Abstract
The objective of this paper is to report a feasibility study on the use of optical fibre and surface plasmon resonance (SPR) for determining detergent efficiency. The concept presented comprises a miniaturised dip-sensor for enabling automated on-line testing. In this way, the effect of formulation, concentration and temperature on the cleaning behavior of various surfactants and commercial cleaners is investigated. For this purpose, the decladded core of an optical fibre was sputtered with gold and afterwards coated with defined layers of stearic acid or animal fat to obtain a kind of model soil. The deposition of stearic acid was performed using a Langmuir–Blodgett through, and the sensor followed on-line the deposition of the respective monolayers by UV/VIS spectroscopy, appearing as a distinct and constant shift in wavelength. Moreover, functional coatings were applied above the gold layer to achieve a variation of the hydrophilicity of the sensor surface. The SPR sensor proved to be easy to use, accurate and flexible. It offers a new solution that could replace the existing methods for detergency sensing and with a customised design it could be a useful industry tool since the small size of the dip sensor promises massive testing. The experiment also showed that functionalising the sensing zone could act as a way to mimic the potential substrates for cleaning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants play a major role in the cleaning industry and a proper validation of the performance of final products is essential. To test the detergent efficiency, i.e. the cleaning power of detergents, a number of testing methods are already available. The methods that are found in the literature have deficiencies which will be mentioned below. One way is the visual comparison of samples by providing rating parameters [1]. The evaluation is based on the human factor and this makes the method unreliable. The chemical analysis of the remaining water after cleaning is a time-consuming method that requires bulky samples. A colourimetric method based on CIELAB has also been applied for evaluating detergents while comparing the colour differences [2]. This technique gives imprecise estimations and has high equipment costs [3]. The accuracy issues arise at each conversion because a standard colour encoding needs multiple colour transforms. Interfacial tension and contact angle measurements have been used for determining the cleaning efficiency, proposing an indirect method for testing that is not sensitive to the washing time and other washing parameters [4]. By using fluorescence measurements the detergency of surfactants has been evaluated quantitatively for actual plant operation [5]. Additionally, another method used surface reflectance for evaluating soiled multifibre fabrics for textile detergency [6]. The lack of sensitivity of these spectroscopic methods results from their inability to distinguish between fluorescent whitening agents and other fluorescent compounds. Moreover the method cannot be used for low concentrations. Also its high cost and the complex training for operators make the method unfavourable. Gas chromatography has also been proposed [7]. The main disadvantages of this destructive method is that the experiment cannot be followed on-line and moreover only a limited number of washing parameters can be tested. The use of hazardous solvents increases the risk for the user, the instrument cost is high, and the time-consuming sample preparation makes it inefficient.
The existing methods for testing detergent efficiency have major disadvantages that make them time-consuming and inaccurate. They are partially limited to small sample quantities and washing parameters, and furthermore they are unable to follow an on-line cleaning process. In the literature, the need for the development of a precise system for evaluation of cleaning process for a routine operation is underlined [8].
There has been pioneering studies on the use of surface plasmon resonance as a tool to determine the interactions of lipid layers with detergents [9–11]. The gradual removal of that kind of “model-dirt” was clearly visible as a shift of the resonance wavelength. For performing the tests, the layer was deposited on the prism of a commercial Biacore instruments (Kretschman configuration). The resonance wavelength was determined using monochromatic light as a function of the incidence angle of the incident beam. The great potential of SPR could thus already be proven in the field of detergency. The SPR platform proposed combines surface chemistry and detergency [12]. However, the set-up used in the above-mentioned studies uses a fixed prism and this might limit the automated and/or flexible measuring regime. A solution to this problem is provided by using of optical fibre sensing technology whereby the “incident angle scanning” of monochromatic beams is replaced by using monochromatic light propagating in a multimode fibre.
Sensor designs based on optics and photonics have undergone extensive research during the last two decades [13–16] due to the wide variety of optical phenomena that one can develop as sensing mechanisms. Unlike the typical communication fibres, which act as passive media for signals, the purpose of a sensing fibre sensor is to generate sensitive responses to a range of chemical and physical changes that occur in the surrounding of the fibre. Among many important advantages, optical fibres have the benefit of being simple and having high flexibility in design. They are preferred due to their light weight, small size, resistance to electromagnetic interference, high sensitivity, easy miniaturisation and cost effectiveness [17]. New alternative fields for standard optical sensing technologies provide better performance than the existing available methods and have the potential for novel innovative devices [18].
SPR optical fiber sensor presents high level of miniaturisation of SPR devices. They were firstly introduced by Jorgenson and Yee [19] and there is extensive literature based on their applications [20].
Experimental Procedures
Materials
For the Langmuir–Blodgett (LB) deposition, stearic acid (Grade 1, purity 99 %) chloroform (spectrophotometric grade 99.8 %), cadmium chloride (CdCl2 AR grade 99.5 %) and sodium bicarbonate (Na2HCO3 ≥99.5 %) were purchased from Sigma–Aldrich. Ultra-pure water (18.3 MΩ cm resistivity) was used as the subphase. It was obtained from a Milli-Q purification system (Millipore-Synergy UV). Both systems were provided by Sigma–Aldrich. Sodium dodecyl sulfate (SDS) and Triton X-100 [C14H22O(C2H4O)n] were the compounds used as cleaning products and they were obtained from Sigma–Aldrich. The animal fat and the two dish cleaning products: A (‘Dreft’ for professional use produced by Procter & Gamble) and B (‘Everyday’ produced by Colruyt group) were obtained from commercial sources. It has to be mentioned that the composition is not known and reverse engineering was not performed.
Sensor Design
In contrast to the Kretschmann design, the sensing surface in the optical fibre sensor is not a flat surface but the curved part of the optical fibre where the cladding has been removed. Different propagation modes are reflected at the gold surface and in the case of the resonance condition, the intensity of the reflected beam at the corresponding wavelength is reduced accordingly. Using the mirror deposited at the end of the fibre, the beam is redirected into the fibre and detected by an optical fibre UV/VIS spectrometer. The mirror itself does not contribute to the SPR signal because the gold layer thickness exceeds that of the sensing area as shown in Fig. 1.
The system consists of the optical sensor and three other spectrometer components: the light source, the bifurcated cable and the spectrometer (detector) connected to a PC. The sensing part has a length of approximately 1 cm and at the polished end of the optical fibre a gold mirror is deposited. Gold deposition was in all cases performed by sputtering. The fibre (Thorlabs-USA, product name FT400UMT) has a 400 μm core diameter and a 0.39 numerical aperture. A final image of the sensor is presented in Fig. 2.
As UV/VIS/NIR light sources, a deuterium and a halogen lamp were used (AvaLight-DH-S, Avantes, The Netherlands). During testing the warm-up of the light source (Avantes, The Netherlands) is crucial, thus the lamp was preheated for 20 min to obtain a stable signal. An optical fibre spectrometer (AvaSpec 2048, Avantes, The Netherlands) was used to collect the transmitted light, with a detection range from 200 to 1100 nm. The signal was acquired and analysed by a PC using the Avantes 7.0 Software (Avantes, The Netherlands). The dark and a reference spectrum in air were saved to be to calibrate the final spectra.
The resolution of the current fibre optic sensor was estimated to be 5 × 10−5 RIU [21]. Refractive index unit (RIU) measures the shifts in SPR wavelengths by refractive index changes. The spectral resolution is 0.1 nm and the average sensitivity is 2000 nm RIU−1. This is in agreement with the literature [22] where the average refraction index resolution is reported to be between 6 × 10−4 and 7 × 10−7 RIU.
Deposition Methods
LB Film Deposition
In order to investigate detergency, a model soil is needed. The LB technique was used for the deposition of the thin target film [23]. It provides a very good thickness control and offers thin-defined solid films. This is essential since a thin layer minimises the cleaning time [24]. Furthermore LB thin film deposition is favoured for optical fibre applications because of the broad range of the functional coatings that can be deposited. The deposition of LB films onto cylindrical surfaces [25] and particularly in optical fibres has been studied before, demonstrating chemical probes and non-linear optical effects [26–29].
All the LB films were prepared with a 5000 KSV (Finland) Langmuir–Blodgett trough. In order to provide a uniform LB multilayer film and to avoid dust deposition on the substrate the sensing area of the sensor was gently cleaned with methanol. A 5-mg/ml stearic acid solution in chloroform was spread onto the subphase to form a cadmium stearate interfacial film. The deposition speed was constant at 5 mm/min. Every layer was dried in air for 15 min after the lifting process and no time had elapsed when the substrate was dipped in water.
Dip Coating
The dip coating method using melted animal fat was evaluated as a faster deposition method. Generally a slower withdrawal speed gives a thicker coating because more coating is pulled onto the surface of the substrate before it has time to flow back into the solution. In our case the liquid is melted animal fat and the substrate is the sensing zone of the sensor. The thickness depends on the fluid viscosity, the fluid density, and the surface tension. The applied coating remains wet for several minutes until the layer dries. When the layer is cured, a new layer can be applied on top of it with another dip coating process. In this way, a multi-layer can be constructed.
Spin Coating Films
The spin coating technique was used for the deposition of thin polymer films onto the sensing zone during the mimicking tests in order to model substrates with varied hydrophilicity. The first step of the process is the deposit of fluid onto the centre of the substrate. Then, the substrate spins at high speed, in our case at 8000 rpm. The centripetal acceleration will cause the spreading of the resin and will enhance the evaporation of the solvent.
Measurement Procedure
The proposed sensor system consists of three major parts: the lipid film, the optical fibre sensor system and a computer. When a SPR probe is calibrated in air and then is dipped in water, a SPR curve is generated. When the sensing zone is clean the SPR transmittance curve is generated at a specific wavelength. When a lipid film is deposited on the sensor surface, the SPR curve will be modified because of the changes in the refractive index and thickness. Less light will couple to the gold surface in this case. If the deposited film is thin (under 300 nm) the change on the sensing zone will be demonstrated either as a shift in wavelength towards shorter wavelengths or, for thicker lipid films, as a dramatic reduction in SPR transmittance intensity. This is the first step of the tests that is the deposition of the lipid film.
Afterward this sensor will be dipped in a cleaning solution. The thickness will change because of the lipid film removal and therefore the SPR transmittance curve will follow the opposite direction with a trend to reach the initial clean state. This second step, the removal of the deposited lipid film, is the core of this research.
The SPR signal thus gives information about the cleaning profile of the surfactant which depends on the experimental conditions such as the concentration of the surfactant, the surfactant type and the temperature. Cleaning profile is defined as the level of soil removal from a substrate after the use of a cleaning agent such as a detergent or a surfactant solution. The cleaning efficiency presented is the mean of three replicate tests, and the standard deviation determines the error.
SPR is an optical technique where plasmons are produced by irradiating light on a thin metal (in this case, gold) layer. The plasmon characteristics are directly related to the reactions and changes in thickness on the gold surface. With a SPR sensor, chemical interactions and physical processes can be followed in real-time. The fibre optic based SPR systems are wavelength based and the surface plasmon excitation generated manifests as a resonant dip in the transmitted spectrum. As the refractive index of the sample on the sensing zone affects the propagation constant of surface plasmons, the wavelength at which the resonant attenuation of the fibre mode occurs is influenced. As a result, changes in the refractive index on the sensing region can be measured by determining the variations in the resonant wavelength or the transmittance intensity.
When melted animal fat is used, the layer is thicker (~500 nm) and no clear SPR curve is generated when the fat covers the sensing area due to saturation [30]. The transmitted output power of SPR based fiber optic sensor has a strong relation with the layer thickness and consequently with the lipid removal.
Thus the measurement with the SPR sensor was focusing on the difference in transmittance, rather than in the shift in SPR resonance wavelength. This analysis (SPR transmittance measurements) is mentioned in the literature and it was preferred for bulky target analytes and thick layers [31]. The removal of soil was defined from the difference between SPR peak intensity (see Fig. 11) before and after the cleaning process respectively as given by Eq. (1):
where T 0 = lower transmittance of light, when the sensor is dipped in the cleaning agent, before the animal fat deposition and T 1 = lower transmittance of light, after the sensor is dipped in the cleaning agent for 10 min, after the animal fat deposition.
As the LB layers are deposited and then removed from the sensing zone, by the surfactant solution, the refractive index changes and the wavelength shift will be used to give the desired information. In the case of LB stearic acid multilayer the layer thickness is around 16 nm—in the penetration depth limit where the SP wave can ‘feel’ the changes on the sensing zone. For the LB solubilisation, the wavelength difference was thus used to follow cleaning efficiency (see Fig. 3). The data was evaluated by using the removal % defined by Eq. (2):
where \(\lambda_{0}\) = wavelength when the sensor is dipped in the cleaning agent, before the LB deposition, \(\lambda_{\text{LB}}\) = wavelength when the sensor was just dipped into the cleaning agent after the LB deposition and \(\lambda_{1}\) = wavelength after the sensor was dipped in the cleaning agent (after cleaning).
After the seven stearic layers were deposited on the sensing zone, the sensor was immersed in a surfactant solution. From this point on, the removal of the lipid layer by solubilisation was measured by the SPR shift in wavelength. The sensor remained dipped inside the surfactant’s solution for 10 min and the cleaning efficiency of the surfactant was followed as a function of time.
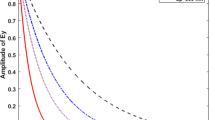
The exponential decay constant, t 1, of the transmittanceversus the cleaning time provides a reliable parameter describing cleaning kinetics (see Fig. 5). The decay constant is defined by Eq. (3) [32]:
Where y 0 is the Y offset transmittance, A is the amplitude, x is the time and t 1 is the time decay constant or cleaning time constant. The quantification in the evaluation of the detergency properties of this study will be based on the above-mentioned Eqs. (1)–(3).
Results and Discussions
The Langmuir–Blodgett Deposition Followed On-Line
The LB deposition of stearic acid on the SPR sensing zone was investigated. The purpose of the tests was to check if the sensor can sense the difference in the LB layers deposition of stearic acid which will act as a kind of model soil. The deposition was followed on-line by the sensor. The shift in wavelength because of the LB deposition of the increasing layers is given by the difference \(\lambda_{i} - \lambda_{0}\). Where \(\lambda_{0}\) = wavelength number at the lower transmittance of light when the sensing zone is dipped in the water subphase, before the LB deposition and \(\lambda_{i}\) = wavelength number at the lower transmittance of light, when the sensor is dipped in the water subphase for i number of LB layers (i = 2, 4, 6).
Figure 3 illustrates transmittance of the light versus wavelength, the four SPR curves show the gold sensing area with 0, 2, 6 and 6 LB deposited layers of cadmium stearate respectively towards the right direction. For the deposition of every two layers a distinct and constant shift in wavelength can be observed. As mentioned above, the sensor was calibrated in air and it is presented as ‘0 layer’. For two, four and six LB deposited layers, spectra were collected when the sensor was dipped in a water bath.
There is a shift in the transmittance minimum value in the SPR curves between the different layers due to the presence of lipid layers and water. In Table 1 and Fig. 4, the wavelength shift is reported for each two layer deposition. The presented results are the mean of three replicate tests and the standard deviation.
Optimum Surfactant Concentration
As expected, the cleaning behavior depends on the concentration of the surfactant. As the surfactant concentration increases the oil/surfactant solution interfacial tension decreases. Moreover, in the literature, it is reported that detergency increases up to a limit after which we observe a saturation effect when regardless of the increasing concentration no difference in cleaning efficiency occurs [33]. Indeed this was also confirmed in this study for stearic acid and animal fat removal where at a specific concentration an optimum performance was observed. The cleaning effectiveness of the aqueous surfactant solutions reaches a saturation state, where it increases to a maximum and after this point it shows a stable cleaning profile.
Stearic Acid LB
Pure water and three different aqueous solutions of SDS were used in different concentrations. The purpose was to test the efficiency of the surfactant concerning the concentration. Long fatty acids such as stearic acids are considered as solid oily contaminants, and in this case the removal mechanism is called solubilisation and occurs only above the critical micelle concentration (CMC) [34]. Solubilisation of the solid organic soil such as the seven multilayer of stearic acid is not taking place under the CMC [35] because no SDS micelles are formed.
The highest concentration (6 × CMC) showed 42.9 % lipid removal and when the concentration is 4 × CMC we observe almost the half (26.9 %) lipid removal and at the CMC 9.1 % removal (Table 2) calculated using Eq. (2). The above trend indicates that increasing concentration improves solubilisation. The plots of the time-dependent removal are given in Fig. 5 and the related parameters of Eq. (3) are presented in Table 3.
In Fig. 5 the cleaning profile of water and SDS at three different concentrations at CMC, 4 × CMC and at 6 × CMC is given. CMC refers to the critical micelle concentration of the SDS surfactant. An important observation was that the SPR wavelength shift is zero in the case of cleaning with pure water in the first 20 min investigated. This confirms that essentially no fatty acid layer is removed without the presence of surfactants. The detergent process appears as an exponential decaying curve when there is an effective removal of the lipid layer. At 4 × CMC the exponential decay constant is 0.68 while at the CMC it is 3.4 [see Eq. (3)]. One can thus obviously conclude that at a concentration higher than CMC SDS cleans better and faster than at the CMC.
Animal Fat
Triton X-100 and SDS enabled the removal of animal fat at all concentrations above the CMC. Under the CMC, no lipid removal was observed, however the penetration of the layer by surfactants leads to swollen layers. However, for increasing concentration it was observed that the cleaning profile improves (Fig. 6).
Particularly, when cleaning with Triton X-100 surfactant solution at the highest concentration investigated, being 10xCMC, 24.5 % of the animal fat layer was removed according to the definition of removal given by (2). At the lowest concentration, 0.1xCMC, no removal was observed; moreover an increase in transmittance was observed that can be translated into the swelling of the layer (Table 4). This is caused by the penetration of the surfactant and water into the fat film. An analogous swelling behavior was observed for 0.9xCMC concentration for the first 2 min and after it was followed by a decrease in the animal fat layer around 3.4 % of the initial stain. The trend explained above shows that also for surfactant concentration around and under the CMC, the molecules can penetrate into the lipid film present after dip coating.
SDS demonstrated an analogous but enhanced cleaning profile where, for 10× CMC concentration, 40.2 % fat animal was removed. At 0.9× CMC, we observed 5.3 % of the animal fat layer and for 0.1× CMC a positive increase of 1.9 % of the animal fat layer that is the swelling of the membrane as explained above (Table 4).
Temperature Dependence
The main purpose of the temperature experiments was to test the removal of the lipid layers at different temperatures since temperature is an important factors affecting cleaning efficiency. Important factors when considering the temperature behavior are the melting point of the functional coatings, the temperature dependence of the SPR sensor and the cloud point. The melting point of pure and dry stearic acid is at 69.6 °C which is higher than the investigated temperature range, 20–40 °C. Moreover the experiments took place at temperatures lower than the animal fat’s melting point to represent only the objective cleaning and not the melting of the lipid. The room temperature was approximately 19 °C and the melting point of the animal fat is 45 °C. It is known that for most soils an increasing temperature causes a reduction in viscosity which makes it easier for the soil to be removed. When the sensing zone covered with animal fat was dipped in heated water at 40 °C, the signal remained stable (tested for about 10 min) and this proves that the sensor will be able to follow the detergency and not the melting of the soil. Also an LB-coated sensor for a temperature scan in water showed no difference in the received signal.
A temperature variation leads to the shift and the broadening of the SPR signal. This occurs due to the temperature dependence of the refractive index. As it is mentioned in the literature [36] the SPR shift due to the change in temperature is in the scale of 1.5 nm and around 1 nm for the SPR peak broadening for 40 °C interval. The SPR shift of the sensor itself (without the coating) due to the increasing temperature was taken into account [12].
All the above issues were taken into account. The results based on our measurements with the SPR sensor, indicate that for increasing temperature both SDS and Triton X-100 show an enhanced cleaning profile (see Fig. 7). Furthermore at higher temperatures the non-ionic Triton X-100 surfactant gives a better profile than the anionic SDS. This is in agreement with literature data where it was shown that the efficiency of nonionic detergents rises considerably at increasing temperature when the performance of the anionic-based detergents remains modest [37].
Influence of Surfaces
In this section, the cleaning efficiency is studied as a function of surface hydrophilicity in order to mimic the nature of the cleaning substrate and investigate its potential influence in detergency process (Fig. 8). Seven stearic acid layers were deposited by the LB method onto three different substrates in order to study their influence on solubilisation.
The layers that were used to functionalise the sensing area were cellulose acetate and polyethylene with contact angles 52° and 104° respectively that represent a hydrophilic and a hydrophobic surface (Fig. 9). The contact angle measurements were performed with a DSA 10 Mk2 drop shape analysis system (Krüss). The gold sensing zone was covered with the functional layers by using the spin-coating technique as it was described previously. The results shown in Table 5 and in Fig. 10 suggest that the difficulty to remove fatty acid from a surface increases for more hydrophobic substrates such as polyethylene. The removal efficiency for the stearic acid layer is higher for cellulose acetate surface than for gold and likewise, the SPR signal shows a higher shift in wavelength than for the polyethylene substrate.
In literature, it is described that the removal efficiency is found to increase with decreasing substrate’s free energy ∆G [38]. Free energy for cellulose acetate is lower than gold and polyethylene accordingly and the removal thus follows the opposite trend. Consequently polyethylene has a worst removal profile compared with cellulose acetate and gold, i.e. and it was more difficult to clean compared with the other substrate [39].
Comparison Between Two Commercial Products
Two final products provided as dish washers were tested and compared for their efficiency to remove animal fat. The composition of the commercial products was not known and no reverse engineering was performed. The removal of the animal fat layer with commercial dish detergents was remarkably different depending on the nature of the product. The two dish washing products/liquids gave an immediate change in the lipid layer thickness upon the transfer of the sensor from air into the detergent’s solution. Product A was found to clean more efficiently than product B from the beginning of the process at the same concentration 1 %. Product A diluted in half concentration 0.5 % (solution C) still proved to have a noticeably better cleaning profile compared to solution B, although the latter has a higher concentration of 1 % (Fig. 11). That means that even if double the amount of the product is used, the cleaning efficiency for product B will remain worse than product A. The cleaning efficiency of the 3 solutions as averages of 3 replicate tests after 10 min of cleaning are presented in Table 6.
References
Cutler G (1986) Detergency: theory and technology. Marcel Dekker, New York
Ilec E, Simoncic B, Hladnik A (2009) Evaluation of surfactant detergency using statistical analysis. Text Res J 79(4):318–325. doi:10.1177/0040517508090886
Adesogan AT, Givens DI, Owen E (2000) Field and laboratory methods for grassland and animal production research. CABI. doi:10.1079/9780851993515.0000
Koran KM, Venosa AD, Luedeker C (2008) Evaluation of detergency, interfacial tension and contact angle for five surface washing agents. Int Oil Spill Conf 1:80. doi:10.7901/2169-3358-2008-1-785
Bradacs LK, Scheteli W (1964) Effectiveness of detergent solutions. J Am Oil Chem Soc 41(3):161. doi:10.1007/BF03024636
Gotoh K (2010) Evaluation of detergency using artificially soiled multifiber fabrics. J Oleo Sci 59(9):477–482. doi:10.5650/jos.59.477
Thompson D, Lemaster C, Allen R, Whittam J (1958) Evaluation of relative shampoo detergency. J Soc Cosmet Chem 36(4):271–286
Jennings WG (1965) Theory and practice of hard-surface cleaning. Advances in food research. Academic Press, New York, pp 325–458. doi:10.1016/S0065-2628(08)60151-2
Sigal GB, Mrksich M, Whitesides GM (1997) Using surface plasmon resonance spectroscopy to measure the association of detergents with self-assembled monolayers of hexadecanethiolate on gold. Langmuir 13(10):2749–2755. doi:10.1021/la961024f
Sonesson AW, Elofsson UM, Brismar H, Callisen TH (2006) Adsorption and mobility of a lipase at a hydrophobic surface in the presence of surfactants. Langmuir 22(13):5810–5817. doi:10.1021/la0531244
Gaspar A, Kecskemeti A, Gomez FA (2013) Use of surface plasmon resonance to study the adsorption of detergents on poly(dimethylsiloxane) surfaces. Electrophoresis 34(8):1249–1252. doi:10.1002/elps.201200573
Patitsa M, Pfeiffer H, Wevers M (2011) Fast and accurate determination of the detergent efficiency by optical fiber sensors. Proc SPIE Int Soc Opt Eng. doi:10.1117/12.884984
Orellana G, Moreno-Bondi MC (2005) Frontiers in chemical sensors: novel principles and techniques. Springer-Verlag. doi:10.1007/3-540-27757-9
Zourob M, Lakhtakia A (2010) Optical guided-wave chemical and biosensors II. Springer-Verlag, Berlin Heidelberg New York
Kiwanuka SS, Laurila T, Kaminski CF (2010) Sensitive method for the kinetic measurement of trace species in liquids using cavity enhanced absorption spectroscopy with broad bandwidth supercontinuum radiation. Anal Chem 82(17):7498–7501. doi:10.1021/ac1012255
Winquist F, Wide P, Lundstrom I (1997) An electronic tongue based on voltammetry. Anal Chim Acta 357(1–2):21–31. doi:10.1016/S0003-2670(97)00498-4
Wolfbeis OS (2004) Fiber-optic chemical sensors and biosensors. Anal Chem 76(12):3269–3283. doi:10.1021/ac800473b
Homola J, Yee SS, Gauglitz G (1999) Surface plasmon resonance sensors: review. Sens Actuators B-Chem 54(1–2):3–15. doi:10.1016/S0925-4005(98)00321-9
Jorgenson RC, Yee SS (1993) A fiberoptic chemical sensor-based on surface-plasmon resonance. Sens Actuators B-Chem 12(3):213–220. doi:10.1016/0925-4005(93)80021-3
Gupta BD, Verma RK (2009) Surface plasmon resonance-based fiber optic sensors: principle, probe designs, and some applications. J Sens. doi:10.1155/2009/979761
Pollet J (2010) Fiber optic surface plasmon resonance. From Plasmon theory to biosensor applications, Doctoraatsproeschrift nt 935, faculty of Biosensing of K.U. Leuven
Grunwald B, Holst G (2004) Fibre optic refractive index microsensor based on white-light SPR excitation. Sens Actuators a-Phys 113(2):174–180. doi:10.1016/j.sna.2004.02.014
Huang YMR (2008) Sensors: advancements in modeling, design issues, fabrication and practical applications. Springer-Verlag. doi:10.1007/978-3-540-69033-7
Ozdemir SK, Turhan-Sayan G (2003) Temperature effects on surface plasmon resonance: design considerations for an optical temperature sensor. J Lightwave Technol 21(3):805–814. doi:10.1109/JLT.2003.809552
Feigin LA, Lvov YM, Troitsky VI (1989) X-Ray and electron-diffraction study of Langmuir-Blodgett films. Harwood Academic Publishers, USA
Partridge M, Wong R, Collins M, James SW, Davis F, Tatam RP et al (2014) Modifying monolayer behaviour by incorporating subphase additives and improving Langmuir-Blodgett thin film deposition on optical fibres. Mater Chem Phys 144(1–2):179–185. doi:10.1016/j.matchemphys.2013.12.040
Mar M, Jorgenson R, Letellier S, Yee S (1993) In-situ characterization of multilayered Langmuir-Blodgett films using a surface plasmon resonance fiber optic sensor. Eng Med Biol Soc Proc 15th Ann Int Conf IEEE. doi:10.1109/IEMBS.1993.979277
Selfridge RH, Kowel ST, Stroeve P, Lam JYS, Higgins BG (1988) Optical fibers coated with non-linear Langmuir-Blodgett films. Thin Solid Films 160(1–2):471–476. doi:10.1016/0040-6090(88)90093-4
Elosua C, Matias I, Bariain C, Arregui F (2006) Volatile organic compound optical fiber sensors: a review. Sensors 1440–1465. doi:10.3390/s6111440
Embuscado ME, Huber KC (2009) Edible films and coatings for food applications. Springer-Verlag, New York. doi:10.1007/978-0-387-92824-1
Lahav M, Vaskevich A, Rubinstein I (2004) Biological sensing using transmission surface plasmon resonance spectroscopy. Langmuir 20(18):7365–7367. doi:10.1021/la0489054
Jennings WG (1959) Circulation cleaning III. The kinetics of a simple detergent system. J Dairy Sci 42(11):1763–1771. doi:10.3168/jds.S0022-0302(59)90806-9
Gotoh K, Nakata Y (2004) Detergency of oily contaminants as assessed with a quartz crystal microbalance and the Langmuir–Blodgett method. J Oleo Sci 53(10):489–495. doi:10.5650/jos.53.489
Gotoh K, Nakazawa N, Tagawa M (2001) Applying the Langmuir–Blodgett technique to a well defined model detergent system. Text Res J. doi:10.1177/004051750107100603
Sharma AK, Gupta BD (2006) Theoretical model of a fiber optic remote sensor based on surface plasmon resonance for temperature detection. Opt Fiber Technol 12(1):87–100. doi:10.1016/j.yofte.2005.07.001
Detergents in the changing scene (1975) J Am Oil Chem Soc 52(5):322A–323A. doi:10.1007/BF02633036
Gotoh K (2005) The role of liquid penetration in detergency of long-chain fatty acid. J Surfactants Deterg 8(4):305–310. doi:10.1007/s11743-005-0360-4
Shimomura K, Onozawa H, Komiyama J, Sehata A (2003) Lipid detergency as observed by the quartz microbalance method. J Home Econom Jpn 54(4):281–289
Acknowledgments
The authors are grateful to the Industrial Research fund (IOF -KP06/11) of KU Leuven for the financial support. In addition, the authors would like to express their gratitude to the Department of Chemistry/‘Molecular and Nanomaterial unit’ of KU Leuven, for providing the Langmuir–Blodgett deposition laboratory facilities. In addition special thanks go to Johan Vanhulst (KU Leuven) for the technical support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Patitsa, M., Pfeiffer, H. & Wevers, M. A Surface Plasmon Resonance Optical Fibre Sensor for Testing Detergent Cleaning Efficiency. J Surfact Deterg 18, 697–706 (2015). https://doi.org/10.1007/s11743-015-1698-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1698-2