Abstract
Inconsistent findings in the studies have been observed concerning the higher dose of statins use in the acute phase of ischemic stroke and transient ischemic attack (TIA). Therefore, we performed a systematic review to assess this issue. A computerized literature search in PubMed, Cochrane Library databases, and EMBASE for randomized controlled trials (RCTs) was conducted. The efficacy outcome indicators were National Institutes of Health Stroke Scale (NIHSS) score, infarct volume, and recurrence of stroke; the safety outcome indicators were intracranial hemorrhage events, cardiovascular and cerebrovascular events, and all-cause death. Pre-specified subgroup analyses were carried out. A total of seven RCTs with 1089 patients were included. Six studies reported the results of the NHISS score. A great reduction was found in NIHSS score in the statins group, and the difference is statistically significant [mean difference (MD) −1.15, 95% confidence interval (CI) −1.64 to −0.66, P < 0.00001]. However, no significant differences in the effect on recurrence of stroke [odds ratio (OR) 1.05, 95% CI 0.65–1.69, P = 0.85] (available in 3 studies), infarct volume [std. mean difference (SMD) 0.04, 95% CI −0.55 to 0.63, P = 0.89] (available in 2 studies), intracerebral hemorrhage events (OR 3.25, 95% CI 0.34–31.52, P = 0.31) (available in 2 studies), cardiovascular and cerebrovascular events (OR 0.70, 95% CI 0.35–1.43, P = 0.33) (available in 2 studies), and all-cause death (OR 1.18, 95% CI 0.60–2.35, P = 0.63) (available in 2 studies) were found. High-dose statin therapy in the acute phase of ischemic stroke and TIA significantly reduce the NIHSS score and improve short-term functional outcome without increasing related adverse events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transient ischemic attack and ischemic stroke are major healthcare problems around the world. Over the past four decades, incidence rates of stroke in middle- and low-income countries have increased more than high-income countries [1]. Meanwhile, stroke is a main cause of disability [2]. Thrombolysis and mechanical thrombectomy are the most effective treatment measures for the opening of occluded vessels. However, as a result of the limitation of the time window, equipment, and technology, most of the patients cannot take advantage of these treatment opportunities. Therefore, to explore safe and reliable medical treatment has been a focus of attention for neurological physicians.
The inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (“statins”) have potential neuroprotective effects in pleiotropic aspects [3], which might play a useful role in the acute phase of ischemic stroke. Statins have benefits that are not only related to changes in lipid level, but also a positive effect on immune-modulation of T lymphocytes and endothelial progenitor stem cells and on vascular inflammation [4]. For example, a neuroprotective effect might regulate cerebral perfusion and improve endothelial function and the stabilization of atherosclerotic plaques [5]. However, early statin therapy is commonly used in ischemic heart disease, and the advantage of statin intake in the acute phase of stroke is controversial [6]. Meanwhile, inconsistent findings in studies have been observed about the efficacy and safety of statins in the acute phase of ischemic stroke and TIA using higher dose of statins [7–9]. Tuttolomondo et al. [7] provide evidence that atorvastatin administered in the acute phase of stroke might improve the functional and prognostic profile. Likewise, a recent study shows that more aggressive statin treatment is associated with a better long-term functional outcome of acute ischemic stroke patients than less aggressive treatment [10]. Nevertheless, data from study of Muscari [9] seem to not entirely support the above point, and the results indicate that there is no short-term benefit of high-dosage statins for patients in the acute phase of ischemic stroke, but a possible beneficial functional effect at 3 months. Therefore, we included the relevant RCTs and conducted a systematic review to assess the efficacy and safety of high-dose statins in the acute phase of ischemic stroke and TIA.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
Search strategy
We searched in MEDLINE (~ June, 2016), Cochrane Library databases (~ June, 2016), and EMBASE (~ June, 2016) for RCTs. Medical Subject Heading (MeSH) terms and free-text terms were used to search in databases, including “Hydroxymethylglutaryl-CoA Reductase Inhibitors”, “statins”, “atorvastatin”, “rosuvastatin”, “simvastatin”, “fluvastatin”, “lovastatin”, “pitavastatin”, “pravastatin”, “cerivastatin”, “stroke”, “dose”, and “intensive”. No other restrictions were applied.
Selection criteria
Two reviewers (W.W. and Y.L.) screened the records independently. The including criteria were: (1) randomized controlled trials (RCTs); (2) patients who were using high-dose statins (Rosuvastatin 20 mg, Atorvastatin ≥80 mg, Simvastatin ≥40 mg) in the acute phase (within the first 2 weeks of onset) of ischemic stroke or TIA; (3) reporting the relevant outcomes (NIHSS score, infarct volume, recurrence of stroke, intracerebral hemorrhage (ICH) events, cardiovascular and cerebrovascular events (ischaemic stroke and ICH were included), and all-cause death); (4) no diet intervention; and (5) full-text available. The final inclusion was obtained by discussion.
Data extraction
Three authors (J.X.F., Y.L., and W.W.) used a standardized extraction form to extract the data independently. Discrepancies were solved by discussion. Following information was extracted from each study: (1) basic characteristics of included studies (author, publication year, design, previous thrombolysis, the previous statins use, date of NIHSS score measurement, disease, treatment time, drug type, dose and patient number in per arm, NIHSS score baseline, and outcome indicators in each study); (2) outcomes of included studies:NIHSS score (3, 5, 7 days), infarct volume, recurrence of stroke, intracerebral hemorrhage (ICH) events, cardiovascular and cerebrovascular events (ischaemic stroke and ICH were included), and all-cause death; (3) information about the risk of bias. When essential data were not reported, we communicated with the original author of the study to get the desired data.
Quality assessment
Two authors (J.X.F. and G.C.) assessed the risk of bias independently. Disagreements were resolved by discussion. Risk of bias in each study was evaluated with the Cochrane risk-of-bias tool [12].
Data synthesis
Risk ratios and odds ratios are similar when the control intervention risks are low and effects are small [12]. Therefore, the endpoints, the results of recurrence of stroke, intracerebral hemorrhage events, cardiovascular and cerebrovascular events, and all-cause death were assessed using the risk ratio (OR) with the 95% CI as dichotomous variables. The endpoint, NIHSS score, was assessed using the mean difference (MD) with the 95% CI as continuous variable. Because of the considerable differences in means between the two groups, we chose the standard mean difference (SMD) with the 95% CI as the summery pooled statistic in the endpoint of infarct volume as continuous variable.
Statistical analysis
A two-tailed P < 0.05 was considered statistically significant, and 95% confidence intervals were calculated. The Cochran Q test was used to evaluate the magnitude of heterogeneity between the studies. Meanwhile, the I 2 statistic was also used to quantify inconsistency. A fixed-effect model was used to calculate pooled estimate; however, Cochran’s P ≤ 0.10 and I 2 > 50% were considered as having significant heterogeneity, and the random-effects model was used to pool the data. Due to the limited number (<10) of included studies, we did not assess the publication bias. Sensitivity analyses were undertaken to test the robustness of the results. We compared the fixed-effects model results with the random-effects model to conduct the sensitivity analyses. Review Manager Software version 5.3 was used to conduct meta-analyses.
Results
Search results
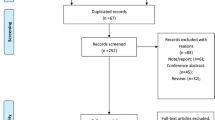
We identified 1164 studies initially, 463 records from PUBMED, 220 records from Cochrane Library, and 481 records from EMBASE database. 326 studies were duplicate studies that were removed. After screening titles and abstracts, 52 potentially relevant studies were identified. After reviewing the full text, seven studies [6–9, 13–15] were included. The selected procedure was shown in Fig. 1.
Characteristics of the included studies
We enrolled a total of 1089 participants: 541 (49.7%) in the intervention group and 548 (50.3%) in the control group. All the control groups were placebo groups. The data of NIHSS score in study Hoe [8], Muscari [9], and Montaner [14], and infarct volume in study Hoe 2016 and Muscari 2011, were obtained by communicating with the authorship. All patients were in acute phase of ischemic stroke or TIA within 48 h except two studies [9, 13] was 96 h and 1 week, respectively. The characteristics of the included trials were shown in Table 1.
Risk of bias in included studies
The risk-of-bias assessment results were presented in Table 2. All studies described the random sequence generation (e.g., randomization table and a computer-generated random list) and were regarded as having a low risk of bias. Five studies [7, 8, 13–15] stated the allocation concealment process. One study [6] was considered as “unclear risk”, because we were unclear whether the envelopes were concealed. Blinding of participants and personnel and outcome assessment in four studies [6, 7, 9, 14] were identified as an unclear risk, because there is no description in these studies. In the domain of incomplete outcome data and selective reporting, all studies were regarded as having a low risk of bias. Meanwhile, in the domain of other biases, all the studies were deemed to have an unclear risk except for two studies [14, 15].
Efficacy outcomes
NIHSS score
Of the seven included studies, six studies [6–9, 13, 14] with 672 participants report the results of the NIHSS score within 7 days. Although use of a clinically relevant cutoff would be the most proper way of dealing with NIHSS data, such data were not available. Therefore, we used the NIHSS score value reported in each study that was expressed as mean ± standard deviation (SD). A fixed-effects model was used in this item. We compared the NIHSS score within 7 days between placebo and statin groups. There was a great reduction in the NIHSS score in statins group than placebo, and the difference is statistically significant (MD −1.15, 95% CI −1.64 to −0.66, P < 0.00001). No significant heterogeneity was observed across the six studies (P = 0.78, I 2 = 0%) The effect of statins may well be different when given shortly after the stroke as compared to a later time. Therefore, we conducted a subgroup analysis according to the time of statins use. The results show that statins significantly reduce the NHISS score when statins used within 48 h of disease onset (MD −1.15, 95% CI −1.65 to −0.66, P < 0.00001), and there is no significant reduction in NHISS score when statins used >48 h of disease onset (MD −0.97, 95% CI −3.88 to 1.94, P = 0.51). The difference between the two subgroups (≤48 vs. >48 h) is not statistically significant (P = 0.90, test for subgroup differences) (Fig. 2a).
Infarct volume
Two studies [8, 9] (n = 351) provide data on infarct volume. Because of the considerable differences in means between the two groups, we chose the standardized mean difference (SMD) with the 95% CI as the summery statistic. There is no significant difference between the two groups (SMD 0.04, 95% CI −0.55–0.63, P = 0.89) with a high heterogeneity (P = 0.03, I 2 = 78%) (Fig. 2b).
Recurrence of stroke
The data of recurrence of stroke are available in three studies [6, 8, 15] (n = 889). Moderate heterogeneity is found (P = 0.16, I 2 = 45%). There is no significant difference between the two groups in the effect on recurrence of stroke (OR 1.05, 95% CI 0.65–1.69; P = 0.85) using the fixed-effects model (Fig. 2c).
Safety outcomes
Intracerebral hemorrhage events
Two studies [6, 8] (n = 497) investigated the incidence of intracerebral hemorrhage events. There is no significant difference between the two groups (OR 3.25, 95% CI 0.34–31.52, P = 0.31), and without heterogeneity (I 2 = 0%, P = 0.97) (Fig. 3a).
Cardiovascular and cerebrovascular events
Among the studies, two studies [6, 8] (n = 497) report the events of cardiovascular and cerebrovascular. No difference is found between the two groups (OR 0.70, 95% CI 0.35–1.43, P = 0.33). No heterogeneity is found, as well. (I 2 = 0%, P = 0.33) (Fig. 3b).
All-cause death
Data on all-cause death are available in two studies [6, 14] (n = 239). The pooled data indicate that there is no significant difference (OR 1.18, 95% CI 0.60–2.35, P = 0.63) between statins and placebo group about the effect on all-cause death. Moderate heterogeneity is found (P = 0.19, I 2 = 42%) in this item (Fig. 3c). We, therefore, used a fixed-effects model.
Discussion
Our systematic review interrogates the related literature to compare the efficacy and safety of high-dose statins in the acute phase (within the first 2 weeks of onset) of ischemic stroke or TIA, and we had the main findings as following: (1) high-dose statins may significantly reduce the NIHSS score, and this means that high-dose statins may improve the short-term neural functional prognosis of acute ischemic stroke. (2) No significant difference is found between the high-dose statins and placebo group in the effect on infarct volume, recurrence of stroke, intracerebral hemorrhage events, cardiovascular and cerebrovascular events, and all-cause death.
Statins have potential beneficial effects lipid profile and inflammation [4, 16]. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) [17] study had confirmed the beneficial effects of statins in patients with the previous stroke. A recent study shows that pretreatment with statins is directly associated with spontaneous neurological recovery at hospital discharge in patients with the first-ever ischemic stroke who do not receive thrombolytic therapy [18]. Pleiotropic effects of statins may be dose-dependent [19]. High-dose statin therapy can bring extra clinical effects to patients with coronary artery diseases, so there is a trend to use high-dose statins [20]. A meta-analysis also indicates that in comparison with the conventional doses of statins, intensive lipid lowering treatment might make a greater reduction in the risk of future stroke after coronary artery disease [21]. The past meta-analysis always evaluated the effect of high-dose statins in coronary heart disease [22] or diabetes patients [23]; however, there is no meta-analysis to assess high-dose statins used in acute phase of ischemic stroke. At the same time, a recent meta-analysis shows that higher dose of statins are associated with the risk of ICH [24], which triggers debates about the feasibility of high-dose statins. Hence, we conducted this meta-analysis to evaluate high-dose statins used in patients in acute phase of ischemic stroke.
National Institute of Health stroke scale (NIHSS) score is a good predictive value for the neurological function of patients with acute cerebral infarction [25], which can estimate the basic neurological abnormalities, consciousness level, and elementary cognitive functions [6]. Our results suggest that high-dose statin therapy used in the acute phase of ischemic stroke and TIA could improve short-term prognosis. An observational study also confirmed the benefit of high-dose statin, and the results suggest that high-dose statin therapy for patients after recanalization is related to a better functional outcome without a higher risk of intracranial hemorrhage [26]. Another study [10] shows that more aggressive statin therapy in acute ischemic stroke patients obtain a better long-term functional outcome. Our results indicate that there was no significant difference between the high statins and placebo group in the effect on infarct volume, recurrence of stroke, and related adverse events. However, these results may be inconsistent with other studies entirely. The SPARCL study [17] shows that high-dose atorvastatin can reduce the recurrence of stroke; however, in another study, high-dose simvastatin has no effect on recurrent stroke [27]. A meta-analysis shows that more aggressive statin treatment reduces major vascular events more than less intensive regimens in patients with coronary artery disease [28]. As for intracerebral hemorrhage events, there are some disagreements. The results of study Scheitz et al. [29] show that statin use prior to acute ischemic stroke (AIS) does not increase the early hemorrhagic complications, and may be associated with a reduced mortality. A meta-analysis of 31 randomized controlled trials also concludes that statin therapy does not increase ICH events, but rather produces a significant reduction in all-cause mortality [30], which is identical with the results of Tziomalos et al. [10] On the contrary, a meta-analysis by Pandit et al. [24] indicates that high-dose statin treatment increases the risk of ICH. However, another study [31] reports that statin therapy decreases the risk of intracerebral hemorrhage events in patients with stroke.
Thus, the benefit of statins in acute stroke remains uncertain and is an issue of great importance to clinical doctors. This study is the first meta-analysis to estimate the effects of high-dose statins for patients in the acute phase of ischemic stroke and transient ischemic attack. The previous reviews and meta-analysis did not pay attention to the high-dose statins in acute ischemic stroke, so we conducted this meta-analysis to provide a reference for clinical medication practice.
There are some limitations in our present meta-analysis. First of all, although we had searched all the relevant studies in this field, the study number is limited, and the total number of patients is not large; thus, the power of our analyses might be restricted. We included seven studies in total in this meta-analysis, and the total participants’ number is 1089. Only one meta-analysis has been conducted considering seven studies. All the remaining analyses have been conducted considering two or three studies. However, the limitation is determined by the number of the studies we searched. There are some related studies in which the full text or the data we needed were not available. We tried to contact the authors to obtain the full-text of these studies; however, no reply was received. This might have caused a publication bias [32, 33]. Second, the heterogeneity of infarct volume is significant. It may be due to the different starting time of statin therapy. We compared the fixed-effects model results with the random-effects model to conduct the sensitivity analyses in other results, and the results were not changed significantly. This shows that our results were robust. In addition, by reason of the small number of included studies, we do not have enough ability to evaluate any publication bias. Moreover, some data on clinically relevant outcomes such as longest follow-up mortality, long-term outcome, and musculoskeletal adverse events, such as myalgia, myopathy, and rhabdomyolysis, were not reported in available studies. The available results of NIHSS score in the study of Kurzepa et al. [34] were not obtained. In addition, the modified Rankin Scale score (mRS) was available in two studies [7, 9], but was evaluated at a different time. Last but not least, analyzing NIHSS score as a continuous variable is an additional limitation of our review, but unfortunately means (± SD) were the only data available in the included studies.
Conclusion
Our meta-analysis emphasizes that high-dose statins therapy in the acute phase of ischemic stroke and TIA might significantly reduce the NIHSS score and improve short-term functional outcome without increasing safety issues. Nevertheless, given the above limitations, the results should be interpreted with caution.
References
Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population- based studies: a systematic review. Lancet Neurol 8:355–369
Meschia JF, Bushnell C, Boden-Albala B et al (2014) Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45:3754–3832
Hong KS, Lee JS (2015) Statins in acute ischemic stroke: a systematic review. J Stroke 17:282–301
Blum A (2014) HMG-CoA reductase inhibitors (statins), inflammation, and endothelial progenitor cells—new mechanistic insights of atherosclerosis. BioFactors 40:295–302
Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L (2007) Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist 13:208–213
Yakusevich VV, Malygin AYu, Lychenko SV, Petrochenko AS, Kabanov AV (2012) The efficacy of high-dose simvastatin in acute period of ischemic stroke. Rational Pharmacother Cardiol 8:4–16
Tuttolomondo A, Di Raimondo D, Pecoraro R, Maida C, Arnao V, Della Corte V, Simonetta I, Corpora F, Di Bona D, Maugeri R, Iacopino DG, Pinto A (2016) Early high-dosage atorvastatin treatment improved serum immune-inflammatory markers and functional outcome in acute ischemic strokes classified as large artery atherosclerotic stroke: a randomized trial. Medicine 95:e3186
Heo JH, Song D, Nam HS, Kim EY, Kim YD, Lee KY, Lee KJ, Yoo J, Kim YN, Lee BC, Yoon BW, Kim JS, Investigators EUREKA (2016) Effect and safety of rosuvastatin in acute ischemic stroke. J Stroke 18:87–95
Muscari A, Puddu GM, Santoro N, Serafini C, Cenni A, Rossi V, Zoli M (2011) The atorvastatin during ischemic stroke study: a pilot randomized controlled trial. Clin Neuropharmacol 34:141–147
Tziomalos K, Giampatzis V, Bouziana SD, Spanou M, Kostaki S, Papadopoulou M, Angelopoulou SM, Konstantara F, Savopoulos C, Hatzitolios AI (2015) Comparative effects of more versus less aggressive treatment with statins on the long-term outcome of patients with acute ischemic stroke. Atherosclerosis 243:65–70
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 updated March 2011. The Cochrane Collaboration 2011. http://www.cochrane-handbook.org. Accessed 06 June 2016
Beer C, Blacker D, Bynevelt M, Hankey GJ, Puddey IB (2012) A randomized placebo controlled trial of early treatment of acute ischemic stroke with atorvastatin and irbesartan. Int J Stroke 7:104–111
Montaner J, Chacón P, Krupinski J, Rubio F, Millán M, Molina CA, Hereu P, Quintana M, Alvarez-Sabín J (2008) Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J Neurol 15:82–90
Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM, Investigators FASTER (2007) Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 6:961–969
Schneider DJ, Ricci MA, Taatjes DJ et al (1997) Changes in arterial expression of fibrinolytic system proteins inatherogenesis. Arterioscler Thromb Vasc Biol 17:3294–3301
Goldstein LB, Amarenco P, Zivin J et al (2009) Statin treatment and stroke outcome in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke 40:3526–3531
Arboix A, García-Eroles L, Oliveres M, Targa C, Balcells M, Massons J (2010) Pretreatment with statins improves early outcome in patients with first-ever ischaemic stroke: a pleiotropic effect of statins or a beneficial effect of hypercholesterolemia? BMC Neurol 10:47
Athyros VG, Kakafika AI, Tziomalos K, Karagiannis A, Mikhailidis DP (2009) Pleiotropic effects of statins—clinical evidence. Curr Pharm Des 15:479–489
Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E (2006) Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 48:438–445
Fernándezde Bobadilla J, Moreno R, Fernández C, Martínez A, Sánchez-Maestre C, Ezpeleta-Echevarri D (2009) Effect of intensive treatment with atorvastatin versus standard doses of statins on the risk of stroke. A meta-analysis from five randomized trials including 25,709 patients. Revue Neurol 48:561–565
Josan K, Majumdar SR, McAlister FA (2008) The efficacy and safety of intensive statin therapy: a meta-analysis of randomized trials. Can Med Assoc J 178:576–584
de Vries FM, Kolthof J, Postma MJ, Denig P, Hak E (2014) Efficacy of standard and intensive statin treatment for the secondary prevention of cardiovascular and cerebrovascular events in diabetes patients: a meta-analysis. PLoS One 9:e111247
Pandit AK, Kumar P, Kumar A, Chakravarty K, Misra S, Prasad K (2016) High-dose statin therapy and risk of intracerebral hemorrhage: a meta-analysis. Acta Neurol Scand 134:22–28
Brott T, Adams HP Jr, Olinger CP, Walker M (1989) Measurement of acute cerebral infarction: a clinical examination scale. Stroke 20:864–870
Kang J, Kim N, Park TH, Bang OY, Lee JS, Lee J, Han MK, Park SH, Gorelick PB, Bae HJ (2015) Early statin use in ischemic stroke patients treated with recanalization therapy: retrospective observational study. BMC Neurol 15:122
Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group (2004) Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 363:757–767
Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R (2010) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376: 1670–1681
Scheitz JF, MacIsaac RL, Abdul-Rahim AH, Siegerink B, Bath PM, Endres M, Lees KR, Nolte CH, VISTA collaboration (2016) Statins and risk of poststroke hemorrhagic complications. Neurology 86:1590–1596
McKinney JS, Kostis WJ (2012) Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke 43:2149–2156
Åsberg S, Eriksson M (2015) Statin therapy and the risk of intracerebral haemorrhage: a nationwide observational study. Int J Stroke Suppl A100:46–49
Fukuma K (2015) Early statin intervention can reduce the early neurological deterioration and recurrence in acute lacunar stroke. Stroke 46:TP65
Ueno Y, Yamashiro K, Tanaka Y (2014) Rationale and design of the EPISTEME trial: efficacy of post-stroke intensive rosuvastatin treatment for aortogenic embolic stroke. Cardiovasc Drugs Ther 28:79–85
Kurzepa J, Bielewicz J, Bartosik-Psujek H, Szczepańska-Szerej A, Stelmasiak Z (2008) Simvastatin inhibits the increase in serum tau protein levels in the acute phase of ischemic stroke. Pharmacol Rep 60:1014–1018
Acknowledgements
Our study had no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures in studies involving human participants are in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration. This article does not contain any studies with animals performed by any of the author.
Informed consent
For this type of study, informed consent is not required.
Rights and permissions
About this article
Cite this article
Fang, Jx., Wang, Eq., Wang, W. et al. The efficacy and safety of high-dose statins in acute phase of ischemic stroke and transient ischemic attack: a systematic review. Intern Emerg Med 12, 679–687 (2017). https://doi.org/10.1007/s11739-017-1650-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-017-1650-8