfactant components for optimal surfactant mixture features, it is very important to perform a systematic study of surfactant formulation properties (CMC and γ). Therefore, the main goal of the present paper is to evaluate the influence as well as possible synergistic effect of amphoteric (CAB) and nonionic [DEA, AO and various ethoxylated alcohols (C12–C15–7EO, C10–7EO and C9–C11–7EO)] surfactants on dishwashing liquid performance and efficiency of anionic surfactant formulation (SLES/AOS) by using conductometric and stalagmometric methods as well as performance testing.
Experimental Procedures
Materials
For determination of performance and efficiency of dishwashing liquids, all commercial chemical raw materials used in this study were obtained from the stated suppliers and used without any further treatment.
Anionic Surfactants
Sodium lauryl ether sulfate (SLES) (C12–C14-alcohol polyethylene glycol ether (2 EO) sulfate sodium-salt, 70 % active substance [Cosmacol AES-70-2-24, Sasol, Italy)].
Sodium α-olefin sulfonate (AOS) (C14–C16 α-olefin sulfonate sodium salt, 90 % active substance [Hostapur OSB, Clariant, Switzerland)].
Amphoteric Surfactants
Cocamidopropyl betaine (CAB) [30 % active substance (Empigen BS/FA, Huntsman, USA)].
Nonionic Surfactants
Cocamide DEA (DEA) [85 % active substance (Empilan 2502, Huntsman, USA)].
Lauramine oxide (AO) [30 % active substance (Empigen OB, Huntsman, USA)].
Ethoxylated alcohols: C12–C15-alcohol polyethylene glycol ethers (7 EO) (C12–C15–7EO) [100 % active substance (Slovasol 257, Sasol, Italy)]; C10-Guebert alcohol polyethylene glycol ethers (7 EO) (C10–7EO) [100 % active substance (Lutensol XP 70, BASF, Germany)]; C9–C11-alcohol polyethylene glycol ethers (7 EO) (C9–C11–7EO), [100 % active substance (Lutensol ON 70, BASF, Germany)].
The tested mixtures of liquid surfactants, noted as formulations 1–6, with appropriate w/w ratio, are presented in Table 1. Total surfactant concentration was 10 % for all samples. The surfactant mixture solutions were prepared in a 100-mL volumetric flask and then diluted in deionized water (18 MΩ cm−1, Milli Q, Millipore) to the desired concentration.
The following chemicals were used for biodegradability determination of dishwashing liquid formulations: ammonium chloride (NH4Cl; Sigma-Aldrich, USA), potassium hydrogen phosphate (K2HPO4; Sigma-Aldrich, USA), magnesium sulfate (MgSO4; Merck, USA), potassium chloride (KCl; Sigma-Aldrich, USA) and ferrous sulfate (FeSO4; Merck, USA). All the chemicals were of analytical grade. For the Zein test procedure, zein protein (from maize; Z3625, Sigma, USA) was used.
Methods
Conductivity and Surface Tension Measurements
Conductivity measurements were applied to determine the CMC in various combinations of surfactants. Those measurements were carried out at 23.0 °C with digital conductivity meter SensION 5 (Hach, USA) with the accuracy ±0.5 %, as well as with the 51975 conductivity probe that uses the 4-ring method. The CMC values at each surfactant formulation composition were determined by using the conventional method (Williams’ method) [23] as well as the method proposed by Carpena et al. [24].
Surface tensions of surfactant mixtures were measured by a stalagmometer (Traube stalagmometer, Neubert-Glass, BN-0330-10-208), while surface tension was determined by the drop counting method. Surface tension measurements were performed at 23.0 °C and both low and regular plate count test dishwashing temperatures of 17.0 and 42.0 °C, respectively. All temperature measurements were carried out with the accuracy ±0.1 °C.
Foam Volume Test
Evaluation of foam ability of surfactant mixtures was done by foam volume measuring after free flows of 0.4 % dishwashing formulations without soil (5 L). The reservoir was placed in such a way that the outlet tube was positioned in the centre of the basin and the distance between its lower edge and bottom was 1 m [8].
Cleaning Performance Test (Plate Count Test)
For cleaning performance testing, all detergent samples were diluted to 4 g L−1 with hard water (16 ± 1°dH) at 17.0 °C for low-temperature washing or heated to 42.0 °C. The plates used were made from white ceramics and, prior to soiling, were washed in a dishwasher (normal program 60 °C) with a normal, low alkaline “all in one” tablet, with no rinse aid. For the plates thus cleaned, the test soil low-fat dosage was 7 g/plate and test soil was normal at 5 g/plate, applied with a pipette. The dishwashing process was done by two trained persons. The soiled plates were cleaned using circular movements of plastic washing up brushes, by cleaning the front side for 10 s with 20 circular movements, cleaning the back side for 3 s with 6 circular movements, and wiping off foam for 15 s. The end point was reached when the foam layer broke permanently on the surface of the dishwashing soak. The number of washed plates (five experiments for each formulation) was written down in test data [8] and presented as mean values.
Testing of Biodegradability (Closed Bottle Test)
The closed bottle test for “ultimate biodegradability” determination of dishwashing liquid surfactant mixtures in an aqueous medium was primarily established according to the standard method [19]. The preadapted microorganism from the river Sava, Belgrade, Serbia was used. The aqueous medium for preparing the cultures had a neutral pH (7.2) and contained minerals and micronutrients to support bacterial activity (2.75 g L−1 NH4Cl, 1.0 g L−1 K2HPO4, 0.252 g L−1 MgSO4, 0.32 g L−1 KCl and 0.0018 g L−1 FeSO4).
Tested dishwashing formulations 1–6 were added to the microorganism media to give a concentration of about 100 mg L−1. Each sample was inoculated with 1 mL of 15 g L−1 suspension of aerobic microorganism. The samples in dark bottles were placed in a temperature-controlled incubator (Velp Scientifica FOC 120E, Italy), and the incubator temperature was maintained at 25 °C. BOD was measured every 5 days during the analyzed time of 28 days (BOD5), by using a Sensor System 6 (Velp Scientifica, Italy).
Testing of Irritability (Zein Test)
In the Zein test procedure, 1 g of protein is solubilized in 10 g L−1 of dishwashing formulation sample. The amount of solubilized protein was determined by Kjejdal analysis [25], and the result of the Zein number procedure was expressed as mg of solubilized protein in 100 mL of sample.
Results and Discussion
In this study, the values of critical micellar concentration and surface tension of different surfactant formulations were obtained. The CMC values of examined mixtures of surfactants were determined from inflections in plots of specific conductivity (κ) versus concentration of surfactant mixture (c). The obtained graphical dependence is a curve which consists of two segments (premicellar and postmicellar); each plot shows the single break point at a certain formulation. The CMC is obtained from the intersection of the fitting lines of the κ/c plots above and below the break point [23]. In addition to the above-mentioned conventional procedure (William’s method), in order to improve the quality of the calculated CMC, the method proposed by Carpena et al. [24] was also used. This method is based on the fit of the experimental raw data to a simple nonlinear function obtained by direct integration of a Boltzmann-type sigmoid function. Fitting to the data was carried out by software package OriginPro 9.0 (OriginLab Corporation, US). The values of CMC obtained by this approach, as well as the values of surface tension of 1.0 % water solutions of surfactant mixtures, are all summarized in Table 2.
From the results, it is obvious that addition of amphoteric and nonionic surfactants generally promoted CMC lowering of the initial SLES/AOS anionic surfactants formulation 1, but nonionic surfactants, of an ethoxylated alcohol type, significantly decreased the CMC of anionic/nonionic formulations 4–6, compared to other mixtures formulated with anionic, amphoteric and nonionic surfactants (formulations 2 and 3).
When an SLES/AOS formulation was used, surface tension was found to be higher (35.3 mN m−1) than with other examined mixtures, probably because of the lower polar charge of the latter. When compared to the SLES/AOS mixture, added amphoteric and nonionic surfactants further reduced surface tension. This suggests a mixed micelle formation between anionic and nonionic surfactants. Comparisons between the obtained surface tension values for 1.0 % water solutions of various formulations at different performance testing temperatures of 17.0, 23.0 and 42.0 °C are presented in Fig. 1.
Differences in surface tensions for each of the formulations at investigated temperatures <5 % because all formulations are totally dissolved even at 17.0 °C and are at higher concentrations than the CMC. On the other hand, differences in various formulations at the same temperature are significant. Generally, formulations with nonionic surfactants of ethoxylated alcohols (formulations 4–6) have a significantly smaller γ in comparison with formulations where they are absent. Anionic/nonionic formulation 6 (SLES/AOS/C9–C11–7EO/AO) at low and regular washing temperatures, 17.0 and 42.0 °C, has more than 55 and 57 % smaller γ, respectively, than binary anionic formulation 1 (SLES/AOS) with higher γ. Compared to formulation 3 (SLES/AOS/DEA/AO), which has the highest γ of all anionic/nonionic formulations at a low washing temperature of 17.0 °C, formulation 6 has 26.6 % smaller γ. At 23.0 °C and regular washing temperature of 42.0 °C, the difference is 27.2 and 28.9 %, respectively. It is very clear that addition of nonionic ethoxylated alcohol surfactants caused a very pronounced synergism in mixed surfactant formulations.
Cleaning Performance
The cleaning performance of various dishwashing formulations at both low and regular temperatures are presented in Table 3 and Fig. 2.
Physical removal of tested soil (normal and low fat) from plate surfaces is based on nonspecific adsorption of surfactants on the interface and specific adsorption of the surfactants on polar solid soil particles. The adsorption leads to an increased soil electrical charge (negative) and an increase of spreading pressure that is active in the adsorbed layers. Then, a disjoining pressure is manifested, and the final result is the removal of soil particle from the plate. For oily soil, which is a primary cleaning problem in dishwashing, reduction of interfacial tension by surfactant adsorption leads to a rolling-up process of cleaning the surface. The best cleaning performance at 42.0 °C was obtained for SLES/AOS/C9-C11-7E/AO (formulation 6) with a surface tension 22.8 mN m−1, because of a lower γ and the lowest CMC, and they are expected to have a better cleaning performance than other formulations.
Cleaning performance of surfactant mixtures can be attributed to the interaction of hydrophilic and hydrophobic groups. The micelle formation and the adsorption on the interface for a single surfactant is a typical property determined by the length of its hydrophobic chain. However, as we can see from the CMC and γ results, mixture properties are more important for detergent application. A synergistic effect is observed, and mixtures have better cleaning performance than single surfactants with the same total concentration. Mixtures of anionic and nonionic surfactants show specific behavior because of significant reduction in the mixture CMC. It is due to the fact that in mixed micelles, the polar group of the ionic surfactant is located further than the polar group in micelles of a pure ionic surfactant, and, therefore, the repulsion energy between the charged groups is smaller. Synergism in CMC reduction in surfactant mixtures is also evident for adsorption on plate interfaces and the result is optimal wetting, rolling up, emulsifying, and overall efficiency. Optimal wetting is a major force for separating oily soil from a plate, because liquids with optimal wetting have a tendency to spread over the surface. As seen from Table 3 for the same total surfactant concentration, the difference in the plate count test at 42.0 °C is about 50 % higher for a mixture of various anionic/nonionic surfactants in comparison with a binary anionic surfactant mixture with the same total concentration; equal results are obtained for both types of test soils (normal and low fat).
Figure 2 presents multiple comparisons for cleaning performance measured as per the plate count test for normal and low-fat soil at both low and regular dishwashing temperatures, 17.0 and 42.0 °C, respectively. The cleaning performance at low temperature was investigated with the same experimental conditions as at 42.0 °C, and the same concentration of formulation (4 mL in 1000 mL).
As for low-temperature washing, the cleaning performance is best for formulation 6 for both normal and low-fat soil, and the difference is almost doubled, 23 plates for formulation 6 and only 12 plates for formulation 1. Formulation 3 (which is the best of the formulations without nonionic ethoxylated alcohol surfactants) performance, when compared to formulation 6, is lower by more than 40 %. If we analyse temperature dependence of cleaning performance, it is obvious that there is a significant difference in formulations with and without ethoxylated alcohols. The formulations with ethoxylated alcohols can be considered as efficient at low temperature, because there is a relatively small difference in the plate count test at 17.0 and 42.0 °C. For both normal and low-fat soil, formulation 6 shows a difference of about 10 %, while for formulation 1, the difference rises to even 50 % better at 42.0 °C than at 17.0 °C. From the presented results, it can be concluded that the SLES/AOS/ethoxylated alcohols/AO formulations 4–6 can be applied for effective dishwashing at low tap water temperature. On the other hand, compared to the same formulation at 17.0 °C, all the dishwashing formulations exhibited better cleaning performance at 42.0 °C. That is mainly because the oily part of soiling is semi-liquefied at 42.0 °C, while at 17.0 °C it is solid, and the semi-liquefied condition makes it easier for a dishwashing formulation to clean the surface through roll-up, solubilization or emulsification.
Foam Ability
The results of surfactant mixture foam ability evaluated at 42.0 °C are presented in Fig. 3.
The lowest foam height was obtained for anionic surfactants mixture SLES/AOS (formulation 1), which generally has excellent foaming characteristics [3]. When amphoteric CAB and nonionic surfactants (DEA, ethoxylated alcohols and AO) are used with anionic surfactants, foaming of the formulations are improved. Formulation 6 has higher foam than formulations 1–3, namely 13 % higher than formulation 1, 8 % than formulation 2, and 5 % than formulation 3. The difference between foam heights of formulations with ethoxylated alcohols (formulations 4–6) is less than 4 %. The resulting synergism can be explained by the presence of betaine and nonionic surfactants, used as secondary surfactants with anionic surfactants that improve foaming of the formulations [3]. Also, formulations 2–6 are formulated with AO, which is a foam stabilizer and provides foam height and stability with no drainage [3]. Although amphoteric (CAB) and nonionic (DEA) surfactants are added in equal and relatively low concentrations as other nonionic surfactants of ethoxylated alcohols type, they do not significantly affect foam height. However, nonionic surfactants of ethoxylated alcohol in equally applied concentrations appreciably improve performance in terms of foam ability. The foam height data presented in Fig. 3 are in good correlation with CMC values (Table 2), because it shows that mixed surfactants with a lower CMC are more efficient foamers, as noted in [4].
Biodegradability of Dishwashing Formulations
Biodegradability after 28 days is shown in Fig. 4. The starting formulation 1 can be readily biodegraded, and 95 % is degraded after 28 days. This result is as expected, considering that the mixture SLES/AOS is formulated with anionic surfactants. The surfactant mixtures formulated with amphoteric and nonionic surfactants (formulations 2–6) were degraded more than 86 %. In addition, formulations with ethoxylated alcohols (4–6) have approximately the same biodegradability, and their degradation is close to formulations 2 and 3.
Pursuant to the EU legislation and Serbian law on detergents, “ultimate aerobic degradation” of surfactant mixture takes place after 28 days if more than 60 % of surfactants degrade to carbon dioxide, mineral salts and water. All formulations are biodegradable, in keeping with the regulations, and can be considered as environmentally safe, with no pollution impact on surface water and soil. However, when it comes to practical usage, biodegradability of formulations with various ethoxylated alcohols which, according to the previously presented results show the best performance and efficiency, is acceptable.
Irritability of Dishwashing Formulations
In dishwashing formulations, amphoteric and nonionic surfactants like betaines and cocamide DEA, respectively, as well as aloe vera, chamomile, lavender and some other herbal extracts are often used for reduction of irritability. As presented in Fig. 5, all analyzed formulations have a Zein number below 200. It can be noticed that formulation SLES/AOS/CAB/AO (formulation 2) has the lowest Zein number, which can be explained by the presence of amphoteric surfactant CAB with lower irritability potential than other examined surfactants [26], as well as nonionic surfactant AO which, although at a low concentration, helps mitigate anionic surfactant irritability [3]. There are no significant differences in Zein number between the SLES/AOS/CAB/AO and SLES/AOS/DEA/AO formulations (formulations 2, 3) and formulations which, instead of the commonly used surfactants for reduction of irritability, contain ethoxylated alcohols (formulations 4–6). The reason is the high concentration of anionic surfactant SLES which has a high Zein number, so the applied concentration of CAB is too low to significantly decrease the Zein number in formulation 2. For the formulations with ethoxylated alcohols (4–6), irritability is primarily determined by the high SLES concentration. When comparing the obtained Zein number for various formulations and recommended limit for dishwashing liquids for normal use, all the formulations are non-irritants and can be considered as safe for consumers’ hands under normal usage. However, formulations are not “care” or “mild” because the Zein number is higher than 150.
In conclusion, the present paper evaluated performance and efficiency of liquid anionic/amphoteric/nonionic and anionic/nonionic surfactant mixtures, starting with a binary SLES/AOS anionic mixture as the initial dishwashing formulation. Amphoteric and nonionic surfactants have a significant influence on the initial performance of an SLES/AOS anionic formulation.
The effect of added surfactants was also investigated by other authors [27–31] in various liquid surfactant mixtures for cleaning hard surfaces, as summarized in Table 4.
In the presented surfactant mixtures, performance in terms of cleaning efficiency and foaming is better than what is achieved by individual surfactants. This synergism, which depends on the composition as well as concentration of applied surfactants, is attributed to the formation of mixed micelles based on reduction of both the surface tension and CMC of a surfactant mixture [32–34].
Generally, nonionic surfactants have a great influence on the performance and efficiency of the initial SLES/AOS anionic formulation. Synergistic behavior of hard surface cleaner formulations, presented in Table 4, is also observed for anionic/amphoteric/nonionic (SLES/AOS/CAB/AO) and anionic/nonionic (SLES/AOS/DEA/AO, SLES/AOS/C12–C15–7EO/AO, SLES/AOS/C10–7EO/AO and SLES/AOS/C9–C11–7EO/AO) dishwashing formulations tested in our study. For these four-component surfactant formulations, noticeable correlation between CMC and γ, as well as cleaning performance, has been observed, especially for the mixture of SLES/AOS/ethoxylated alcohols/AO. The lower obtained CMC values indicate that they display better dishwashing performance, even in the presence of relatively low concentrations of added ethoxylated alcohols. This effect is very important in terms of practical application, since it is shown that these dishwashing formulations can be used in considerably lower concentrations. However, ethoxylated alcohols improved performance of the starting SLES/AOS formulation in terms of foam and soil removal and showed better cleaning application properties. But, on the other hand, biodegradability and irritability of these formulations were not significantly changed in comparison to the starting formulation. Preferred formulation 6 (SLES/AOS/C9–C11–7EO/AO) is a non-irritant for usual purposes, exhibits excellent cleaning properties and highly satisfying foaming performance as a liquid dishwashing detergent. From an application point of view, at both low and regular dishwashing temperatures, using anionic/nonionic SLES/AOS/ethoxylated alcohols/AO surfactant mixtures is recommended for better performance and efficiency of liquid dishwashing formulations.
References
Falbe J (1987) Surfactants in consumer products. Springer, Berlin. doi:10.1007/978-3-642-71545-7
Yuan XY, Rosen MJ (1988) Dynamic surface tension of aqueous surfactant solutions: I. Basic parameters. J Colloid Interface Sci 124:652–659. doi:10.1016/0021-9797(88)90203-2
Gambogi J, Kennedy S, Ambundo E (2009) Dishwashing with detergents. In: Zoller U (ed) Handbook of detergents, Part E: applications. Taylor & Francis Group, Boca Raton, pp 39–65
Rosen MJ (2004) Surfactants and interfacial phenomenon, 3rd edn. Wiley, New Jersey, pp 16–28
Szewczyk G, Burke J, Sackariason K (2005) Liquid dish cleaning compositions. US Patent 6,884,764 Serial No. 10/653,819
Meyers D (2005) Surfactant science and technology, 3rd edn. VCH, New York
Day JM (1975) Strength of bonding of food soils to dishes. J Oil Fat Ind 52:461–464. doi:10.1007/BF02637490
Nitsch C, Huttmann G (2002) Recommendation for the quality assessment of the cleaning performance of hand dishwashing detergent. SOFW J 128(5):11–15
IKW (1999) Methods for ascertaining the cleaning performance of dishwasher detergents. SOFW J 125(1):52–59
Jakubicki GJ, Warschewski D (1991) EP 0487169A1 Concentrated liquid detergent composition containing alkyl benzene sulfonate and magnesium, Colgate-Palmolive Co., Publication number 0 487 169 A1
Pancheri EJ, Oh Young S, Wise RM (1990) Liquid detergent composition containing polymeric surfactant. US Patent 4,904,359 Serial No. 07/218,437
Lai KY (1986) High foaming nonionic surfactant based liquid detergent. Colgate-Palmolive Co., US Patent 4,595,526
ASTM D4009-92 (2011) Standard guide for foam stability of hand dishwashing detergents. In: Annual book of ASTM standards, vol 15.04. Soaps and Other Detergents; Polishes; Leather; Resilient Floor Coverings, ASTM International, West Conshohocken. doi:10.1520/D4009-92R06
ASTM D1173-07 (2015) Standard test method for foaming properties of surface-active agents. In: Annual book of ASTM standards, vol 15.04. Soaps and Other Detergents; Polishes; Leather; Resilient Floor Coverings, ASTM International, West Conshohocken. doi:10.1520/D1173-07R15
SRPS ISO 696:2000 Surface active agents: Measurement of foaming power—Modified Ross-Miles method. Institute for Standardization of Serbia. http://www.iss.rs/en/standard/?natstandard_document_id=15636
Bikerman JJ (1973) Measurement of foaminess. Foams 10:65–97. doi:10.1007/978-3-642-86734-7_3
Ansett RM, Schuck EJ (1966) A miniature dishwashing evaluation method. J Am Oil Chem Soc 43:576–580. doi:10.1007/BF02641191
SRPS EN 12728:2012 Surface active agents: determination of foaming power—perforated disc beating method. Institute for Standardization of Serbia. http://www.iss.rs/en/standard/?natstandard_document_id=43410
SRPS EN ISO 10707:2009 Water quality: evaluation in an aqueous medium of the “ultimate” aerobic biodegradability of organic compounds—method by analysis of biochemical oxygen demand (closed bottle test), Institute for Standardization of Serbia. http://www.iss.rs/en/standard/?natstandard_document_id=23286
SRPS ISO 5815:1994: Water quality: determination of biochemical oxygen demand after 5 days (BOD5)—dilution and seeding method. Institute for Standardization of Serbia. http://www.iss.rs/en/standard/?natstandard_document_id=12876
Grammer-West N, Fitzpatrick JE, Jackson RL, Horton H, Damiano MA (1996) Comparison of the irritancy of hand dishwashing liquids with modified patch testing method. J Am Acad Dermatol 35:258–260. doi:10.1016/S0190-9622(96)90344-8
Cronin M (2005) Report on the Review of the Status of the Development of Alternatives to using Animals in Chemical Safety Testing and Identification of New Areas for Development or Research in the Context of the Proposed REACH Regulation. Liverpool John Moores University and The Fund for the Replacement of Animals in Medical Experiments, Liverpool, p 42
Williams R, Phillips JN, Mysels KJ (1955) The critical micelle concentration of sodium lauryl sulphate at 25° C. Trans Faraday Soc 51:728–737. doi:10.1039/TF9555100728
Carpena P, Aguiar J, Bernaola-Galván P, Carnero Ruiz C (2002) Problems associated with the treatment of conductivity-concentration data in surfactant solutions: simulations and experiments. Langmuir 18:6054–6058. doi:10.1021/la025770y
AOAC (1995) Official method 920.87—protein (total) in flour, final action. In: Cunniff P (ed) Official methods of analysis of the association of official analytical chemists, 16th edn. AOAC, Arlington, pp 12–13
Mild Surfactants for Personal Care Applications, Public ICC Personal Care, Clariant. http://www.essentialingredients.com/pdf/clariantmildsurfactants.pdf
Nazanin J, Behrooz A, Farrokh BM (2013) Synergism and performance optimization in liquid detergents containing binary mixtures of anionic–nonionic, and anionic–cationic surfactants. J Surfactants Deterg 16:115–121. doi:10.1007/s11743-012-1371-y
Huei LW, Salmiah A (2004) Dishwashing performance of mixed palm stearin sulfonated methyl esters—nonylphenol ethoxylate alcohol. J Surfactants Deterg 7:53–58. doi:10.1007/s11743-004-0288-8
Goloub TP, Pugh RJ, Zhmud BV (2000) Micellar interactions in nonionic/ionic mixed surfactant systems. J Colloid Interface Sci 229:72–81. doi:10.1006/jcis.2000.6954
Tamura T, Iihara T, Nishida S, Ohta S (1999) Cleaning performance and foaming properties of lauroylamidopropylbetaine/nonionics mixed systems. J Surfactants Deterg 2:207–211. doi:10.1007/s11743-999-0075-6
Bozetine I, Ahmed Zaid T, Chitour CE, Canselier JP (2008) Optimization of an alkylpolyglucoside-based dishwashing detergent formulation. J Surfactants Deterg 11:299–305. doi:10.1007/s11743-008-1089-z
Rosen MJ, Zhu BY (1984) Synergism in binary mixtures of surfactants-III: betaine-containing systems. J Colloid Interface Sci 99:427–434. doi:10.1016/0021-9797(84)90129-2
Rosen MJ, Murphy DS (1986) Synergism in binary mixtures of surfactants V. Two-phase liquid-liquid systems at low surfactant concentrations. J Colloid Interface Sci 110:224–236. doi:10.1016/0021-9797(86)90371-1
Lucassen-Reynders EH (1982) Surface interactions in mixed surfactant systems. J Colloid Interface Sci 85:178–186. doi:10.1016/0021-9797(82)90246-6
Acknowledgments
This work was partially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant no. 172015).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Blagojević, S.N., Blagojević, S.M. & Pejić, N.D. Performance and Efficiency of Anionic Dishwashing Liquids with Amphoteric and Nonionic Surfactants. J Surfact Deterg 19, 363–372 (2016). https://doi.org/10.1007/s11743-015-1784-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1784-5