Abstract
Current diagnostic biomarkers for ACS are mainly represented by troponin I and troponin T. Dosing of these two molecules often leads to false positive results, since their plasma levels can increase in several different systemic settings. Therefore, identification of new markers able to detect patients with acute coronary syndromes is an emerging priority. On this view, many studies have been performed on different microRNAs, mitochondrial peptides, inflammatory cytokines and adhesion molecules with very promising results. Besides their introduction in screening programs, further studies are now needed in the acute setting, beyond or in association with troponin levels. This will help to better discriminate the real occurrence of an ACS in many patients accessing the emergency department for chest pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To date, cardiovascular diseases represent the leading cause of death worldwide and, according to the World Health Organization, ischemic heart disease (IHD) and stroke rank among the top. Despite several attempts to realize reliable prediction models, we still cannot rely on efficient tools to estimate patient’s risk of developing acute coronary syndromes (ACS) for all individuals [1].
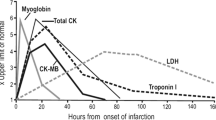
While current diagnostic biomarkers for ACS are mainly represented by troponin I (TnI) and troponin T (TnT). Dosing of these two molecules often leads to false positive results, since their plasma levels can increase in several different settings, such as heart failure, chronic kidney disease and sepsis and many other conditions [2, 3]. Moreover, blood levels of cardiac troponins only rise after myocardial cell death, a process that usually takes place after 2–4 h from the ischemic event, and they remain detectable in the peripheral blood for days [4]. Therefore, identification of new strategies to early detect patients with ACS is an emerging priority as it may allow to start an immediate treatment thus reducing mortality [1]; in this regard, a large number of studies have been conducted to identify a set of new biomarkers useful for an early assessment of ACS. In this review, we provide an update of the most recent literature about those novel biomarkers, with a special focus on microRNAs (Mir), mitochondrial peptides, inflammatory cytokines and adhesion molecules.
MicroRNAs
According to our research, miR-146a, miR-26a, miR-499 and miR-34 were the most studied (Table 1).
As for miR-146a, we found three studies that observed how it is involved in the development of coronary heart disease (CAD). A study on murine models demonstrated that miR-146a is involved in MI development by targeting two important toll-like receptors, thus inducing an inflammatory state that drives to atherosclerotic plaque formation [5]. Raitoharju et al. showed that miR-146a is significantly upregulated in coronary atherosclerotic plaques when compared with normal internal thoracic arteries [6], while Xue et al. studied patients with acute myocardial infarction (AMI) compared with healthy controls, observing how miR-146a represents an optimal diagnostic biomarker for this condition [7].
The same study also pointed out how miR-26a is a good biomarker in the early detection of AMI [7], even though, more recently, Chiang et al. observed that its expression is reduced in the infarct area of the heart in murine models [8]. The role of MiR-499 role in CAD is currently equivocal; while Wang et al. showed that it protects from H2O2-induced heart injury following MI in mice [9], Zhang et al. observed that its downregulation protects endothelial cells from inflammatory damage during CAD in a large cohort of patients and controls [10]. This is why its role in ACS warrants further investigation. MiR-34 family seems to be involved mostly in chronic heart diseases [11], despite results from studies in murine models suggest that targeting this particular miR and its substrate PNUTS might confer a cardio-protective effect after MI [12].
Studies on patients affected by CAD and in healthy controls showed that miR-133b and miR-720 express a negative correlation with the risk of CAD, while miR-21 and miR-145-3p correlate with a higher risk of acute cardiovascular events. Moreover, miR-133b and miR-21 seem to be early detectable in course of CAD [13, 14].
Similarly, Chen et al. demonstrated that miR-3113-5p represents a stable marker for an early diagnosis of cardiac injury following an acute cardiovascular event in mice [15].
Evidences about the cardioprotective role of miR-214 after CAD have been shown by Aurora et al. in murine models [16], while Hullinger et al. suggested that knocking out the expression of miR-15 may guarantee the same effect [17].
Finally, long non-coding RNAs (lcnRNAs) were shown to be good biomarkers for IHD, as observed by Yang et al.; in particular, they identified Coromarker as a stable, sensitive, and specific biomarker for CAD [18]. Similarly, Li et al. observed how Lipcar expresses a positive correlation with Tns and a negative correlation with left ventricular ejection fraction after MI [19].
Mitochondrial peptides
MOTS-c, SHLPs and Humanin are the most analyzed mitochondrial peptides that have been investigated as potential biomarkers for CAD (Table 2).
MOTS-c mainly produces metabolic effects: it prevents insulin resistance by enhancing glucose uptake and utilization, but also stimulates fatty acids oxidation and inhibits oxidative respiration [20]. However, MOTS-c has also been shown to protect against coronary endothelial dysfunction by reducing the release of inflammatory cytokines and adhesion molecules [21].
As for SHLPs (small humanin-like peptides), they are encoded by the same mDNA region of humanin, but seem to provide different biological effects. Several types of SHLPs have been found in different tissues, but only a very limited number of studies are available about these molecules [22].
Humanin has been thought to be beneficial in attenuating stress caused by ischemia/reperfusion injury [23], and there are evidences that treatment with humanin reduces both infarct size and loss of cardiac function following an ischemic damage [24]. Initially believed to guarantee a neuroprotective effect, Humanin also acts as a protector of vascular system from disease processes and toxic damage [25, 26]. In a study involving 40 patients undergoing coronary angiography whose endothelial function was tested, Widmer et al. demonstrated that preserved human coronary endothelial function is uniquely associated with higher systemic humanin levels [27].
Concerning ACS, several studies demonstrated that MDPs have a protective role in myocardial ischemia–reperfusion injury, probably by activating the AMPK-endothelial NO synthase-mediated signaling and regulating apoptotic factors. Indeed, administration of humanin to mice after a MI determined an improvement in left ventricular function and a decrease in the infarct area [24]. In particular, ischemic-reperfusion injury is mainly mediated by oxidative stress and Humanin has shown to alleviate mitochondrial damage induced by ROS [28].
Cytokines
Among all, interleukin (IL)-17 family seems to be the most involved in the inflammatory processes associated with CAD, despite other cytokines have been investigated as well (Table 3).
IL-17A was shown to be increased in plasma of patients with MI or unstable angina in a study focused on patients suffering from ACS and in controls [29]; at the same time, IL-17A levels increase in the infarct area of the heart after a MI and after left coronary artery ligation and reperfusion on murine models [30]. In these experiments, anti-IL-17A immunoglobulins and IL-17A knockout markedly ameliorate ischemia/reperfusion injury [31].
Analyzing coronary arteries from patients with atherosclerosis and healthy individuals, Xu et al. observed that IL-17E is significantly higher in coronary arteries and plasma of patients suffering from CAD compared to controls, and that the increase levels were proportional to the severity of the disease [32].
From the analysis of a large cohort of subjects with CAD and healthy controls, Tajfard et al. obtained results showing how IL-6 and MCP-1 can be considered predictors of high mortality in CAD patients [33] (Table 3).
In a randomized, double-blind trial, Ridker and his team demonstrated that reducing the inflammatory process through the anti-IL-1β canakinumab leads to a significantly lower rate of recurrence of cardiovascular events, thus identifying IL-1β as a major responsible for CAD [34] (Table 3).
Interleukin-1 receptor antagonist (IL-1RA) has also been fully investigated in the last years. IL-1RA is an endogenous inhibitor of IL-1β and acts as a counter-regulator of IL-1β activity. While dosage of IL-1β is very difficult to be obtained, due to its extremely low plasma levels, the level of circulating IL-1RA can be easily and reliably quantified. Consequently, IL-1RA may serve as a surrogate parameter for high IL-1β activity (Schofer et al. [35]). Promising results came from phase III studies on IL-1 blockade in patients with MI, thus identifying this biomarker as a therapeutic target (Leo et al. [36]). Zi-Heng et al. have demonstrated that IL-1 blockade treatment decrease cardiovascular risk in a recent meta-analysis. In fact, they analyzed eight randomized controlled trials including 15,647 participants and measured the effect of IL-1 blockade on different parameters. Results showed that IL-1 blockade reduce the risk of MACE (RR 0.88, 95% CI 0.82–0.94), unstable angina (RR 0.80, 95% CI 0.66–0.98) and heart failure (RR 0.44, 95% CI 0.22–0.87) [37]. Similar results were obtained by Herder et al., who reported the occurrence of a positive association between IL-1RA levels and cardiovascular diseases [38]. Schofer et al. conducted a study aimed at assessing the prognostic impact of IL-1RA in patients with CAD. By studying 1337 patients, they demonstrated that patients with IL-1RA levels in the highest tertile showed a higher prevalence of ACS, were more commonly treated with PCI and had a lower ventricular ejection fraction, and higher C reactive protein levels. Moreover, a significant association was found between IL-1RA levels and all cause of mortality (adjusted HR 1.45; 95% CI 1.16–1.82) and cardiovascular mortality (adjusted HR 1.93, 95% CI 1.33–2.80) [35]. A study performed by Hiort et al. demonstrated that high levels of eight biomarkers, including IL-1RA increased probability of non-obstructive coronary arteries 3 months after occurrence of MI [39]. Concerning mechanisms by which IL-1RA protect myocardial ischemia, Quian et al. have recently demonstrated that it may downregulate inositol trisphosphate three receptors, attenuating Ca++ overload and the consequent systolic and diastolic dysfunction and inhibit of apoptosis in injured cardiomyocytes thus reducing myocardial infarct size in vivo [40].
Adhesion molecules
Lately, some studies demonstrated that ICAM-1 and VCAM-1 take part in the inflammatory process that leads to the development of atherosclerotic plaque.
As for ICAM-1, its gene rs5498 has been studied in correlation with CAD, but controversial results have been obtained, since some authors identified this molecule as a risk factor [41], while others as a protective factor [42]. A meta-analysis by Yin et al. however, showed that ICAM-1 gene K469E is associated with an increased risk of acute cardiovascular events [37]. Finally, a recent study on human coronary arteries obtained from patients with different grades of atherosclerosis and/or sudden cardiac death demonstrated that the expression of ICAM-1 and 0X40L is positively correlated with the stability of the atherosclerotic plaque and sudden coronary death [43] (Table 4).
VCAM-1 expression on endothelial cells and formation of microparticles at the site of coronary plaque positively correlate with the extent of vascular inflammation in patients with MI, as provided by a study from Radeche et al. [44]. Furthermore, serum VCAM-1 seems to be associated with the extent of coronary lesions and it may represent an alternative to improve the cardiovascular risk classification in patients without CAD [45] (Table 4).
Discussion
Despite its high prevalence, diagnosis of IHD still represents a real problem worldwide; current tests are not able to identify all patients affected by this condition, thus explaining such a high mortality [3]. This is why looking for novel biomarkers may be a wise strategy for an early detection of patients at a high risk of ACS development.
MiRs are small, non-coding RNAs that regulate gene expression at a post-transcriptional level: they bind to specific, complementary mRNAs in order to modulate the translational process leading to protein synthesis. Therefore, miRs play a fundamental role in developmental timing, cell death, cell proliferation, hematopoiesis, and patterning of the nervous system [41]. Recently, different miRs have been shown to take part in the development of several cardiovascular conditions, including of IHD and MI, leading to the conclusion that their dosage in the peripheral blood offers the opportunity to monitor the biological status of the cardiovascular system [42,43,44,45]. In a recent review, De Rosa et al. described that some cardiac-specific and muscle-specific miRs might represent useful biomarkers for patients with ACS, since they show a good correlation with Tns. They also proposed miRs as an alternative diagnostic tool to high-sensitivity Tns (hsTns), considering their earlier detectability in the peripheral blood. Some studies, in fact, showed a very early myocardial release of some miRs within 30 min from symptom onset, with consequent significant elevation of miRs at a time where hsTn was still negative [46]. Furthermore, some research teams have reported that miRs can also be used as a marker of progression and prognosis of the disease [47, 48].
In our review, we identified a large number of miRs potentially involved in atherosclerosis and CAD; some of these may have a cardioprotective role, while others seem to correlate with a major heart injury during or after an acute cardiovascular event. Moreover, miRs are apparently easy and early detectable in the peripheral blood of patients with a CAD, and some of them express a direct correlation with the severity of the disease. Therefore, detection of specific clusters of miRs could represent in the future a useful tool to assess the risk of developing an acute cardiovascular event. Nevertheless, larger studies are needed to better understand the correlation between these molecule levels and IHD risk, and to identify only miRs really associated with the occurrence of acute cardiovascular events.
Mitochondrial-derived peptides (MDPs) are a new class of small peptides encoded by mitochondrial DNA that play a cytoprotective role and take part in a number of cellular processes such as cell survival, metabolism, response to stressors, and inflammation [22, 24]. Due to their numerous metabolic effects, some authors pointed out how MDPs are so important actors in the pathogenesis of atherosclerosis, thus considering them as optimal potential biomarkers for those conditions. From our research analysis, MOTS-c and humanin are the most studied so far, and both of them seem to act in protecting heart tissue from the damage occurring during and after a MI.
Currently, there is a common agreement upon the inflammatory process as a crucial phenomenon in atherosclerotic plaque formation and rupture, then causing ACS. Therefore, many inflammatory mediators have been investigated as potential biomarkers for IHD and possible target for novel therapies [34, 49,50,51, 54]. Our results show that IL-17 family better correlate with the development of CAD, since both IL-17A and IL-17E plasma levels are increased in such patients. IL-17, indeed, is produced by Th-17 lymphocytes, whose role in atherosclerosis is already well known [52,53,54]; nevertheless, a specific correlation with acute cardiovascular events may only be hypothesized and further investigation is needed in order to confirm this hypothesis. The same association has been found for IL-6 and IL-1β as well; the latter also represents a potential therapeutic target to reduce the rate of future cardiac ischemic attacks. Very promising results have also been reported for IL-1RA, which may discriminate cardiovascular risk and represent a potential therapeutic target in patients with ACS.
ICAM-1 and VCAM-1 are adhesion molecules involved in the leukocyte recruitment process during inflammation, which allow leukocytes to firmly adhere to the endothelium before their transmigration across blood vessels. Thus, it is reasonable to suppose that they might have a role in perpetuating the inflammatory process that leads to atherosclerosis and MI.
Despite the role of ICAM-1 is still controversial, it positively correlates with the stability of the atherosclerotic plaque and sudden coronary death. As for VCAM-1, instead, studies agree that its expression positively correlate with the extent of coronary plaques, thus suggesting a potential but concrete employment of VCAM-1 for risk assessment of CAD.
In conclusion, we currently need to exert a more precise assessment of CV risk and to identify biomarkers more precise that Ths for a fast and safe rule out of patients presenting in ED with chest pain. There are now several potential novel biomarkers able to early detect patients at a high risk to develop ACS, beyond Tns [4]. Besides their introduction in screening programs, further studies are now needed to test their predictive value in the acute setting, beyond or in association with Tn levels. This will help to better identify patients with ACS accessing the emergency department.
References
Velle-Forbord T et al (2019) Circulating microRNAs as predictive biomarkers of myocardial infarction: evidence from the HUNT study. Atherosclerosis. https://doi.org/10.1016/j.atherosclerosis.2019.07.024
Long B, Long DA, Tannenbaum L, Koyfman A (2019) An emergency medicine approach to troponin elevation due to causes other than occlusion myocardial infarction. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.12.007
Roffi M et al (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. https://doi.org/10.1093/eurheartj/ehv320
Gilardi E, Iacomini P, Marsiliani D, De Marco G, Covino M (2014) Biomarkers in the prediction and management of acute coronary syndromes: current perspectives. Res Reports Clin Cardiol. https://doi.org/10.2147/rrcc.s36294
Wang X et al (2013) Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc Res. https://doi.org/10.1093/cvr/cvs356
Raitoharju E et al (2011) MiR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere vascular study. Atherosclerosis. https://doi.org/10.1016/j.atherosclerosis.2011.07.020
Xue S et al (2019) Circulating miR-26a-1, miR-146a and miR-199a-1 are potential candidate biomarkers for acute myocardial infarction. Mol Med 25:1–12
Chiang MH et al (2020) miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia–telangiectasia mutated in myocardial infarction. J Cell Physiol. https://doi.org/10.1002/jcp.29537
Wang J et al (2014) miR-499 protects cardiomyocytes from H2O2-induced apoptosis via its effects on Pdcd4 and Pacs2. RNA Biol. https://doi.org/10.4161/rna.28300
Zhang YH, He K, Shi G (2017) Effects of microRNA-499 on the inflammatory damage of endothelial cells during coronary artery disease via the targeting of PDCD4 through the NF-Κβ/TNF-α signaling pathway. Cell Physiol Biochem. https://doi.org/10.1159/000484588
Bernardo BC et al (2012) Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1206432109
Boon RA et al (2013) MicroRNA-34a regulates cardiac ageing and function. Nature. https://doi.org/10.1038/nature11919
Kumar D et al (2020) Circulatory miR-133b and miR-21 as novel biomarkers in early prediction and diagnosis of coronary artery disease. Genes (Basel) 11(2):164
Gigante B et al (2020) MicroRNA signatures predict early major coronary events in middle-aged men and women. Cell Death Dis 11:10–12
Chen Y, Ye X, Yan F (2019) MicroRNA 3113-5p is a novel marker for early cardiac ischemia/reperfusion injury. Diagn Pathol. https://doi.org/10.1186/s13000-019-0894-1
Aurora AB et al (2012) MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. https://doi.org/10.1172/JCI59327
Hullinger TG et al (2012) Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. https://doi.org/10.1161/CIRCRESAHA.111.244442
Yang Y et al (2015) Plasma long non-coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin Sci. https://doi.org/10.1042/CS20150121
Li M et al (2018) Circulating long noncoding RNA LIPCAR acts as a novel biomarker in patients with ST-segment elevation myocardial infarction. Med Sci Monit. https://doi.org/10.12659/MSM.909348
Lee C, Kim KH, Cohen P (2016) MOTS-c: a novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radical Biol Med. https://doi.org/10.1016/j.freeradbiomed.2016.05.015
Qin Q et al (2018) Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2017.12.001
Yang Y et al (2019) The role of mitochondria-derived peptides in cardiovascular disease: recent updates. Biomed Pharmacother 117:109075
Yen K, Lee C, Mehta H, Cohen P (2012) The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. https://doi.org/10.1530/JME-12-0203
Muzumdar RH et al (2010) Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. https://doi.org/10.1161/ATVBAHA.110.205997
Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M (2009) Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor α/WSX-1/gp130. Mol Biol Cell. https://doi.org/10.1091/mbc.E09-02-0168
Lee C, Yen K, Cohen P (2013) Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab. https://doi.org/10.1016/j.tem.2013.01.005
Widmer RJ et al (2013) Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol 304(3):H393–H397
Thummasorn S, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N (2017) High-dose Humanin analogue applied during ischemia exerts cardioprotection against ischemia/reperfusion injury by reducing mitochondrial dysfunction. Cardiovasc Ther. https://doi.org/10.1111/1755-5922.12289
Liang J et al (2009) Myeloperoxidase (MPO) and interleukin-17 (IL-17) plasma levels are increased in patients with acute coronary syndromes. J Int Med Res. https://doi.org/10.1177/147323000903700331
Ávalos AM et al (2012) IL-17A levels increase in the infarcted region of the left ventricle in a rat model of myocardial infarction. Biol Res. https://doi.org/10.4067/S0716-97602012000200012
Liao YH et al (2012) Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2011.10.863
Xu Y et al (2020) The expression of interleukin-25 increases in human coronary artery disease and is associated with the severity of coronary stenosis. Anatol J Cardiol 23:151–159
Tajfard M et al (2017) Serum concentrations of MCP-1 and IL-6 in combination predict the presence of coronary artery disease and mortality in subjects undergoing coronary angiography. Mol Cell Biochem 435:37–45
Ridker PM et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. https://doi.org/10.1056/NEJMoa1707914
Schofer N, Ludwig S, Rübsamen N, Schnabel R, Lackner KJ, Ruprecht HJ, Bickel C, Landmesser U, Blankenberg S, Zeller T (2018) Prognostic impact of interleukin-1 receptor antagonist in patients with documented coronary artery disease. Int J Cardiol. 257:24–29
Buckley LF, Abbate A (2018) Interleukin-1 blockade in cardiovascular diseases: from bench to bedside. Bio Drugs 32:111–118
Zheng Z-H, Zeng X, Nie X-Y, Cheng Y-J, Liu J, Lin X-X, Yao H, Ji C-C, Chen X-M, Jun F, Su-Hua Wu (2019) Interleukin-1 blockade treatment decreasing cardiovascular risk. Clin Cardiol 42:942–951
Herder C, Heras Gala TL, Carstensen-Kirberg M, Huth C, Zierer A, Wahl S, Sudduth-Klinger J, Kuulasmaa K, Peretz D, Ligthart S, Bongaerts BWC, Dehghan A, Arfan Ikram M, Jula A, Kee F, Pietilä A, Saarela O, Zeller T, Blankenberg S, Meisinger C, Peters A, Roden M, Salomaa V, Koenig W, Thorand B (2017) Circulating levels of interleukin 1-receptor antagonist and risk of cardiovascular disease: meta-analysis of six population-based cohorts. Arterioscler Thromb Vasc Biol. 37:1222–1227
Hjort M, Eggers KM, Lindhagen L, Agewall S, Brolin EB, Collste O, Daniel M, Ekenbäck C, Frick M, Henareh L, Hofman-Bang C, Malmqvist K, Spaak J, Sörensson P, Hassan SY, Tornvall P, Lindahl B (2019) Increased inflammatory activity in patients 3 months after myocardial infarction with nonobstructive coronary arteries. Clin Chem. 65:1023–1030
Qian W, Zhao C, Li D, Dai R (2018) Mechanism of interleukin-1 receptor antagonist protection against myocardial ischaemia/reperfusion-induced injury. Arch Cardiovasc Dis 111:545–554
Hulok A et al (2014) Soluble cell adhesion molecules—does estimating sVCAM-1 and sICAM-1 concentration provide additional information about cardiovascular risk in patients with coronary artery disease? Adv Clin Exp Med 23:735–741
Liu A, Wan A, Feng A, Rui R, Zhou B (2018) ICAM-1 gene rs5498 polymorphism decreases the risk of coronary artery disease. Medicine (United States). https://doi.org/10.1097/MD.0000000000012523
Yin DL et al (2019) Association between the ICAM-1 gene polymorphism and coronary heart disease risk: a meta-analysis. Biosci Rep 39:1–7
Wang Y et al (2019) The role of OX40L and ICAM-1 in the stability of coronary atherosclerotic plaques and their relationship with sudden coronary death. BMC Cardiovasc Disord. https://doi.org/10.1186/s12872-019-1251-8
Radecke CE et al (2015) Coronary artery endothelial cells and microparticles increase expression of VCAM-1 in myocardial infarction. Thromb Haemost. https://doi.org/10.1160/TH14-02-0151
Dos Santos JC et al (2018) Relationship between circulating VCAM-1, ICAM-1, E-selectin and MMP9 and the extent of coronary lesions. Clinics. https://doi.org/10.6061/clinics/2018/e203
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Cheng Y et al (2010) A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci. https://doi.org/10.1042/CS20090645
Dong S et al (2009) MicroRNA expression signature and the role of MicroRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. https://doi.org/10.1074/jbc.M109.027896
Iaconetti C, Gareri C, Polimeni A, Indolfi C (2013) Non-coding RNAs: the ‘dark matter’ of cardiovascular pathophysiology. Int J Mol Sci. https://doi.org/10.3390/ijms141019987
Ren J et al (2013) Signature of circulating MicroRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS ONE. https://doi.org/10.1371/journal.pone.0080738
De Rosa S, Curcio A, Indolfi C (2014) Emerging role of micrornas in cardiovascular diseases. Circ J 78:567–575
Islas JF, Moreno-Cuevas JE (2018) A microRNA perspective on cardiovascular development and diseases: an update. Int J Mol Sci. https://doi.org/10.3390/ijms19072075
Sun T et al (2017) The role of microRNAs in myocardial infarction: from molecular mechanism to clinical application. Int J Mol Sci. https://doi.org/10.3390/ijms18040745
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Statement of human and animal rights
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piccioni, A., Valletta, F., Zanza, C. et al. Novel biomarkers to assess the risk for acute coronary syndrome: beyond troponins. Intern Emerg Med 15, 1193–1199 (2020). https://doi.org/10.1007/s11739-020-02422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-020-02422-z