Abstract
In developed countries, more than 80% of cases of acute pericarditis remain without an established diagnosis after a conventional and standard diagnostic approach. These cases are generally labelled as ‘idiopathic’, i.e. without a known cause. This lack of information is a matter of concern for both patients and clinicians. Some years ago, this term reflected the state of the art of scientific knowledge on the topic. Advances have changed this point of view, in light of available molecular techniques like polymerase chain reaction able to identify viral cardiotropic agents in pericardial fluid and biopsies. Furthermore, the remarkable efficacy of interleukin-1 antagonists, a therapy targeting the innate immune response, suggests clinical and pathogenic similarity between a proportion of patients with idiopathic recurrent pericarditis and classical autoinflammatory diseases. So, it seems useful to discuss the pros and cons of using the term “idiopathic” in light of the new knowledge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pericarditis encompasses many diseases with distinct causes. In the developed world, the most common category is presumed to be viral or idiopathic. This terminology reflects the traditional consensus that, even though pericarditis is often precipitated by a virus, the mechanisms that perpetuate inflammation and lead to recurrence are poorly understood [1, 2]. At best, the term “presumed viral pericarditis”, reflects a partial understanding of the pathophysiology, and at worst it may be misleading by overemphasizing an aspect of the pathogenesis that is neither related to severity nor a target for treatment.
Considering that testing for viruses with or without anti-viral therapy is rarely indicated in pericarditis, many clinicians prefer the term idiopathic. However, more recently in three important contexts, discussions of pericarditis have contradicted this term. First, similarities between idiopathic recurrent pericarditis (IRP) and autoinflammatory diseases are now recognized [3]. Second, and more importantly, therapies that target the innate immune system such as the anti-interleukin (IL)-1 agents, have demonstrated efficacy [4,5,6,7]. Third, molecular tools like polymerase chain reaction (PCR) are capable of identifying cardiotropic viral agents in the pericardial fluid and cardiac tissue [8].
In this review, we debate whether adherence to the term idiopathic pericarditis reflects simple inertia or an appropriately tempered response to the emerging understanding of this disease. Specifically, how convincing is the evidence that IRP is actually an autoinflammatory or an autoimmune disease? And most importantly, what is the clinical relevance for the clinician to abandon the term “idiopathic pericarditis?”
Pathophysiology
The immune-mediated pathophysiology of IRP is still not completely understood and may involve both autoinflammatory mechanisms of the innate immune system and the autoimmune mechanisms of the adaptive immune system. The inflammatory response of the innate immune system, typical of the so-called “autoinflammatory diseases”, is predominantly mediated by cytokines, mainly IL-1, while the inflammatory response of the adaptive immune system, typical of the so-called “autoimmune diseases”, is predominantly mediated by autoantibodies or autoreactive T lymphocytes [9]. Even if these two immune systems can overlap, patients with autoinflammatory disease have a predilection for inappropriate innate immunity and respond to therapies that target the primary mediators of disease, such as excessive IL-1 production.
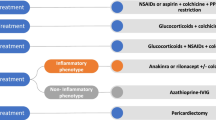
The innate immune system represents an immediate response to many diverse damage- and pathogen-associated molecular patterns (DAMPs and PAMPs) with downstream activation of the inflammasomes, cytosolic macromolecular structures composed of an adaptor protein, procaspase 1, and a sensor molecule [10] (Fig. 1). The sensor molecule contains a nucleotide-binding oligomerization domain-like receptor, nod-like receptor (NLR) and, when this sensor molecule is activated by DAMPs or PAMPs, the precursor pro-IL-1β is cleaved to active IL-1β. Circulating IL-1 then recruits cells of a myeloid lineage, namely neutrophils and monocytes, to the site of injury [11].
Proposed simplified pathogenesis of idiopathic recurrent pericarditis. PAMPs pathogens-associated molecular patterns, DAMPs damage-associated molecular patterns, TLR toll-like receptor, NLR nod-like receptor, AHA anti-heart antibodies, AIDA anti-intercalated-disk antibodies, NSAIDs nonsteroidal anti-inflammatory drugs, APC antigen-presenting cell
Autoinflammatory diseases, which are a heterogeneous group of diseases, are characterized by periodic inflammation mediated through the inflammasomes [12]. Prototypical autoinflammatory diseases include cryopyrin-associated periodic syndromes, which may result from a gain of function mutation in the NLR cryopyrin domain containing three sensor molecule, and familial Mediterranean fever (FMF), which may result from missense mutations in the gene encoding pyrin. Despite constitutive activation of the inflammasome, these diseases are characterized by intermittent inflammatory attacks with fever, serositis, and oligoarthritis [13]. This spontaneous onset of symptoms may also be seen in patients with IRP. Conversely, pericarditis occurs frequently in patients with typical autoinflammatory diseases [13]. Some cases of autoinflammatory disease with onset in adulthood might be related to the presence of low-penetrance mutations, and this might be the case of IRP [14].
These similarities and the efficacy of colchicine in the treatments of FMF relapses prompted investigators to pursue the “autoinflammatory” way in IRP 30 years ago [15]. Subsequent clinical trials with colchicine have consistently demonstrated a 50% relative risk reduction for pericarditis recurrence [1, 16]. Colchicine has properties of disrupting tubulin fibres, but also to modulate innate immunity blocking the processing of IL-1β [17]. More recently, anakinra, a short-acting IL-1 receptor antagonist, has shown interestingly good results in controlling very quickly the acute attack and decreasing relapses in IRP [18]. The success of therapies that target the innate immune response and the similarities to autoinflammatory diseases have bolstered the putative hypothesis that a subset of IRP might be a form of autoinflammatory disease mainly based on the activation of the innate immune system with a pivotal role of IL-1 [2, 3, 6]. On the other hand, simultaneous activation of the adaptive immune system has been hypothesized in some cases, since anti-heart autoantibodies (AHA) or anti-intercalated disk autoantibodies (AIDA), usually detected in autoimmune diseases, are found in 67.5% of adult IRP patients [19]. It has been shown that the presence of AIDA or high titre of AHA in IRP is associated with a higher number of recurrences and hospitalizations, respectively [19]. We can therefore speculate that in a proportion of patients with IRP, the inflammatory mechanism may also activate the “autoimmune” pathway. Nevertheless, these autoantibodies may simply be biomarkers without a pathogenic role [20].
The pros
The term “idiopathic” is used to describe diseases of unknown cause or mechanism. The term is derived from the Greek ιδιος (idios) “one’s own”, and πάθος (pathos) “suffering”; so idiopathy means approximately “a disease of its own kind”. The term “idiopathic pericarditis” has been used for centuries and can be found in PubMed since 1950.
While it is obvious that no diseases are really “idiopathic”, rather this is simply a statement of our awareness of the ignorance of the causes and mechanisms, this term has been widely used and accepted in pericardial diseases to report cases with an unknown cause following exclusion of the many common and treatable conditions (usually systemic inflammatory diseases, neoplastic diseases, tuberculosis, or other bacterial causes).
In Western countries with a low prevalence of tuberculosis, > 80% of cases of acute pericarditis remain without an established diagnosis after a conventional diagnostic approach, and this percentage also remains > 50% for those admitted to hospital [21,22,23]. The majority of these cases have a self-limiting, benign course with an overall good prognosis. However, the treatment, based on nonsteroidal anti-inflammatory drugs (NSAID) plus colchicine, remains unaffected by the absence of an established diagnosis [1].
While this ignorance of the cause should not encourage stopping efforts to improve our knowledge of aetiology and pathophysiology of pericarditis, it remains true that our understanding of the innate and adaptive immune responses in patients with pericarditis is still limited [2, 24], even acknowledging the proven efficacy of anti-IL-1 agents such as anakinra [5, 18].
On this basis, we think that the old term “idiopathic” is still a suitable working term for such cases, while we endeavour to study the disease and improve our basic and clinical knowledge of it; at the moment, this term identifies a group of patients with similar treatment and prognosis and has an honest labelling as “idiopathic”: what we still do not know.
The cons
The term “idiopathic” can sound reassuring for doctors, who are well aware that a definite aetiology is not recognized in the vast majority of such diseases. On the other hand, the term idiopathic is often alarming and confusing for many patients, because it implies the concept of uncertainty, regarding not only aetiology, but also therapy and particularly long-term prognosis [25].
Moreover, the term idiopathic masks and also amplifies our ignorance. In a biopsy study including 259 patients with a large pericardial effusion, the underlying cause was identified by molecular and immunohistological methods: 12% viral, 35% defined as autoreactive/lymphocytic, 2% bacterial, 15% traumatic, 28% malignant, and 8% other [8]. This demonstrates that when pericardial fluid or cardiac tissue is available, an aetiological final diagnosis can often be made.
In acute pericarditis, most cases are presumably viral in the first attack. Identifying the causative virus is generally not a productive endeavour, given the lack of any impact for treatment implications.
Recurrences are sometimes heralded by repeated viral infections, but they often occur due to rapid tapering of drugs, e.g. NSAIDs used at low doses, or given only orally and not intravenously in hospitalized patients, or, very frequently, they are due to a too rapidly tapered drug regimen (NSAIDs and particularly corticosteroids) [1, 2, 26, 27]. Other cases of recurrences are seen in patients with a predisposing genetic background [2, 28]. Possible non-invasive clues for autoimmunity are antinuclear antibodies (ANA, 43% of adults) [29], AHA [19] or AIDA (67.5% of adults), dry eyes, arthralgias, and a subacute course. Conversely, clues for an autoinflammatory pathogenesis are acute attacks followed by complete resolution, strikingly elevated C-reactive protein (CRP), high fever, and pleuropulmonary and systemic involvement [6]; in these patients, generally autoantibodies cannot be detected and familiar occurrence has been reported in 10% of the cases [28]. This clinical picture is particularly typical of paediatric cases of IRP [30], and this new pathogenetic paradigm has been initially proposed just for children [31]. Such phenotype looks strikingly similar to those observed in some autoinflammatory diseases, such as FMF, or tumour necrosis factor receptor-associated periodic syndrome (TRAPS), conditions where the inflammasome and IL-1 play a pivotal role. Typical mutations of these entities are, however, rare in IRP [32, 33], but new, still unknown mutations may be present [14].
These patients may have a diathesis related to the presence of genes encoding proteins involved in activation/regulation of inflammatory pathways; this diathesis may induce an exuberant autoinflammatory response [2, 3, 6], initiated nonspecifically by many different stimuli: virus, bacteria, trauma, minor intrapericardial bleeding (often iatrogenic), surgery, tissue necrosis, pleural or peritoneal inflammation, excessive cold, and finally inflammasome activation [2, 3, 6] (Fig. 1).
Anakinra is an IL-1 recombinant receptor antagonist that has been proposed as a potential treatment for IRP [5, 31]. We recently published a randomized controlled trial (AIR TRIP) showing that anakinra is effective in patients with cortico-dependent, colchicine-resistant IRP with elevated CRP [18]. For this disease, the term idiopathic seems somehow inappropriate when treated with anti-IL1 agents [5, 6, 18, 24, 31]. It seems in fact not appropriate to label as idiopathic a condition with a typical clinical course, good prognosis, and a spectacular response to a monotherapy with an anti-IL-1 agent [25]. The pathogenesis of recurrent acute pericarditis in a proportion of patients is comparable to most other inflammatory diseases, and we may consider abandoning the term idiopathic in this setting. Acceptable terms might be “autoinflammatory pericarditis” for the typical phenotype previously described, or “autoimmune pericarditis”, for those cases without “autoinflammatory” features and with positive autoimmune serology (e.g. organ-specific anti-heart or non-organ-specific autoantibodies); an option would be to simply use the term “acute pericarditis” and “recurrent pericarditis”, with no other adjective or attribute.
Discussion
Should we abandon the term “idiopathic” in case of acute/recurrent pericarditis in 2018 [25]? Is this term obsolete in the era of molecular diagnostics and with targeted drugs that block the IL-1 pathway in IRP patients [3, 5, 6, 18, 31, 34]? On the other hand, the term “idiopathic” is familiar to cardiologists, and has been included in the European guidelines on pericardial diseases (1) and in the American and European imaging guidelines on pericardial disease [35, 36]. The pro and the con arguments to keep the term “idiopathic” or abandon it are briefly presented in this paper.
Patients with these diseases are much tuned to social media [37], i.e. Facebook (Menlo Park, CA), with support groups on pericarditis. They often feel frustrated with their condition. Clinicians usually suggest that the cause of their pericardial condition is idiopathic or possibly viral, and then propose the shotgun treatment approach with anti-inflammatory medications including triple therapy (NSAIDs, colchicine, and steroids) [38].
What does current scientific evidence suggest for IRP? Even if the initial cause is thought to be a viral agent in most cases, in a large biopsy-proven series it was found that viruses may account for only a minority of cases [8]. Furthermore, IRP relapses are often seen following rapid tapering of the anti-inflammatory medications in the absence of a proven viral reinfection [2, 26, 27]. Recent advances have recognized that IRP may be related to autoinflammatory or autoimmune causes in patients with different clinical presentations. The autoinflammatory mechanism may result from activation of the inflammasome by a cardiotropic virus or a nonspecific agent in a genetically predisposed individual who has abnormal innate immunity. This will cause release of pro-inflammatory cytokines including interleukins that bring neutrophils and macrophages to the injured area [2, 3, 6]. Anakinra, an IL-1 receptor blocker, has shown in the AIRTRIP study its high efficacy, suggesting the pivotal role of this cytokine of the innate immunity in IRP promise in alleviating the symptoms as shown in the AIRTRIP study with targeted therapy against this pivotal cytokine of the innate immunity [18]. Similarly, the recent CANTOS study with canakinumab (human monoclonal antibody to interleukin 1 β) holds promise for targeting inflammation in coronary artery disease patients [39].
In summary, in the era of precision medicine, the adoption of terms such as ‘idiopathic’ actually highlights gaps in current scientific knowledge. However, this is not exclusive of pericardial diseases. In the specific context of IRP, recent basic and clinical research data allow us to take steps forward towards understanding the specific mechanisms leading to pathogenesis. The goal (and our proposal) for the near future is to gradually abandon the term ‘idiopathic’ acute/recurrent pericarditis and to adopt new terms attributed to the pathophysiology of the disease such as “autoinflammatory” or “autoimmune” as soon as we are able to identify such mechanisms in specific patient subsets. Further studies should focus on the actual existence of these two subgroups providing evidence of their clinical and pathogenic difference.
References
Adler Y, Charron P, Imazio M, ESC Scientific Document Group et al (2015) ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 36:2921–2964. https://doi.org/10.1093/eurheartj/ehv318
Cremer PC, Kumar A, Kontzias A et al (2016) Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol 68:2311–2328. https://doi.org/10.1016/j.jacc.2016.07.785
Xu B, Harb SC, Cremer PC (2017) New insights into pericarditis: mechanisms of injury and therapeutic targets. Curr Cardiol Rep. https://doi.org/10.1007/s11886-017-0866-6
Imazio M, Belli R, Brucato A et al (2014) Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomized trial. Lancet 383:2232–2237
Lazaros G, Imazio M, Brucato A et al (2016) Anakinra: an emerging option for refractory idiopathic recurrent pericarditis: a systematic review of published evidence. J Cardiovasc Med (Hagerstown) 17:256–262. https://doi.org/10.2459/JCM.0000000000000266
Brucato A, Emmi G, Cantarini L et al (2018) Management of idiopathic recurrent pericarditis in adults and in children: a role for IL-1 receptor antagonism. Intern Emerg Med. https://doi.org/10.1007/s11739-018-1842-x
Vitale A, Insalaco A, Sfriso P et al (2016) A snapshot on the on-label and off-label use of the interleukin-1 inhibitors in Italy among rheumatologists and pediatric rheumatologists: a nationwide multi-center retrospective observational study. Front Pharmacol 7:380 (eCollection 2016)
Maisch B, Rupp H, Ristic A et al (2013) Pericardioscopy and epi- and pericardial biopsy—a new window to the heart improving etiological diagnoses and permitting targeted intrapericardial therapy. Heart Fail Rev 18:317–328. https://doi.org/10.1007/s10741-013-9382-y
Van Kempen TS, Wenink MH, Leijten EFA, Radstake TR, Boes M (2015) Perception of self: distinguishing autoimmunity from autoinflammation. Nat Rev Rheumatol 11:483–492. https://doi.org/10.1038/nrrheum.2015.60
Muruve DA, Petrilli V, Zaiss AK et al (2008) The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103–107. https://doi.org/10.1038/nature06664
Park H, Bourla AB, Kastner DL, Colbert RA, Siegel RM (2012) Lighting the fires within: the cell biology of autoinflammatory diseases. Nat Rev Immunol 12:570–580. https://doi.org/10.1038/nri3261
Rigante D, Lopalco G, Vitale A et al (2014) Untangling the web of systemic autoinflammatory diseases. Mediat Inflamm 2014:948154. https://doi.org/10.1155/2014/948154 (Epub 2014 Jul 15)
Ozen S, Demirkaya E, Amaryan G et al (2014) For the Paediatric Rheumatology International Trials Organisation (PRINTO), Eurofever Project. Results from a multicenter international registry of familial Mediterranean fever: impact of environment on the expression of a monogenic disease in children. Ann Rheum Dis 73:662–667. https://doi.org/10.1136/annrheumdis-2012-202708
Cantarini L, Vitale A, Lucherini OM et al (2015) The labyrinth of autoinflammatory disorders: a snapshot on the activity of a third-level center in Italy. Clin Rheumatol 34:17–28. https://doi.org/10.1007/s10067-014-2721-0
Rodriguez de la Serna A, Guindo Soldevila J, Marti Claramunt V, Bayés de Luna A (1987) Colchicine for recurrent pericarditis. Lancet 2:1517
Verma S, Eikelboom JW, Nidorf SM et al (2015) Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 15:96. https://doi.org/10.1186/s12872-015-0068-3
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241. https://doi.org/10.1038/nature04516
Brucato A, Imazio M, Gattorno M et al (2016) Effect of Anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 316:1906–1912. https://doi.org/10.1001/jama.2016.15826
Caforio AL, Brucato A, Imazio M et al (2010) Anti-heart and anti-intercalated disk autoantibodies: evidence for autoimmunity in idiopathic recurrent acute pericarditis. Heart 96:779–784. https://doi.org/10.1136/hrt.2009.187138
Caforio AL, Adler Y, Agostini C et al (2017) Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 38:2649–2662. https://doi.org/10.1093/eurheartj/ehx321
Imazio M, Cecchi E, Demichelis B et al (2007) Indicators of poor prognosis of acute pericarditis. Circulation 115:2739–2744. https://doi.org/10.1161/CIRCULATIONAHA.106.662114
Fardman A, Charron P, Imazio M, Adler Y (2016) European guidelines on pericardial diseases: a focused review of novel aspects. Curr Cardiol Rep 18:46. https://doi.org/10.1007/s11886-016-0721-1
Gouriet F, Levy PY, Casalta JP et al (2015) Etiology of pericarditis in a prospective cohort of 1162 cases. Am J Med 128:784. https://doi.org/10.1016/j.amjmed.2015.01.040
Lotan D, Wasserstrum Y, Fardman A, Kogan M, Adler Y (2016) Usefulness of novel immunotherapeutic strategies for idiopathic recurrent pericarditis. Am J Cardiol 117:861–866. https://doi.org/10.1016/j.amjcard.2015.12.012
Brucato A, Maisch B, Valenti A (2017) Acute and recurrent pericarditis. Still idiopathic? J Am Coll Cardiol 69:2775–2776. https://doi.org/10.1016/j.jacc.2017.02.072
Brucato A, Brambilla G, Adler Y, Spodick DH, Canesi B (2006) Therapy for recurrent acute pericarditis: a rheumatological solution? Clin Exp Rheumatol 24:45–50
Imazio M, Brucato A, Cumetti D et al (2008) Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation 118:667–671. https://doi.org/10.1161/CIRCULATIONAHA.107.761064
Brucato A, Brambilla G (2005) Recurrent idiopathic pericarditis: familial occurrence. Int J Cardiol 102:529. https://doi.org/10.1016/j.ijcard.2004.06.012
Imazio M, Brucato A, Doria A et al (2009) Antinuclear antibodies in recurrent idiopathic pericarditis: prevalence and clinical significance. Int J Cardiol 136:289–293. https://doi.org/10.1016/j.ijcard.2008.05.020
Imazio M, Brucato A, Pluymaekers N et al (2016) Recurrent pericarditis in children and adolescents: a multicentre cohort study. J Cardiovasc Med Hagerstown Md 17:707–712. https://doi.org/10.2459/jcm.0000000000000300
Picco P, Brisca G, Traverso F, Loy A, Gattorno M, Martini A (2009) Successful treatment of idiopathic recurrent pericarditis in children with interleukin-1beta receptor antagonist (anakinra): an unrecognized autoinflammatory disease? Arthritis Rheum 60:264–268. https://doi.org/10.1002/art.24174
Brucato A, Shinar Y, Brambilla G et al (2005) Idiopathic recurrent acute pericarditis: familial Mediterranean fever mutations and disease evolution in a large cohort of Caucasian patients. Lupus 14:670–674. https://doi.org/10.1191/0961203305lu2197oa
Cantarini L, Lucherini OM, Brucato A et al (2012) Clues to detect tumor necrosis factor receptor-associated periodic syndrome (TRAPS) among patients with idiopathic recurrent acute pericarditis: results of a multicentre study. Clin Res Cardiol 101:525–531. https://doi.org/10.1007/s00392-012-0422-8
Emmi G, Urban ML, Imazio M et al (2018) Use of interleukin-1 blockers in pericardial and cardiovascular diseases. Curr Cardiol Rep 20(8):61. https://doi.org/10.1007/s11886-018-1007-6
Klein AL, Abbara S, Agler DA et al (2013) American society of echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the society for cardiovascular magnetic resonance and society of cardiovascular computed tomography. J Am Soc Echocardiogr 26(965–1012):e15. https://doi.org/10.1016/j.echo.2013.06.023
Cosyns B, Plein S, Nihoyanopoulos P et al (2015) European association of cardiovascular imaging (EACVI) position paper: multimodality imaging in pericardial disease. Eur Heart J Cardiovasc Imaging 16:12–31. https://doi.org/10.1093/ehjci/jeu128
Chung JE (2014) Social networking in online support groups for health: how online social networking benefits patients. J Health Commun 19:639–659. https://doi.org/10.1080/10810730.2012.757396
Imazio M, Gribaudo E, Gaita F (2017) Recurrent pericarditis. Prog Cardiovasc Dis 59:360–368. https://doi.org/10.1016/j.pcad.2016.10.001
Ridker PM, Everett BM, Thuren T et al (2017) Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 21:1119–1131. https://doi.org/10.1056/NEJMoa1707914
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Antonio Luca Brucato has received research grants from Acarpia, Sobi, and Lilly, and speaker fees from Sobi, Menarini, and Novartis; Massimo Imazio has received an institutional research grant from Acarpia e SOBI. Paul C. Cremer, Yehuda Adler, Bernhard Maisch, and George Lazaros and Alida L.P. Caforio declare that they have no conflict of interest; Alberto Martini has no conflict of interest to declare, since starting from 1 March 2016 he has become the Scientific Director of the G. Gaslini Hospital; therefore, his role does not allow rendering private consultancies resulting in personal income. He has performed consultancy activities on behalf of the Gaslini Institute for the companies listed below. Abbvie, Biogen, Boehringer, Bristol Myers and Squibb, EMD Serono, Janssen, Novartis, Pfizer, and R-Pharm. The money received for these activities are directly transferred to the Gaslini Institute’s bank account; Marco Gattorno has received research grants and speaker fees from Novartis and Sobi; Renzo Marcolongo has received fee for consultancy from SOBI; Giacomo Emmi has received fees for consultancy from SOBI, GSK, and Novartis; Allan L. Klein has received research grant from Kiniksa. The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Brucato, A., Imazio, M., Cremer, P.C. et al. Recurrent pericarditis: still idiopathic? The pros and cons of a well-honoured term. Intern Emerg Med 13, 839–844 (2018). https://doi.org/10.1007/s11739-018-1907-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-1907-x