Abstract
The etiology of pericardial effusions remains unresolved in many cases because not the full spectrum of diagnostic methods including cytology, histology, immunohistology and PCR on cardiotropic agents, which are currently available, used in many institutions. After comprehensive clinical workup and use of imaging methods, such as echocardiography and cardiac MRI, pericardiocentesis and epicardial and pericardial biopsy were carried out under pericardioscopical control of the biopsy site. Biopsies and fluid were evaluated by cytological, histological, immunological and molecular (PCR) methods in 259 patients of our tertiary referral center following an identical clinical pathway, diagnostic and therapeutic algorithm in all cases. A standard clinical pathway and the same diagnostic and therapeutic algorithms were used in all cases. When all methods are applied to patients with pericardial effusions, “idiopathic” pericardial effusion is no longer a relevant diagnosis. Autoreactive and lymphocytic pericardial effusions are the leading diagnosis in 35 % of patients in the prospective Marburg registry, followed by malignant effusions in 28 % of cases. Viral genome was assessed in fluid and epi- as well as pericardial biopsies in 12 %, followed by post-traumatic/iatrogenic effusions in 15 % and purulent/bacterial effusions in only 2 %. Pericardioscopy permits the macroscopic inspection of the pulsating heart and its disease-associated macroscopic alterations. It also permits safe and targeted biopsy for further investigations of the tissue. Therapy, tailored to the individual etiology, can be selected such as intrapericardial instillation in autoreactive effusions with triamcinolone and with cisplatin or thiotepa in neoplastic effusions. With this approach the recurrence of pericardial effusion can be avoided effectively. A comprehensive approach to the diagnosis of pericardial effusions in conjunction with pericardioscopy for targeted tissue sampling is the prerequisite for an etiologically based intrapericardial and systemic treatment, which improves outcome and prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and historical development of pericardioscopy
Percutaneous pericardioscopy opens a window to the “heart in action” in patients with pericardial effusion. As a diagnostic tool it permits the visualization of the pericardial sac with its epicardial and pericardial layers. It enables the interventional pericardiologist to take targeted biopsies from both layers, the pericardium and the epicardium, thus increasing the probability to get disease-specific results. The optical control minimizes complications, because the biopsy site can be inspected beforehand and after taking the biopsy sample [1]. It also allows the rhythmologist to select the site for ablation or implantation of an electrode for ablation therapy.
In 1957 the surgeon Johannes Volkmann performed [2] and 1 year later the pharmacologist Nikolai Petrovich Synitsin described [3] pericardial endoscopy with rigid instruments. Valuable contributions to the technique were added by Santos and Frater [4], Kondos et al. [5, 6], Little and Ferguson [7], Millaire et al. [8] and [9], Wurtz et al. [10], Nugue et al. [11], Porte [12] and Seferovic et al. [13]. Maisch et al. reported already in 1991 on flexible pericardioscopy by the transcutaneous subxiphoid approach in local anesthesia [14, 15], and we developed protocols for further analyses by histology, immunohistochemistry and molecular biology techniques for the biopsy and fluid samples as routine methods [16, 17]. Additional diagnostic progress came from the use of the atraumatic Touhy needle in small effusions [1], the PerDUCER [18] and the Marburg AttachLifter even in dry pericardium [1, 19]. Further input resulted from video documentation through the flexible fiberglass instruments instead of photography [20–26]. Pericardioscopy has been classified as IIa indication in patients with unknown pericardial diseases by the ESC guidelines, the only pericarditis guidelines worldwide [27]. With the application of histological, immunohistological and molecular investigations, biopsies and fluid made available through pericardioscopy, cytology, epi- and pericardial biopsy-specific intrapericardial treatment became a unique therapeutical approach in a selected compartment of the heart with little side effects [28–30].
Methods
After comprehensive clinical workup by imaging methods (echocardiography, cardiac MRI), pericardiocentesis and epicardial and pericardial biopsy were carried out under pericardioscopical control of the biopsy site. Biopsies and fluid were evaluated by cytological, histological, immunological and molecular (PCR) methods in 259 patients of a referral center following an identical clinical pathway, diagnostic and therapeutic algorithm in all cases.
Diagnostic and therapeutic algorithms
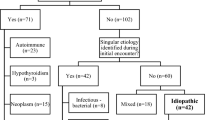
Since more than 20 years, we apply the same protocol for the diagnosis and treatment of the diseases mentioned in Fig. 1: When clinical symptoms such as precordial pain, palpitations, sinus tachycardia, dyspnea, physical weakness, hypotension, venous congestion and pulsus paradoxus (decrease in pulse amplitude or blood pressure during inspiration) point to pericardial syndromes, echocardiography is obligatory. If aortic dissection is excluded and pericardial effusion is demonstrated, we follow the clinical pathway in Fig. 1 for cardiac tamponade and large effusions, which is in essence identical with the ESC guidelines published in [27]. We use, however, all currently available technical options for pericardioscopy and pericardial and epicardial biopsy and for intrapericardial and systemic treatment [28–32].
If the effusion is very small, which would make the pericardial access more difficult, we offer the patient the diagnostic assessment by endomyocardial biopsy (EMB) under the conditions in Fig. 2. Left ventricular EMB is carried out most of the time in the context of heart catheterization and coronary angiography, together with left and right ventricular angiography. Here we follow the routine protocol of the European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID) in suspected myocarditis and inflammatory cardiomyopathy and the diagnostic and therapeutical algorithms outlined for left or right ventricular endomyocardial biopsy recently [28].
Flow chart from diagnosis to systemic treatment in small pericardial effusions in suspected perimyocarditis. ADV adenovirus, CMV cytomegalovirus, EBV epstein barr virus, EF ejectionfraction, EV enterovirus, HHV 6, herpes humanus virus 6, MRI magnetic resonance imaging, PM perimyocarditis, PE pericardial effusion, VT ventricular tachycardia
Patients in the Marburg pericardial disease registry
The pericardial disease registry, Marburg, reported here, comprises 259 patients (152 males, age 57 ± 14.8 years) (Fig. 3). It may be representative for a specialized tertiary referral center in Europe for pericardial and myocardial diseases. This contribution focuses on pericardial effusions large enough to permit pericardiocentesis and pericardioscopy with epi- and pericardial biopsy. The most important cohort are patients, who have been classified as autoreactive or lymphocytic virus-negative pericardial effusions with 35 %. Since in all patients PCR on cardiotropic agents was carried out in both biopsy and pericardial fluid—and both were negative—but either anticardiac antibodies or lymphocytes were identified in fluid and/or biopsy, the nomenclature autoreactive is justified for them. In instituions, which do not follow this detailed workup, the correct diagnosis could have been missed and they might have carried the label idiopathic. The second largest cohort comprises malignant effusion with 28 %. Bacterial infections are very rare, tuberculosis has been observed twice and borreliosis with effusions 3 times only in years prior to this consecutive analysis presented here. HIV patients with pericardial effusion were not seen in our center. The PCR-virus-positive cohort constitutes patients carrying the genome of Parvovirus B19 (n = 25), Epstein Barr Virus (EBV; n = 19), cytomegalovirus (CMV; n = 1), influenza (n = 1), hepatitis C (n = 1), Parvovirus B19 and EBV in 3 cases and HHV 6 and EBV in 1 case as double infections. The group of traumatic pericardial effusion comprised pericardial hemorrhage after pacemaker implantation in 15, after interventions in 16, after myocardial infarction in six and after direct chest trauma from accidents in four cases. Further details can be derived from the contribution by Pankuweit et al. in this issue.
Two of the patients in the group “other” suffered from a hypereosinophilic syndrome with pericardial effusion.
The medical pretreatment before pericardial puncture
Pretreatment which sedates the patients and takes away any pain during the procedure allows to perform pericardioscopy without the need of an anesthesiologist. After obtaining informed onsent and on-site echocardiography, sedation of the patient start with the intravenous injection of 5–15 mg midazolam (Dormicum®), 10 mg metoclopramid (Paspertin®) and 10 mg morphine. Local anesthesia is applied to the subxiphoid access site. If oxygen saturation drops, the patient is supplied with oxygen through a mask. If blood pressure is below 100 mmHg because of tamponade and premedication, we infuse saline intravenously.
Pericardiocentesis
For a safe entry into the pericardial sac, the puncturing needle is advanced under radiological control substernally in the direction of the left shoulder. The by far best radiologic setting to advance the puncturing Touhy needle to the diaphragm and the pericardial sac is using a biplane X-ray system. The p.a. view is helpful for the orientation of the trajectory of the needle to the left shoulder, and the lateral view is essential to target the halophenomenon (Fig. 4). To visualize the diaphragm, one injects a small volume of a mixture of contrast medium and saline (1:1) after the mandrel of the Touhy needle has been removed. When crossing the diaphragm, the rounded head of the Touhy needle should face the heart. At this time, the needle is advanced under constant gentle aspiration to see whether the pericardial space has been entered. If a hemorrhagic aspirate is evacuated, it may be difficult to distinguish it from blood of a cardiac chamber. Then it is advisable to inject some contrast medium through the needle, to record a pressure curve and to determine the hemoglobin concentration. After the pericardial sac is entered, a regular guide-wire with a rounded tip is advanced. Consequently, a 7 F introducer sheath is inserted over the wire. For safety reasons another flexible guide-wire is inserted via the same introducer sheath. The wires should be placed such that they form a loop from the anterior retrosternal space to the posterior antevertebral part of the pericardial space with the tip of wire at the lowest inferior location obtainable. The introducer sheath now holds two wires. Then the sheath is withdrawn over the two wires and placed over one of the guide-wires only. For the introduction of the sheath into the pericardial sac, the complete introducer set (perforator and sheath) is advanced over the wire, whereas the other guide-wire is just left in place. Over the sheath with one guide-wire, a pigtail catheter is introduced in the pericardial sac. Then the intrapericardial pressure is measured before the evacuation of the pericardial fluid to document tamponade at the beginning and the evacuation of it at the end [1].
a The halo phenomenon as equivalent of the epicardial fatpat (red arrows). The red arrows mark the rim of the pericardial effusion, the green arrow mark the Touhy needle entering the pericardial sac in the 90 degree lateral view. Use the lateral view and the halo phenomenon (red arrows). Note: The needle approaches the pericardium slowly (green arrow); steady manual aspiration is essential; stop the needle as soon as the effusion is aspirated; exchange for soft J-tip guidewire and after dilatation for a multi-holed pigtail catheter. b Verification of entry of the Touhy needle in the pericardial sac by injecting a small amount of contrast medium. Modified from [1] Fig. 5.2a, b (page 57) Fluoroscopic guidance of pericardiocentesis using the epicardial halo phonomenon in the lateral angiographic view. Red arrows are pointing to the halo phonemon, the green arrow points to the Touhy needle
If the pericardial sac has been missed and the needle has entered a cardiac chamber, the puncturing needle is slowly retracted by injecting small amounts of contrast medium. When the retracting Touhy needle gives its contrast medium no longer to the penetrated chamber but to the pericardial space, one tries to insert again a soft tip guide-wire over it followed by a pigtail catheter.
The pericardial fluid is then drained. For better visualization by the pericardioscope, which will be introduced later after widening the puncture site, rinsing of the pericardial sac by at least 100 ml saline is obligatory. Instillation of a limited amount of air improves visibility, but tamponade by air insufflations must be avoided, however.
For pericardioscopy the entry site of pericardiocentesis must be widened by the consecutive introduction of perforators up to 16 F. The final 16 F introducer set is inserted over the wire, and the perforator is exchanged with pericardioscope, which is still guided through the pericardial space by one of the wires. Since the fiberglass instrument can be curved and twisted when the guide-wire is still in place, a perforation of cardiac tissue is virtually impossible [1].
The pericardioscope and different light sources for investigation
Despite almost 50 years of worldwide use of endoscopy of the pericardium, no specific pericardioscopical device has reached the market from any endoscope-producing company.
Historically rigid endoscopes and mediastinoscopes were used since 1955 [3] and 1957 [2]. For flexible pericardioscopy, the interventional pericardiologists were forced to use devices designed for pulmonary endoscopy or urethroscopy with a small outer diameter of less than 5 mm, but large enough to accommodate a working channel for aimed pericardial and epicardial biopsy with a minimum of 1.2–2 mm and a second channel for the guide-wire [1].
Our experience with pericardial endoscopy started with a rigid device, initially used for urethroscopy more than two decades ago [14]. It allowed photo documentation of near-normal pericardium (Fig. 5a) and of diseased epicardium (Fig. 5b with protrusions and Fig. 5c with neovascularization and petechiae), but was limited in its range, since it could not adapt to the curved anatomy of the pulsating heart and the danger of causing a lesion to the epicardium by the rigid device was a major concern. The use of a 16 F Storz® flexible endoscope (AF 1101181) together with the digital AIDA image capture system permitted the inspection of the complete interior of the pericardial sac. In the sedated patient the glass-fiber instrument is introduced over the wire, which will run through one of the two channels of the instrument. The second channel is used for the bioptome (Cordis or HM bioptome as for endomyocardial biopsy) or bioptomes with a thorn inside the jaws to grasp the rigid pericardial tissue. The macroscopic “landscape” of the epi- and pericardium can be appreciated under white and blue light. Protrusions, petechiae and fibrinous threads can be identified and selected for subsequent biopsy sampling. By blue light investigation, the white surface represents fibrin or collagen tissue, whereby the dark spots are diagnostic for malignant or inflamed tissue or areas with petechial bleeding (Fig. 6a, b).
a Pericardial layer with a slight increase in vasculature assessed by pericardioscopy (from [1] p. 114 with permission). b Epicardial layer with protrusions from a metastasis in breast cancer (from [1] p. 114 with permission). c Epicardial neovascularization with little petechiae in malignant pericardial effusion in a patient with bronchus carcinoma (from [1] p. 114 with permission). d Calcification in constrictive-effusive tuberculous pericarditis. e Advancing the bioptome through the pericardioscope. The fingers of the left hand advance the bioptome, the fingers of the right hand open and close the jaws of the bioptome under radiological and endoscopical control. f At the epicardial site the bioptome approaches perpendicular to the epicardial surface
a Blue light evaluation of malignant pericardial effusion. The dark spots mark areas of bleeding, inflammation or malignancy. The white areas indicate fibrosis or fibrin. b Same biopsy site under white light illumination. With this mode, protrusion can be identified better, but epicardial fat or fibrin cannot be distinguished from malignant protrusions
Results and discussion
Macroscopic evaluation of pericardioscopical features
Macroscopic features of pericardioscopy can be correlated with the definite diagnosis after additional histological, immunohistological, cytological and virological diagnosis. A hemorrhagic effusion with protrusions (Fig. 5b) and neovascularizations with small islets of bleeding (Figs. 5c, 6a, b) are associated with a malignant effusion. Of note, neovascularization can also be found in 20 % of radiogenic and 27 % of viral or autoimmune effusions [32] (Table 1). Fibrin deposits are clearly nonspecific for any of the investigated cohorts. Historically, they have been a hallmark of uremic pericardial effusion as “Zottenherz”.
Biopsy sampling
Sampling of the biopsies should be done from both the epicardium and the pericardium. The pericardial specimens can be collected best by advancing the bioptome close to the cardiac silhouette in the posterior–anterior plane, then opening the jaws of the bioptome advancing it to the outer rim of the silhouette and pressing it to the pericardium (fig. 7 from [1] page 124 fig. 9.2b with permission). Most of the time the biopsy will catch either fibrin layers or collagen tissue, which then has to undergo histology, immunohistology and PCR assessment (see Pankuweit et al. in this issue). Seferovic et al. [13] have analyzed tissue from the pericardial site only and compared three different sampling modalities at that time without using PCR for viral genomes. Although the groups were small in size, the results were obvious: (a) Pericardioscopically targeted biopsy was better than a fluoroscopically guided pericardial biopsy. (b) The etiological information was best the more pericardial biopsies were taken under pericardioscopical control (Table 2).
Our institution has focused initially on biopsying preferentially the epicardial layer with the concept that the yield for viral DNA or RNA and for specific findings is better. To get well-sized biopsy samples, the forceps of the bioptome should grasp the tissue perpendicularly (Fig. 5e and f). When comparing retrospectively the yield of a definite diagnosis by collecting pericardial and epicardial samples, the concept of a better diagnostic yield by epicardial specimens holds for the diagnosis of viral perimyocarditis but not for neoplastic pericardial disease. In our most recent analysis epimyocardial biopsies from the dark spots sampled at blue light proved to be more informative than random sampling (unpublished) (Fig. 6a and b).
The combination of cytology and epi- and pericardial tissue sampling together gave the best results.
Pericardial fluid analysis
Pericardial effusion occurs when either the production of pericardial fluid increases or its clearance is reduced or both occur simultaneously. It may be clear as in hydropericardium, pale or yellowish as in viral or autoreactive effusions or look milky as in chylopericardium [1, 32–34]. Serosanguinous or hemorrhagic effusions can be found in malignant as well as post-pericardiotomy, rheumatologic and traumatic effusions but may also occur because of iatrogenic lesions during pericardiocentesis into an originally nonhemorrhagic fluid [1, 35].
Analysis of bacteria in pericardial fluid can be restricted to cases of sepsis, cases of endocarditis with suspected abscess formation, patients after surgical interventions, suspected tuberculosis and immunosuppressed patients or HIV-positive patients [1, 27]. Blood cultures should be always taken simultaneously beforehand and before the administration of antibiotics.
Pericardial fluid cytology
Fluid cytology may separate malignant from nonmalignant effusion. When neoplastic cells are detected, the specificity is high, but their sensitivity may vary due to treatment or sampling error [1]. A negative report does not eliminate malignant pericardial effusion from the differential diagnosis in tumor patients due to a fairly large proportion of false-negative findings [36, 37] or the fact that nonmalignant effusions can also occur after radiation therapy [38] or by infection under immunosuppressive therapy. Then differential diagnosis is truly a challenge with therapeutic implications. In a meta-analysis of four studies from 93 tumor patients, 51 % were attributed to primary and secondary malignancies, while in the remaining 49 % other pericardial disorders were found, including 15 % of “idiopathic” pericarditis. Accordingly, they calculated for a cytological examination of pericardial effusions in tumor patients a specificity of 100 % and a sensitivity of only 87 % [39]. In contrast, Krikorian et al. [40] described in 123 patients specific etiologies such as malignancy, bacterial infection, chylous effusions and hemopericardium in only 24 % of cases, thus leaving the term “idiopathic” effusion for too many “unknown” etiologies. Unfortunately viral or autoreactive effusions remained unconsidered. These etiologies should have been investigated and included in the diagnostic repertoire nowadays [1].
This diagnostic dilemma can be solved best in a synoptic approach to cytology, epi- and pericardial biopsy, PCR analysis of the fluid and the biopsy, pathohistology and immunohistology of the tissue specimens [32].
Biomarkers and biochemical markers in pericardial fluid
The fluid may contain an abundance of pro- or anti-inflammatory cytokines and biomarkers of inflammation, heart failure and malignancy. Since no generally accepted levels for tumor markers in pericardial effusion yet exist, mean values of normal concentrations, standard deviations and a clear-cut association with malignancy or other forms of pericardial inflammation have to be established in each institution. It is well accepted that tumor markers in pleural and pericardial fluids can be above “normal” not only in malignancy but also in pericardial inflammation. We have analyzed simultaneously in suspected malignant pericardial effusion CEA (carcinoembryonic antigen), NSE (neuron-specific enolase), CA 125 (carbohydrate antigen 125), CYFRA 21-1 (cytokeratin-19 fragments) and AFP (alpha-fetoprotein) [for details see 1]. Karatolios et al. have undertaken this differentiation for some biochemical markers and biomarkers in this issue of HFR.
Lymphocytes and cardiac autoantibodies in the pericardial fluid and peripheral blood
The dominant cell population in autoreactive and viral pericardial effusions are lymphocytes [1]. They can also be found in tuberculous and bacterial effusion [41]. In lupus erythematodes or rheumatic arthritis patients, elevated concentrations of antinuclear antibodies and anti-DNA antibodies, immune complexes and cryoglobulins were found [42], but even in high titers, ANA may not be diagnostic of lupus serositis, because they were also described in malignant effusions [43].
In myocarditis and perimyocarditis, antisarcolemmal (ASA), antimyolemmal (AMLA) and antifibrillary antibodies (AFA) of different immunoglobulin subclasses can be detected to varying degrees in the peripheral blood of patients [44–47]. They are signatures of autoreactive forms of myocarditis, if demonstrated both in endomyocardial biopsies and in the peripheral blood [44–47]. We interpret them, when detected in the pericardial effusion and in blood samples also as part of an active humoral immune process, if they fix complement and are of the IgG subclass, and/or if they are of the IgM subclass [1]. Examples of bound ASAs (Fig. 8a), circulating and intrapericardial AMLAs (Fig. 8b) and AFAs (Fig. 8c) from the pericardial effusion are given in Fig. 8a, b and c. If detected in sera they indicate a recent activation of B cells both in the peripheral blood. If they are bound to the epicardial biopsy and fix complement, they are indicative of a humoral immune process also directed to the epi- and pericardium (see below). Pericardial disease with epimyocardial biopsy has not been part of the consensus statement of the cardiologic societies [48], but was considered so far only in our and the European classification as a relevant prognostic component in heart diseases [28, 44–47, 49, 50].
a Demonstration of bound antisarcolemmal antibodies (ASAs) detected in a 62-year-old male patient with recurring autoreactive pericardial effusions in the epicardial biopsy. The ASAs belonged to the IgG subclass, titer 1:80, and fixed complement. b Antimyolemmal antibodies (AMLA) detected in pericardial fluid of the same patient as in a with a virus negative, therefore autoreactive effusion of the IgG subclass, titer 1:80. c Antifibrillary antibodies in a 54-year-old female patient after radiation therapy in mamma carcinoma and pericardial effusion without cytologic evidence for tumor cells; titer 1:40
Histology
The demonstration of metastases or tumor in the biopsy specimens from the pericardium or epicardium is diagnostic for a malignant pericardial effusion. This is self-evident. We recently analyzed biopsies in 68 patients, who had a tumor anamnesis. Interestingly, the histology of the 42 patients, who were classified later by a combination of cytology and histology as being affected from a malignant effusion, histology was diagnostic in 10 patients (24 %) only. The larger contribution to the diagnosis was given by 37 of the 42 patients (88 %) by cytology. But only the combination of both, cytology and histology, constituted the final diagnosis [32]. The exclusion of tumor in pericardial fluid and epi- and pericardial biopsy permitted the diagnosis of radiogenic pericardial effusion in 15 patients on the one hand and of viral pericarditis (virus-positive by PCR) or autoimmune (PCR-negative) pericarditis in 11 patients.
Immunohistology
The binding of immunoglobulins and complement to the surface of the epicardium and sarcolemma (Fig. 8a) as well as the presence of lymphocytes (CD 45R0; T4-helper or T8-suppressor T cells) in the epicardial (more frequent) and in the pericardial layers (rare) was considered indicative for an ongoing autoimmune process, when a malignant process was ruled out. In malignant effusions an abundance of T cells, neutrophils and macrophages was not uncommon.
Molecular analysis by PCR
The detailed methods and the results have been described by Pankuweit et al. in this issue. The most often detected virus was parvovirus B19 in 49 % of cases (n = 25) followed by EBV in 37 % of cases (n = 19). PCR was tested in all patients, both in biopsies and in the pericardial fluid.
Clinical pathway and treatment algorithms
Intrapericardial disease-specific treatment
In patients with large effusions of unknown origin with or without tamponade, we followed the clinical pathway outlined in Fig. 1. After pericardiocentesis the pericardial sac was rinsed regularly with at least 100 ml saline to dilute inflammatory cytokines, cells and eventual infective agents. After biopsies were taken, the pigtail catheter was left in place, 80 mg gentamycin was given intrapericardially (i.p.), and the patient was transferred to the ICU for further monitoring. The puncture site was kept under sterile conditions, and fluid was withdrawn from the pericardium at least once daily until less than 25 ml fluid per day could be aspirated (prolonged drainage). The patient was also given antibiotic prophylaxis i.v. in all cases.
As soon as the results from cytology, histology, immunohistology and PCR permitted a definitive and final diagnosis, disease-specific intrapericardial and/or systemic treatment was decided and, after obtaining informed consent, given to the patient. Figure 1 outlines the treatment strata.
In neoplastic pericardial effusion, most of them due to bronchus carcinoma (22/42) or breast cancer (8/42), 30 mg/m2 cisplatin in 100 ml of 0.9 % saline was given in a single slow injection through the intrapericardially located pigtail catheter. It was kept there for 24 h and then evacuated. Cisplatin is an alkylating agent with additional antitelomerase activity. In our study [29, 31] 41 of the 42 patients could be promptly discharged. In the mean follow-up of 8.5 + 3.2 months, none of the patients treated with cisplatin died due to cardiac tamponade, and relapses within 6-month follow-up were recorded in eight patients only. Three of them suffered from breast cancer, one from lung cancer and four from other tumors. The success rate of intrapericardial cisplatin corresponds to similar data reported from smaller groups of patients with lung cancer [51, 52]. Cisplatin was highly effective immediately together with pericardiocentesis but less successful after 6 months in the prevention of a recurring effusion in three of our eight patients with breast cancer, who had to undergo a second pericardial puncture [31]. Colleoni et al. reported on the instillation of thiotepa (15 mg on days 1, 3 and 5), which is also an alkylating agent with sclerosing and cytostatic activity, an immediate response to the treatment of nine breast cancer and 11 lung cancer patients, but also a recurrence in three patients during follow-up [53]. Their data correspond to work by Girardi et al. [54] and Martinoni et al. [55].
The disease-specific intrapericardial treatment for autoreactive and lymphocytic pericarditis with crystalloid triamcinolone is an excellent anti-inflammatory treatment in patients with a negative PCR on viral genomes in biopsy and pericardial fluid. It avoids systemic side effects in many cases and allows a high local dose. The two treatment regimens in 84 patients with 600 and 300 mg/m2 body surface showed comparable high success rates: Recurrence was prevented in 92.6 % of the patient group treated with 600 mg/m2 and 86.7 % of those treated with 300 mg/m2 after 3 months and 86.0 versus 82.1 % after 1 year. The instillation was retained in the pericardium, and due to the crystalloid suspension, triamcinolone stays active for 3–4 weeks. Nevertheless, it was well tolerated, even though it was accompanied by 0.5 mg colchicine tid after drainage of the effusion. Transient iatrogenic Cushing was observed more frequently in patients receiving the higher intrapericardial dose (29.6 vs. 13.3 %).
Pericardiocentesis in uremic pericarditis is a rare necessity nowadays due to effective hemo- or peritoneal dialysis. In the rare refractory cases, evacuation of the effusion and triamcinolone instillation has been reported to be effective [56–59].
Purulent pericarditis is a rare, acute and fulminant illness, which is fatal if untreated. Predisposing conditions include immunosuppression, alcohol abuse and cardiac surgery with superinfection. Despite prompt percutaneous pericardiocentesis, extensive rinsing of the pericardial cavity, best by surgical drainage with permanent saline irrigation and maximal systemic antibiotic treatment, the prognosis is still poor. Intrapericardial antibiotic instillation is not enough [1], and mortality rate is about 40–50 % [60]. Instead of surgery, pericardiocentesis and frequent irrigation of the pericardial cavity with urokinase, streptokinase or r-tpa was successfully attempted in some small series of patients [60–64]. Intrapericardial urokinase has been also reported to prevent later constriction by Cui et al. [65].
In viral pericardial effusion, pericardiocentesis, saline rinsing and gentamycin as sclerosing therapy are nonspecific intrapericardial treatment options. Further systemic antiviral treatment is similar to the treatment for myocarditis by first line i.v. immunoglobulin therapy in enteroviral, CMV, EBV and Parvo B19 infection and an attempt with oral valganociclovir in HHV 6 perimyocarditis and viral perimyocarditis refractory to immunoglobulins. These treatments are still in evaluation in controlled or randomized trials (Figs. 1 and 2) [28, 47].
Perspectives
To access normal pericardium in the absence of any effusion is still an exception, but it has been successfully attempted by rhythmologists for epicardial mapping and ablation therapy by using the Touhy needle [66, 67]. Intrapericardial access has been attempted by the PerDUCER with partial success only [68]. Novel approaches have been the Marburg Attacher and AttachLifter [69] both with endoscopic visualization of the pericardium before lifting the epicardium away by controlled suction. Thus, a safe entry of the puncturing needle is warranted followed by a flexible guide-wire. For locomotion in the pericardial sac and on the surface of the epicardium, refined instruments have been designed such as the Heart-Lander, which has been tested so far in porcine hearts only [70, 71]. Epicardial ablation of ventricular tachycardia [66, 67] or localized application of stem cells or growth factor cocktails may become a reality in the future not far away [69].
Conclusions
By application of cytological, histological, immunohistological, molecular, viral and bacterial methods, idiopathic pericardial effusion is no longer an accepted diagnosis. On the basis of a correct etiological diagnosis, treatment algorithms have been developed for the different subgroups permitting causative therapy, thus improving prognosis as well.
References
Maisch B, Ristic AD, Seferovic M, Tsang SMT (2011) Interventional pericardiology. Pericardiocentesis, pericardioscopy, pericardial biopsy balloon pericardiotomy and intrapericardial therapy. Springer, Heidelberg
Volkmann J (1957) Perikardioskopie und Kontrastdarstellung des Herzbeutels mit anatomischen Grundlagen [Pericardioscopy and contrast imaging of the pericardium including essentials of its anatomy]. Z Aerztliche Fortb 24:1105–1108
Sinitsyn NP (1958) Method of implantation of the cannula into the thorax for visual observation of coronary circulation [in Russian]. Biull Eksp Biol Med 39(3):74–76
Santos GH, Frater RWM (1977) The subxiphoid approach in the treatment of pericardial effusion. Ann Thorac Surg 23:467–470
Kondos G, Rich S, Levitsky S (1986) Flexible fiberoptic pericardioscopy for the diagnosis of pericardial disease. J Am Coll Cardiol 7:432–434
Kondos G, Rich S, Levitsky S (1986) Flexible fiberoptic pericardioscopy. Chest 90(5):787–788
Little AG, Ferguson MK (1986) Pericardioscopy as adjunct to pericardial window. Chest 89(1):53–55
Millaire A, Wurtz A, Brullard B et al (1988) Value of pericardioscopy in pericardial effusion. Arch Mal Coeur 81:1071–1076
Millaire A, Wurtz A, de Groote P, Saudemont A, Chambon A, Ducloux G (1992) Malignant pericardial effusions: usefulness of pericardioscopy. Am Heart J 124(4):1030–1034
Wurtz A, Chambon JP, Millaire A, Saudemont A, Ducloux G (1992) Pericardioscopy: techniques, indications and results. Apropos of an experience with 70 cases. Ann Chir 46(2):188–193
Nugue O, Millaire A, Porte H et al (1996) Pericardioscopy in the etiologic diagnosis of pericardial effusion in 141 consecutive patients. Circulation 94(7):1635–1641
Porte HL, Janecki-Delebecq TJ, Finzi L, Metois DG, Millaire A, Wurtz AJ (1999) Pericardioscopy for primary management of pericardial effusion in cancer patients. Eur J Cardiothorac Surg 16(3):287–291
Seferovic PM, Ristic AD, Maksimovic R, Tatic V, Ostojic M, Kanjuh V (2003) Diagnostic value of pericardial biopsy: improvement with extensive sampling enabled by pericardioscopy. Circulation 107:978–983
Maisch B, Drude L (1991) Pericardioscopy—a new diagnostic tool in inflammatory diseases of the pericardium. Eur Heart J 12(Suppl. D):2–6
Maisch B, Drude L (1992) Pericardioscopy—a new window to the heart in inflammatory cardiac diseases. Herz 17:71–78
Maisch B, Bethge C, Drude L, Hufnagel G, Herzum M, Schönian U (1994) Pericardioscopy and epicardial biopsy—new diagnostic tools in pericardial and perimyocardial disease. Eur Heart J 15(suppl. C):68–73
Maisch B, Karatolius K (2008) New possibilities of diagnostics and therapy of pericarditis. Internist (Berl) 49(1):17–26
Maisch B, Ristic AD, Rupp H, Spodick DH (2001) Pericardial access using the PerDUCER and flexible percutaneous pericardioscopy. Am J Cardiol 88(11):1323–1326
Rupp H, Rupp O, Alter P, Jung N, Pankuweit S, Maisch B (2010) Regeneration by stem cells. Need for minimal invasive access(AttachLifter) the normal pericardial cavity. Herz 7:458–466
Nataf P, Cacoub P, Regan M et al (1998) Video-thoracoscopic pericardial window in the diagnosis and treatment of pericardial effusions. Am J Cardiol 82:125–126
Robles R, Pinero A, Lujan JA et al (1997) Thoracoscopic partial pericardiectomy in the diagnosis and management of pericardial effusion. Surg Endosc 11:253–256
Geissbühler K, Leiser A, Fuhrer J, Ris HB (1998) Video-assisted thoracoscopic pericardial fenestration for loculated or recurrent effusions. Eur J Cardiothorac Surg 14:403–408
Ohno K, Utsumi T, Sasaki Y, Suzuki Y (2005) Videopericardioscopy using endothoracic sonography for lung cancer staging. Ann Thorac Surg 79(5):1780–1782
Karthik S, Milton R, Papagiannopoulos K (2005) Simultaneous double video mediastinoscopy and video mediastinotomy–a step forward. Eur J Cardiothorac Surg 27(5):920–922
Georghiou GP, Stamler A, Sharoni E et al (2005) Video-assisted thoracoscopic pericardial window for diagnosis and management of pericardial effusions. Ann Thorac Surg 80:607–610
Pego-Fernandes PM, Mariani AW, Fernandez F, Ianni BM, Stolf NG, Jatene FB (2008) The role of videopericardioscopy in evaluating indeterminate pericardial effusions. Heart Surg Forum 11(1):E62–E65
Maisch B, Seferović PM, Ristić AD et al (2004) Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. European Society of Cardiology Guidelines: diagnosis and management of the pericardial diseases. Executive summary. Eur Heart 25(7):587–610
Maisch B, Pankuweit S (2012) Current treatment options in (peri)myocarditis and inflammatory cardiomyopathy. Herz 37:644–656
Maisch B, Pankuweit S, Brilla C et al (1999) Intrapericardial treatment of inflammatory and neoplastic pericarditis guided by pericardioscopy and epicardial biopsy—results from a pilot study. Clin Cardiol 22(suppl 1):17–22
Maisch B, Ristic AD, Pankuweit S (2002) Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone: the way to avoid side effects of systemic corticosteroid therapy. Eur Heart J 23:1503–1508
Maisch B, Ristic AD, Pankuweit S, Neubauer A, Moll R (2002) Neoplastic pericardial effusion: efficacy and safety of intrapericardial treatment with cisplatin. Eur Heart J 23:1625–1631
Maisch B, Ristic A, Pankuweit S (2010) Evaluation and management of pericardial effusion in patients with neoplastic disease. Prog Cardiovasc Dis 53(2):157–163
Spodick DH (1997) Diagnostic interpretation of pericardial fluids. Chest 111:1156–1157
Kjeldsberg CR, Knight JA (eds) (1993) Body fluids, 3rd edn. American Society of Clinical Pathologists Press, Chicago, pp 159–254
Meyers DG, Meyers RE, Prendergast TW (1997) The usefulness of diagnostic test on pericardial fluid. Chest 111:1213–1221
Reyes CV, Strinden C, Banerji M (1981) The role of cytology in neoplastic tamponade. Acta Cytol 26:299–302
Theologides A (1978) Neoplastic cardiac tamponade. Semin Oncol 5:181–192
Posner WR, Cohen GI, Skarin AT (1981) Pericardial disease in patients with cancer: the differential diagnosis of malignant from idiopathic and radiation-induced pericarditis. Am J Med 71:407–413
Meyers DG, Boyska DJ (1989) Diagnostic usefulness of pericardial fluid cytology. Chest 95:1142–1143
Krikorian JG, Hancock EW (1978) Pericardiocentesis. Am J Med 65:808–814
Corey GR, Campbell PT, Van Trigt P et al (1993) Etiology of large pericardial effusions. Am J Med 95:209–213
Thadani U, Iveson JM, Wright V (1975) Cardiac tamponade, constrictive pericarditis and pericardial resection in rheumatic arthritis. Medicine (Baltimore) 54:261–270
Leventhal LJ, De Marco DM, Zurier RB (1990) Antinuclear antibodies in pericardial fluid from a patient with primary cardiac lymphoma. Arch Intern Med 150:113–114
Maisch B, Trostel-Soeder R, Stechemesser E et al (1982) Diagnostic relevance of humoral and cell-mediated immune reactions in patients with acute viral myocarditis. Clin Exp Immunol 48:533–545
Maisch B (1987) The sarcolemma as antigen in the secondary immunopathogenesis of myopericarditis. Eur Heart J 8(suppl J):155–165
Maisch B, Bauer E, Cirsi M et al (1993) Cytolytic crossreactive antibodies directed against the cardiac membrane and viral proteins in coxsackievirus B3 and B4 myocarditis. Characterization and pathogenetic relevance. Circulation 87(suppl 5):IV49–IV65
Maisch B, Noutsias M, Ruppert V, Richter A, Pankuweit S (2012) Cardiomyopathies: classification, diagnosis, and treatment. Heart Fail Clin 8:53–78
Cooper LT, Baughman KL, Feldman AM et al (2007) The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 116:2216–2233
Maisch B, Outzen H, Roth D et al (1991) Prognostic determinants in conventionally treated myocarditis and perimyocarditis—focus on antimyolemmal antibodies. Eur Heart J 12(Suppl B):81–87
Maisch B, Richter A, Koelsch S et al (2006) Management of patients with suspected (peri-)myocarditis and inflammatory dilated cardiomyopathy. Herz 31:881–890
Tomkowski W, Szturmowicz M, Fijalkowska A, Burkowski J, Filipecki S (1997) New approaches to the management and treatment of malignant pericardial effusion. Support Care Cancer 5(1):64–66
Tondini M, Rocco G, Bianchi C, Severi C, Corbellini D (1995) Intracavitary cisplatin (CDDO) in the treatment of metastatic pericardial involvement from breast and lung cancer. Monaldi Arch Chest Dis 50(2):86–88
Colleoni M, Martinelli G, Beretta F et al (1998) Intracavitary chemotherapy with thiotepa in malignant pericardial effusions: an active and well-tolerated regimen. J Clin Oncol 16:2371–23716
Girardi LN, Ginsberg RJ, Burt ME (1997) Pericardiocentesis and intrapericardial sclerosis: effective therapy for malignant pericardial effusions. Ann Thorac Surg 64(5):1422–1427
Martioni A, Cipolla CM, Cardinale D, Civelli M, Lamantia G, Colleoni M, Fiorentini C (2004) Long-term results of intrapericardial chemotherapeutic treatment of malignant pericardial effusions with thiotepa. Chest 126(5):1412–1416
Peraino RA (1983) Pericardial effusion in patients treated with maintenance dialysis. Am J Nephrol 3(6):319–322
Fuller TJ, Knochel JP, Brennan JP, Fetner CD, White MG (1976) Reversal of intractable uremic pericarditis by triamcinolone hexacetonide. Arch Intern Med 136(9):979–982
Buselmeier TJ, Davin TD, Simmons RL, Najarian JS, Kjellstrand CM (1978) Treatment of intractable uremic pericardial effusion. Avoidance of pericardiectomy with local steroid instillation. JAMA 240(13):1358–1359
Jr Quigg RJ, Idelson BA, Yoburn DC, Hymes JL, Schick EC, Bernard DB (1985) Local steroids in dialysis-associated pericardial effusion. A single intrapericardial administration of triamcinolone. Arch Intern Med 145(12):2249–2250
Defouilloy C, Meyer G, Starna M et al (1997) Intrapericardial fibrinolysis: a useful treatment in the management of purulent pericarditis. Intensive Care Med 23(1):5–10
Ustunsoy H, Celkan MA, Sivrikoz MC et al (2002) Intrapericardial fibrinolytic therapy in purulent pericarditis. Eur J Cardio-thorac Surg 22(3):373–376
Schafer M, Lepoir M, Delabays A, Ruchat P, Schaller MD, Borccard AF (2002) Intrapericardial urokinase irrigation and systemic corticosteroids: an alternative to pericardectomy for persistent fibrino-purulent pericarditis. Cardiovasc Surg 10(5):508–511
Ekim H, Demirbag R (2004) Intrapericardial streptokinase for purulent pericarditis. Surg Today 34:569–572
Tomkowski WZ, Gralec R, Kuca P, Burokowsi J, Orlowski T, Kurzyna M (2004) Effectiveness of intrapericardial administration of streptokinase in purulent pericarditis. Herz 29(8):802–805
Cui Hb, Chen XY, Cui CC et al (2005) Prevention of pericardial constriction by transcatheter intrapericardial fibrinolysis with urokinase. Chin Med Sci 20(1):5–10
Sosa E, Scanavacc M, d’Avila A (1996) A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol 7(6):531–536
Sosa E, Scanavacca M, d’Avila A (1999) Different ways of approaching the normal pericardial space. Circulation 100(24):e115–e116
Maisch B, Ristic AD, Rupp H, Spodick DH (2001) Pericardial access using the PerDUCER and flexible percutaneous pericardioscopy. Am J Cardiol 88:1323–1326
Rupp H, Rupp TP, Alter P, Jung N, Pankuweit S, Maisch B (2010) Intrapericardial procedures for cardiac regeneration by stem cells. Herz 35:458–466
Patronik NA, Riviere CN, El Qarra S, Zenati MA(2005). The Heart-Lander: a novel epicardial crawling robot for myocardial injection. Proceedings of the 19th International Congress of Computer Assisted Radiology and Surgery, Elsevier1291C: 735–739
Ota T, Patronik NA, Schwarzman D, Riviere CN, Zenat MI (2008) Minimally invasive epicardial injections using a novel semiautonomous robotic device. Circulation 118(14 Suppl):115–120
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maisch, B., Rupp, H., Ristic, A. et al. Pericardioscopy and epi- and pericardial biopsy—a new window to the heart improving etiological diagnoses and permitting targeted intrapericardial therapy. Heart Fail Rev 18, 317–328 (2013). https://doi.org/10.1007/s10741-013-9382-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-013-9382-y