Abstract
Whether the anemia increases the risk of mortality in patients with acute heart failure (AHF) remains unclear. This study aims to explore the relationship between anemia and outcomes in patients with AHF including subgroup analysis. This study included 3279 patients with hemoglobin available from the Beijing Acute Heart Failure Registry (Beijing AHF Registry) study. The primary endpoint was all-cause mortality in 1 year, and the secondary endpoint was 1-year all-cause events including all-cause death and readmission. Logistic regression models were applied to describe related variables of anemia in patients with AHF. Multivariate Cox proportional hazards models described associations of anemia with clinical outcomes in the overall cohort and subgroups. 45.4% of the patients were found anemic. They were older and had more comorbidities than non-anemic patients. Variables including older age, female, chronic kidney dysfunction (CKD), lower hematocrit, lower albumin, with loop diuretics applied, without beta-blockers, angiotensin-converting enzyme inhibitors /angiotensin receptor blockers (ACEIs/ARBs) and spironolactone applied in the emergency department (ED) were associated with anemia in AHF patients. Anemic patients had higher 1-year mortality (38.4% vs. 27.2%, p < 0.0001) and 1-year events rates (63.2% vs. 56.7%, p < 0.0001). After adjusted for covariates, anemia was associated with the increase of 1-year mortality (hazard ratio [HR] 1.278; 95% confidence interval [CI] 1.114–1.465; p = 0.0005) and 1-year events (HR 1.136; 95% CI 1.025–1.259; p = 0.0154). The severer anemia patients had higher risks both of 1-year mortality and events. In the subgroup analysis, the independent associations of anemia with 1-year mortality were shown in the subgroups including age < 75 years, male, body mass index < 25 kg/m2 and BMI ≥ 25 kg/m2, New York Heart Association (NYHA) functional class I–II and NYHA functional class III–IV, with and without cardiovascular ischemia, heart rate (HR) < 100 bpm and HR ≥ 100 bpm, systolic blood pressure (SBP) < 120 mmHg and SBP ≥ 120 mmHg, left ventricular ejection fraction (LVEF) < 40% and LVEF ≥ 40%, serum creatinine (Scr) < 133 umol/l, and with diuretics use, with and without beta-blockers use, without ACEIs/ARBs use in the ED. Anemia is associated with older age, female, CKD, volume overload, malnutrition, with loop diuretics, without beta-blockers, ACEIs/ARBs and spironolactone administration, and higher mortality and readmission in AHF. The risk associations are particular significantly obvious in younger, male, overweight, preserved LVEF, lower Scr, with diuretics and beta-blockers, without ACEIs/ARBs administration subgroups.
Clinical trial No. ChiCTR-RIC-17014222
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is a common condition in patients with chronic heart failure (HF) regardless of the level of left ventricular ejection fraction (LVEF) and is an efficient predictor of readmission and mortality [1,2,3,4]. Although almost half of patients hospitalized with acute heart failure (AHF) are anemic [5], the impact of anemia on prognostic still remains unclear. Data from large registries and randomized controlled trials have shown controversial results implying anemia played a different role in associating with both long-term [6] and short-term [5, 7,8,9,10] with age, gender and renal function adjusted mortality. The pathogenesis of HF-associated anemia considered multifaceted, for instance, volume overload dilution, chronic kidney dysfunction (CKD), malnutrition, and drugs such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEIs/ARBs) might be the key confounders of mortality in patients with AHF [11]. However, very limited evidence from literature unveiled the relationship of anemia and long-term mortality with key confounders adjusted in this patient population. There are two studies showed that the presence of anemia on admission was associated with an increased risk of mortality [6, 12]; in contrast, a recent report indicated that the relationship between anemia and 1-year outcomes was not associated in multivariate analysis, despite a significant association in univariate analysis [13]. To understand the risk association of anemia and 1-year outcomes in patients with AHF, we evaluated the independent effect of anemia on the clinical outcomes in patients with AHF.
Methods
Study design and data collection
The Beijing Acute Heart Failure Registry (Beijing AHF Registry) study was an observational, patient-centered, prospective, multicenter registry study recruited in a total of 3335 patients with AHF admitted in 14 emergency departments (EDs) in Beijing from 1st January 2011 to 23rd September 2012. It was designed to clarify the profile of AHF patients in Beijing, including demographics and clinical characteristics, current treatments, morbidity or mortality in ED stay and during the follow-up [14]. This study was conducted under the principles of the Declaration of Helsinki. Ethic approval was obtained from Institutional Review Board Approval of Fuwai Hospital (2010, approval number: 218). Data were collected only after detailed information regarding the study was provided and a signed written informed consent has been obtained from each patient.
Patients and definitions
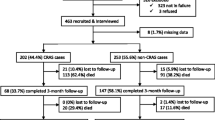
Patients presented any signs and symptoms of HF, as well as one of the following criteria, were defined as AHF: (i) symptomatic lung congestion confirmed via chest X-ray or (ii) objective findings consistent with left ventricular systolic dysfunction. This study analyzed patients enrolled in the Beijing AHF Registry with whose data on hemoglobin (Hb) at admission were available (Fig. 1). According to the World Health Organization (WHO) classification, anemia was defined as Hb < 13.0 g/dl for men and Hb < 12.0 g/dl for women. Among the anemia patients, mild anemia was defined as Hb ≥ 9.1 g/dl, moderate anemia was defined as 6.1 g/dl ≤ Hb < 9 g/dl, and severe anemia was defined as Hb < 6 g/dl [15]. The lower hematocrit was defined as hematocrit < 40% for men and hematocrit < 37% for women. The primary endpoint was all-cause mortality in 1 year, and the secondary endpoint was all-cause events including all-cause death and readmission in 1 year.
Statistical analysis
Data were reported as the median (with interquartile range, IQR) for continuous variables, or as numbers (percentages) for categorical variables. Mann–Whitney U test and Pearson chi-square (χ2) test for continuous and categorical variables were used respectively to estimate the differences between patients with or without anemia in the baseline. Kaplan–Meier curve and log-rank test were used to analyze the outcomes. Univariate and multivariate logistic regression models were used to analyze the correlation between anemia and age, gender, CKD, white blood cell (WBC) counts, hematocrit, albumin, loop diuretics use, beta-blockers use, ACEIs/ARBs use, spironolactone use in ED. Unadjusted and adjusted Cox proportional hazards regression models were used to estimate the impact of anemia and Hb on clinical outcomes in patients with AHF. Adjustment covariates in the multivariable model included age, female, body mass index (BMI), New York Heart Association (NYHA) functional class IV, cardiovascular ischemia, cardiomyopathy, diabetes mellitus, heart rate (HR), systolic blood pressure (SBP), serum creatinine (Scr), WBC count, hematocrit, diuretics use, beta-blockers use, and ACEIs/ARBs use in the ED. In addition, we performed multivariable Cox regression models for anemia relevant to AHF outcomes in various subgroups divided by age (stratified at 75 years), gender, BMI (stratified at 25 kg/m2), NYHA functional class (I–II vs. III–IV), cardiovascular ischemia, HR (stratified at 100 bpm) SBP (stratified at 120 mmHg), LVEF (stratified at 40%), Scr (stratified at 133 umol/l), diuretic use, beta-blockers and ACEIs/ARBs use in the EDs. All subgroup analyses were fully adjusted with the same covariates in the main multivariable models. Statistical analyses were performed using SAS software (version 9.3, Cary, NC). A 2-sided p value < 0.05 was considered to be statistically significant.
Results
Study population
Of 3335 patients who were originally enrolled in the registry, patients were serially excluded for unavailable Hb data at admission (n = 56). Thus, a total of 3279 patients were eligible for this study, 1490 (45.4%) patients were diagnosed with anemia based on the World Health Organization criteria (Table 1). Among the total cohort, 1137 (34.68%), 295 (9%) and 58 (1.77%) patients were diagnosed with mild, moderate and severe anemia, respectively. The median age of the study population was 71 (IQR 58–79) years old, with 46.9% female, and the median follow-up period was 372 (IQR 366–581) days.
Clinical characteristics
Baseline characteristics of AHF population are shown in Table 1. The median Hb of the non-anemic patients was 14.1 (13.3–15.1) g/dl, compared with 10.7 (9.1–11.6) g/dl for anemic patients. The anemic group was significantly older, more female patients, and comorbidities of previous ischemia, diabetes mellitus, hypertension and CKD. Compared with the non-anemic group, lower HR, higher SBP and LVEF, lower hematocrit and serum albumin, higher Scr and BNP/N-terminal pro-BNP (NT-proBNP) was observed in anemic patients. Patients who received intensive diuretics, vasodilators and inotropes intravenously instead of orally in the ED, had higher in-hospital mortality and less compliance after discharged.
Associations between clinical variables and anemia in AHF patients
In multivariate logistic analysis, characteristics including older age, female, CKD, hematocrit and albumin decreasing, loop diuretics applied and beta-blockers, ACEIs/ARBs, spironolactone unapplied in ED were independently associated with the occurrence of anemia in AHF patients. While, previous ischemia, lower WBC count was significantly associated with anemia in univariate logistic regression analysis (Table 2).
Associations between anemia and outcomes in AHF patients
The mortality and events including all-cause death and readmission in 1 year both showed significantly higher rates in the patients with anemia (mortality 38.4% vs. 27.2%; events 63.2% vs. 56.7%; p < 0.0001) (Fig. 2a and b). The severer anemia patients had poorer outcomes both of 1-year mortality and events rate. (Fig. 2c and d).
Kaplan–Meier curves of clinical outcomes. a All-cause mortality in 1 year of patients with and without anemia; b all-cause events in 1 year of patients with and without anemia; c all-cause mortality in 1 year of patients with mild, moderate and severe anemia; d all-cause events in 1 year of patients with mild, moderate and severe anemia. Significant differences in outcomes were displayed between patients with and without anemia. Significant differences in outcomes wee also displayed in patients with mild, moderate and severe anemia
In Cox regression models, anemia was independently associated with mortality (adjusted hazard ratio [HR] 1.278; 95% confidence interval [CI] 1.114–1.465; p = 0.0005) and all-cause events (adjusted HR 1.136; 95% CI 1.025–1.259; p = 0.0154). Moreover, a higher level of hemoglobin (per 1 g/dl) was independently associated with decreasing the risk of all-cause mortality (adjusted HR 0.938; 95% CI 0.912–0.964; p < 0.0001) and all-cause events (adjusted HR 0.965; 95% CI 0.945–0.986; p = 0.0011). The severer anemia patients had higher risks of both 1-year all-cause mortality and events (Table 3).
Figure 3 showed the independent impact of baseline anemia on the risk of mortality rate was significant in the subgroup of patients who were younger than 75 years old, male gender, with BMI < 25 kg/m2 and BMI ≥ 25 kg/m2, with NYHA functional class I-II and NYHA functional class III-IV, with and without cardiovascular ischemia, with HR < 100 bpm and HR ≥ 100 bpm, with SBP < 120 mmHg and SBP ≥ 120 mmHg, with LVEF < 40% and LVEF ≥ 40$, with Scr < 133 umol/l, with diuretics use in ED, with and without beta-blockers use in ED and without ACEIs/ARBs use in ED.
Discussion
In this population-based sample of patients hospitalized with AHF, anemia was an independent risk factor for death and events. Approximately half of the AHF patients had anemia on admission. Compared with non-anemia, anemic patients were significantly older, more female, with more comorbidities, presented a lower HR, higher SBP and an elevated BNP/NT-proBNP level, and received less guideline-recommended HF therapies. Several characteristics of AHF patients including older age, male, CKD, hematocrit and albumin decreasing, loop diuretics applied and beta-blockers, ACEIs/ARBs, spironolactone unapplied in ED were independently associated with anemia on admission. Higher mortality and events (all-cause death or readmission rate) were found in patients with anemia, and correspondence with the severity of anemia. On admission anemia was independently associated with a 27.8% increased risk of mortality and 13.6% increased risk of events in 1 year in patients with AHF.
Previous studies showed that the prevalence of anemia was relatively high in AHF patients (33–59.6%) who were older, not overweight, with ischemia etiology, with preserved ejection fraction and elevated creatinine, with intensive diuretics use [5, 6, 16, 17]. The prevalence of anemia in our data (47.3%) and baseline characteristics of patients with anemia were similar to these previous reports. Although pathogeneses of anemia in AHF are still unclear, hemodilution, inflammation, malnutrition (iron deficiency) and erythropoietin levels might be the main reasons for anemia in patients with AHF, and erythropoietin levels were affected by kidney function, medication of ACEIs/ARBs and beta-blockers [18]. In our study, CKD as well as hematocrit and albumin decreasing, on loop diuretics in ED, malnutrition and volume overload were all independently associated with anemia in AHF patients. Contrary to previous report [18], medication of beta-blockers, ACEIs/ARBs and spironolactone was less likely to develop anemia in patients with AHF. This may because patients with guideline-recommended therapy were more compliance with doctors’ advice and less severe ill. More evidence should be investigated in the impact of guideline-recommended medications on anemia occurrence in patients with AHF. Interestingly, HF patients with iron deficiency who treated with intravenous iron showed an improvement in the quality of life, exercise tolerance, and NYHA [19, 20]. Moreover, in the CONFIRM-HF trial [21], intravenous iron therapy was associated with a remarkable reduction in rehospitalizations for worsening HF and with an insignificant reduction of mortality.
A study of 182 patients from Von Haehling [12] showed patients with moderate or severe anemia had a significantly increased 12-month mortality after adjusting for age, NYHA functional class, systolic and diastolic blood pressure, and creatinine, which indicated anemia was an independent predictor of death. This finding is similar to ours. Recent reports [6, 12, 13] indicated that the anemia was associated with increased 1-year mortality. While independent associations between anemia and 1-year clinical outcomes were also showed after covariate adjusted in our study. Furthermore, events were increasing correspondence to the severity of anemia. And independent associations of continuous hemoglobin levels with clinical outcomes were also displayed in our study. Similar results could be found in the previous report, there was an inverse relationship between the risk of death and hemoglobin level that seems “almost linear” [8]. The continuous hemoglobin levels may reflect the over load volume, malnutrition, and severity of illness in the certain degree. In addition, anemia was diagnosed with certain levels of hemoglobin. The points above could explain the linear relationship between hemoglobin and outcomes risks.
Consistent with a previous study [6], we also found that the presence of anemia on admission was associated with a significantly increased mortality in patients with age < 75 years than 75 years or older. Anemia appears to be more common in older patients with AHF, however, the interaction of anemia, age and clinical outcomes is still unclear. Similar to Duncan [22] found that anemia affected mortality in patients of both genders with chronic HF but this effect is weaker in women, the impact of baseline anemia on mortality was more significant in male patients.
Whether the impact of anemia differs by heart failure with preserved (HFpEF) or reduced (HFrEF) ejection fraction is uncertain. The associations of anemia with 1-year mortality were both independently significant in patients with HFrEF and HFpEF, and the risk was higher in the HFpEF subgroup in our study. In an observational cohort, anemia was associated with patients with HFrEF instead of HFpEF after adjusted analysis [23]. While in another HFrEF cohort, there was no association of anemia at admission with all-cause mortality [17]. The incremental risks of death associated with anemia are more pronounced in AHF patients classified with HFpEF than HFrEF [24]. Previous report showed that anemic patients with HFpEF were less often treated with diuretics, and that anemia was only associated with diuretics in patients with HFrEF [24]. This raises the possibility that hemodilutional anemia prevalence may differ by HF type. In a small study involving 46 ambulatory HF patients identified with anemia, the examination of plasma volume by I-131 labeled albumin revealed 12% with HFpEF to be hemodilutional, compared to 41% with HFrEF [25]. In the PROTECT trial, involving 1969 patients hospitalized with HFrEF, hemoglobin levels were closely monitored by measurements on days 1–4 and day 7 of the hospitalization. Short term improvement in hemoglobin levels was attributed to diuresis and abatement of hemodilution, and associated with better mortality outcome [26]. The disparity in relative outcomes that we observed among anemic HFpEF and HFrEF patients may relate to differing anemia etiologies.
Low erythropoietin levels are associated with CKD and inhibition of angiotensin II [18]. That is, in the subgroups of AHF patients with Scr ≥ 133 umol/l and with ACEIs/ARBs medication were more likely to be anemic. While CKD and ACEIs/ARBs medication were two important independent prognostic factors for AHF, that anemia contributes little to mortality relative to these two confounders.
Study limitations
This is a retrospective study collecting the patient data from a registry. The dynamic changes of Hb level in follow up were not considered. Second, the pathogeny of anemia was unknown, especially data on iron deficiency were not collected. Because different types of anemia may have different prognostic impacts, this issue also remains to be investigated in future studies. Finally, this is an observational study with Chinese AHF patients, caution should be taken when generalizing the present findings to other populations.
Conclusions
Anemia is an independent risk factor for 1-year outcomes in patients with AHF. It is associated with age, cardiovascular ischemia and comorbidities, and increased the mortality and events in AHF, particularly in younger, male, less severe heart failure, and overweight individuals.
References
Felker GM, Shaw LK, Stough WG, O'Connor CM (2006) Anemia in patients with heart failure and preserved systolic function. Am Heart J 151(2):457–462
O'Meara E, Clayton T, McEntegart MB, McMurray JJV, Lang CC, Roger SD, Young JB, Solomon SD, Granger CB, Ostergren J, Olofsson B, Michelson EL, Pocock S, Yusuf S, Swedberg K, Pfeffer MA, for the CHARM Committees, and Investigators (2006) Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 113(7):986–994
Anand IS, Kuskowski MA, Rector TS, Florea VG, Glazer RD, Hester A, Chiang YT, Aknay N, Maggioni AP, Opasich C, Latini R, Cohn JN (2005) Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation 112(8):1121–1127
Mozaffarian D, Nye R, Levy WC (2003) Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J Am Coll Cardiol 41(11):1933–1939
Young JB, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O'Connor CM, She L, Sun JL, Yancy CW, Fonarow GC, for the OPTIMIZE-HF Investigators, and Coordinators (2008) Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE-HF registry). Am J Cardiol 101(2):223–230
Kajimoto K, Sato N, Takano T, Investigators of the Acute Decompensated Heart Failure Syndromes Registry (2015) Association between anemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care 4(6):568–576
Felker GM, Gattis WA, Leimberger JD, Adams KF, Cuffe MS, Gheorghiade M, O'Connor CM (2003) Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol 92(5):625–628
Tarantini L, Oliva F, Cantoni S, Cioffi G, Agnoletto V, Alunni G, De Cian F, Di Lenarda A, Lucci D, Pulignano G, Scelsi L, Maggioni AP, Tavazzi L (2013) Prevalence and prognostic role of anaemia in patients with acute heart failure and preserved or depressed ventricular function. Intern Emerg Med 8(2):147–155
Kosiborod M, Curtis JP, Wang Y, Smith GL, Masoudi FA, Foody JM, Havranek EP, Krumholz HM (2005) Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med 165(19):2237–2244
Silva RP, Barbosa PHU, Kimura OS, Sobrinho CRMR, Sousa Neto JD, Silva FAL, Silva Júnior GB, Mota RMS, Daher EF (2007) Prevalance of anemia and its association with cardio-renal syndrome. Int J Cardiol 120(2):232–236
Desai AS (2013) Hemoglobin concentration in acute decompensated heart failure: a marker of volume status? J Am Coll Cardiol 61(19):1982–1984
von Haehling S, Schefold JC, Hodoscek LM, Doehner W, Mannaa M, Anker SD, Lainscak M (2010) Anaemia is an independent predictor of death in patients hospitalized for acute heart failure. Clinical Res Cardiol 99(2):107–113
Tymińska A, Kapłon-Cieślicka A, Ozierański K, Peller M, Balsam P, Marchel M, Crespo-Leiro MG, Maggioni AP, Jankowska EA, Drożdż J, Filipiak KJ, Opolski G (2017) Anemia at hospital admission and its relation to outcomes in patients with heart failure (from the Polish Cohort of 2 European Society of Cardiology Heart Failure Registries). Am J Cardiol 119(12):2021–2029
Wang G-G, Wang S-J, Qin J, Li C-S, Yu X-Z, Shen H, Yang L-P, Fu Y, Zheng Y-A, Zhao B, Yu D-M, Qin F-J, Zhou D-G, Li Y, Liu F-J, Li W, Zhao W, Gao X, Wang Z, Jin M, Zeng H, Li Y, Wang G-X, Zhou H, Sun X-L, Wang P-B, Woo K-S (2017) Characteristics, management, and outcomes of acute heart failure in the emergency department: a Multicenter Registry Study with 1-year follow-up in a Chinese Cohort in Beijing. Chin Med J 130(16):1894–1901
Kazory A, Ross EA (2009) Anemia: the point of convergence or divergence for kidney disease and heart failure? J Am Coll Cardiol 53(8):639–647
Migone de Amicis M, Chivite D, Corbella X, Cappellini MD, Formiga F (2017) Anemia is a mortality prognostic factor in patients initially hospitalized for acute heart failure. Intern Emerg Med 12(6):749–756
Mentz RJ, Greene SJ, Ambrosy AP, Vaduganathan M, Subacius HP, Swedberg K, Maggioni AP, Nodari S, Ponikowski P, Anker SD, Butler J, Gheorghiade M (2014) Clinical profile and prognostic value of anemia at the time of admission and discharge among patients hospitalized for heart failure with reduced ejection fraction: findings from the EVEREST trial. Circ Heart Fail 7(3):401–408
Sîrbu O, Floria M, Dascalita P, Stoica A, Adascalitei P, Sorodoc V, Sorodoc L (2018) Anemia in heart failure—from guidelines to controversies and challenges. Anatol J Cardiol 20(1):52–59
Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, de Albuquerque D, Bocchi E, Vilas-Boas F, Moura LZ, Montera MW, Rassi S, Clausell N (2013) IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol 168(4):3439–3442
Gutzwiller FS, Pfeil AM, Comin-Colet J, Ponikowski P, Filippatos G, Mori C, Braunhofer PG, Szucs TD, Schwenkglenks M, Anker SD (2013) Determinants of quality of life of patients with heart failure and iron deficiency treated with ferric carboxymaltose: FAIR-HF sub-analysis. Int J Cardiol 168(4):3878–3883
Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, for the CONFIRM-HF Investigators (2015) Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 36(11):657–668
Duncan JA, Levin A (2005) Sex, haemoglobin and kidney disease: new perspectives. Eur J Clin Invest 35(Suppl 3):52–57
Kajimoto K, Sato N, Takano T, Investigators of the Acute Decompensated Heart Failure Syndromes Registry (2016) Association of anemia and renal dysfunction with in-hospital mortality among patients hospitalized for acute heart failure syndromes with preserved or reduced ejection fraction. Eur Heart J Acute Cardiovasc Care 5(7):89–99
Caughey MC, Avery CL, Ni H, Solomon SD, Matsushita K, Wruck LM, Rosamond WD, Loehr LR (2014) Outcomes of patients with anemia and acute decompensated heart failure with preserved versus reduced ejection fraction (from the ARIC study community surveillance). Am J Cardiol 114(12):1850–1854
Abramov D, Cohen RS, Katz SD, Mancini D, Maurer MS (2008) Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am J Cardiol 102(8):1069–1072
van der Meer P, Postmus D, Ponikowski P, Cleland JG, O'Connor CM, Cotter G, Metra M, Davison BA, Givertz MM, Mansoor GA, Teerlink JR, Massie BM, Hillege HL, Voors AA (2013) The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol 61(19):1973–1981
Acknowledgements
We thank all the study investigators for their contributions. We also appreciated Wei Li for his statistical assistance.
Funding
This study was supported by the Capital Health Development Research Fund (No. 2009-SHF04), Beijing Municipal Commission of Health and Family, Beijing, China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Statement of human and animal rights
Ethic approval was obtained from Institutional Review Board Approval of Fuwai Hospital (2010, approval number: 218).
Informed consent
Data were collected only after detailed information regarding the study was provided and a signed written informed consent has been obtained from each patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ye, Sd., Wang, SJ., Wang, GG. et al. Association between anemia and outcome in patients hospitalized for acute heart failure syndromes: findings from Beijing Acute Heart Failure Registry (Beijing AHF Registry). Intern Emerg Med 16, 183–192 (2021). https://doi.org/10.1007/s11739-020-02343-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-020-02343-x