Abstract
We aimed to explore the role of procalcitonin (PCT) for the diagnosis of Candida spp. bloodstream infections in a population of critically ill septic patients admitted to internal medicine units. This is a retrospective case–control study considering all cases of candidemia identified in three internal medicine units, from January 1st 2012 to May 31st 2016. For each case of candidemia, two patients with bacteremic sepsis were included in the study as control cases. The end point of the study was to evaluate the diagnostic performance of PCT for the diagnosis of Candida spp. blood stream infections in patients with objectively documented sepsis. Sixty-four patients with candidemia and 128 controls with bacteremia were enrolled. Median and interquartile range (IQR) PCT values are significantly lower in patients with candidemia (0.73; IQR 0.26–1.85 ng/mL) than in those with bacteremia (4.48; IQR 1.10–18.26 ng/mL). At ROC curve analysis, values of PCT greater than 2.5 ng/mL had a negative predictive value (NPV) of 98.3% with an AUC of 0.76 (0.68–0.84 95% CI) for the identification of Candida spp. from blood cultures. At multivariate analysis, a PCT value <2.5 ng/mL showed an odds ratio of 8.57 (95% CI 3.09–23.70; p < 0.0001) for candidemia. In septic patients at risk of Candida infection, a PCT value lower than 2.5 ng/mL should raise the suspicion of candidemia, adding value for considering prompt initiation of antifungal therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida species (spp.) are among the most common causes of invasive bloodstream (BSI) and deep tissue infection. Data from the ECDC point prevalence survey of healthcare-associated infections and antimicrobial use in acute care hospitals in Europe, carried out in 2011–2012, shows that Candida spp. are the third most frequent cause of bloodstream infection in Italy [1]. Long considered an opportunistic pathogen, it is now recognized as an active component of disease featuring several virulence factors among which is the ability to form a characteristic biofilm in which the microorganisms grow as a highly structured and organized functional community (REF). Overall, it is estimated that this infection is accountable for up to 50% mortality among critically ill patients worldwide [2–5].
To date, the gold standard for diagnosis of invasive candidiasis remains growth in blood cultures; yet it has been recognized that this method features a relatively low sensitivity (yielding a positive result only when blood samples collect a sufficient concentration of viable Candida from systemic circulation) and a 3- to 5-day lag time for identification, which can represent a delay in initiation of antifungal therapy and impact on patient outcome [6, 7]. It has been estimated that, relying on conventional culturing methods, approximately 50% of candidemia cases remain undiagnosed [7].
In recent years, several studies have considered the use of surrogate diagnostic markers for earlier detection of invasive Candida infections, including β-1,3-d-glucan, mannan antigen in association with anti-mannan antibodies, and Candida albicans germ tube (CAGT) antibodies [8]. Unfortunately they apparently feature a high false positive rate (especially on single testing), require multiple determinations, and have limited availability across hospitals [8–11]. Another alternative that is receiving increasing attention is procalcitonin (PCT), a 116-amino acid prohormone of the calcium metabolism regulator calcitonin that is expressed in response to lipopolysaccharide- and bacteria-induced cytokines, and is down regulated in patients with viral infections [12, 13]. To date PCT is becoming broadly recognized as the serological marker of reference for the diagnosis, prognostication and management of septic bacterial infections across several settings [14–18]. So far, however, studies exploring the role of PCT in fungal sepsis are limited, and data supporting the usefulness of PCT in Candida spp. bloodstream infections are lacking or provide inconclusive results [14, 19–22].
Here we report a case–control study that aims to explore the role of PCT for the diagnosis of Candida spp. bloodstream infections in a population of critically ill septic patients admitted to internal medicine units.
Methods
This is a retrospective case–control study carried out in three internal medicine units (a total of 97 beds) of the University and General Hospital of Careggi, Florence, Italy, enrolling all cases of sepsis due to candidemia identified from January 1st 2012 to May 31st 2016. Sepsis, defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection according to the current definition, was classified either as bacterial or Candida spp. infection, based on the results of microbiological cultures. Bacterial and fungal blood cultures were performed using the BACTEC system (Benex Limited, Shannon, Ireland); identification of isolates and susceptibility testing were performed using standard laboratory techniques (MCM last).
Each case of candidemia was matched to two cases of bacterial blood stream sepsis, as to gender, age (±5 years) and time of hospitalization (±20 days) as control cases. Laboratory data were collected from the database of the Clinical Microbiology and Virology Unit of the Careggi hospital, which serves the three units of Internal Medicine involved in the study. Demographic, clinical and laboratory characteristics were collected from electronic medical records (ArchiMed Web Based DataBase© by B. Dannaoui). Neutropenic patients (neutrophils <1000/mm) were excluded from the study.
As foreseen by our hospital protocol, PCT measurements were performed as part of routine laboratory workup for all patients upon admission to the Internal Medicine ward (same day or following morning, according to scheduled nursing routine). PCT measurements considered for the analysis were those available within 24 h since blood sampling, which subsequently documented isolation of Candida or bacterial species. PCT was measured using the Vidas PCT kit (Brahms d-agnostics, Berlin, Germany) in accordance to manufacturer’s instructions as described elsewhere [23].
The study protocol was submitted and approved by our Local Ethics Committee (SepsiMed Study protocol number 0018329), and the study was performed in agreement with the principles set in the Declaration of Helsinki.
The primary end point of the study is to evaluate the diagnostic performance of PCT for the diagnosis of Candida spp. blood stream infections in patients with objectively documented sepsis.
Statistical analysis
Variables were expressed as mean ± standard deviation or as median and interquartile ranges (25th–75th). In general, statistical comparisons were performed using Student’s t test and one-way ANOVA models for the comparison of continuous normally distributed variables and Mann–Whitney U test for continuous not normally distributed variables. The Chi-square test or Fisher’s exact test were used for the comparison of categorical variables. A whisker box-plot chart was used to graphically render results. A receiver-operating-characteristic (ROC) curve analysis was used to obtain the most accurate PCT cut-off for the identification of candidemia. Odds ratio (OR) of variables and 95% confidence intervals (CIs) were calculated using univariate and multivariate logistic regression analysis. Multivariate analysis was performed using a stepwise forward regression model, with an entry probability for each variable set at 0.05. All p values were two-tailed and considered significant when <0.05 (95% CI). All analyses were performed using SPSS statistical software 21.0 (SPSS, Chicago, Illinois, USA).
Results
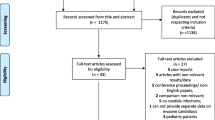
Overall, the study enrolled 64 cases with confirmed candidemia and 128 controls with bacterial sepsis.
The mean age was 71.7 years and both genders were equally represented. In general, patients had several comorbidities, which however did not significantly differ among the two patient groups, except for pancreatitis and severe cognitive impairment that were more common in patients with candidemia, and for diabetes and chronic kidney disease (CKD) that were more common in patients with bacterial sepsis (Table 1). Notably, a concomitant or recent Clostridium difficile infection was more common in patients with candidemia compared to those with bacterial sepsis. Patients with candidemia had a lower score at malnutrition universal screening tool (MUST), indicating higher risk of or overt malnutrition [24]. The presence of medical devices (i.e., central venous catheter and urinary catheter) and total parenteral nutrition were more frequent among patients with candidemia. Patients with candidemia also had been exposed to antimicrobial drugs more frequently within the month preceding the septic index event. Furthermore, patients with candidemia more often suffered a severe clinical presentation of the infectious process, as indicated by a higher prevalence of severe sepsis; they had a longer duration of hospitalization and a tendency to higher in-hospital mortality compared to patients with bacterial sepsis (Table 1).
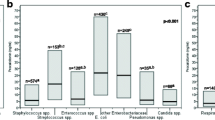
Among the patients of the control group with bacterial sepsis, the distribution of bacterial pathogens was the following: 70 (54.7%) Gram negative, 52 (40.6%) Gram positive, and 6 (4.7%) polymicrobial. Among the 64 patients with candidemia, 69 isolates of Candida spp were found, with species distribution featuring the most widespread to be C. albicans (42 isolates, 60.9%), while the remaining were due to C. parapsilosis in 16 (23.1%), C. glabrata in 8 (11.6%), C. tropicalis in 2 (2.9%), and C. krusei in 1 case (1.4%). Of note, there were five patients with concomitant isolation of more than two Candida species in blood cultures: three cases with C. albicans and C. glabrata, one case of C. albicans and C. parapsilosis, and one case of C. parapsilosis and C. krusei co-infection.
Mean and median PCT values were significantly lower in patients with candidemia than in those with bacteremia. Mean PCT values were 3.37 ± 10.10 and 18.14 ± 38.23 ng/mL (p < 0.001), whereas median-interquartile range values were 0.73 (0.26–1.85) ng/mL and 4.48 (1.10–18.26) ng/mL (p = 0.006) in patients with candidemia and bacteremia, respectively (Fig. 1). At ROC curve analysis, values of PCT greater or equal to 2.5 ng/mL showed a sensitivity of 78.3%, a specificity of 72%, a positive predictive value (PPV) of 15.1%, and a negative predictive value (NPV) of 98.3% with an AUC of 0.76 (0.68–0.84 95% CI) for the identification of Candida spp. by blood cultures (Fig. 2). Results of univariate and multivariate analysis are shown in Table 2. Of note, at multivariate analysis, a PCT value <2.5 ng/mL showed a relative association (odds ratio) with candidemia of 8.57 (95% CI 3.09–23.70; p < 0.0001).
Discussion
Few studies addressed the role of PCT testing in the diagnosis of Candida blood stream infections in patients with sepsis. This case–control study aims to investigate this marker’s behavior in patients with candidemia and its performance as a diagnostic marker.
Findings from the study evidence significantly lower PCT values in patients with candidemia compared to those with bacteremia.
The reasons for this lower response in PCT values to fungal infections is not completely understood; however, it is likely that fungal infections might trigger a different pattern and extent of cytokines response from that elicited by Gram-negative or by Gram-positive bacteria and other pro-inflammatory cytokines [25, 26]. Another hypothesis could be that patients with fungal disease have an impaired immune response to infectious agents, which would be coherent with the clinical observation of the “frail phenotype” of patient suffering of invasive candidiasis [27]. It is a common clinical observation that patients with candidemia in internal medicine units more frequently have signs of malnutrition or a lower body mass index, have suffered a prolonged hospitalization with multiple contacts with predisposing drugs (i.e., antibiotics, steroids) and with the healthcare facilities. Indeed, Candida blood stream infection is often a late event in the course of the disease process.
In this study, a PCT value ≥2.5 ng/mL has a negative predictive value of 98% for candidemia, and at multivariate analysis a value below that threshold increases eightfold the probability of recovery Candida spp. in blood cultures (OR 8.57, 95% CI 3.09–23.70; p < 0.0001).
Similar results have been observed in septic patients admitted to intensive care units as reported by Charles and coworker in a small retrospective study on 50 patients with sepsis (35 with bacteremia and 15 with candidemia) admitted to a single intensive care unit [20], and recently by Cortegiani et al. in a larger retrospective cohort of septic patients from a series of ICU patients as well [22]. The PCT cut-off values for the identification of candidemia reported in the studies by Charles and Cortegiani are <5.5 and <6.08 ng/mL, with negative predictive values of 96.3 and 100% for bacterial infections, respectively.
However, differently from the studies cited above, our population is a typical representation of patients admitted to internal medicine units that differ from patients of acute settings as the ICU, as they feature a lower disease severity, older age, greater burden of comorbidities, and repeated contacts with healthcare facilities. Many patients with candidemia enrolled in studies from ICUs have undergone abdominal surgical intervention, which is a leading risk factor for candidemia in ICU, whereas this is not so common in Internal Medicine patients. It is well known that the severity of the inflammatory process and abdominal surgical interventions per se, may increase PCT levels independently from an underlying infectious process [28]. These observations could help explain the lower PCT cut-off value found in our study as discriminating between bacterial infection and Candida blood stream infections with respect to the studies carried out in ICUs.
Findings from our study seem of value, since Candida spp. is an increasingly recognized cause of severe infection in patients admitted to internal medicine units. Candidemia is associated with a very high mortality rate in this setting (ranging from 20 to 50%) [29], and a rapid diagnosis with prompt starting of antifungal therapy has been shown to be a crucial step to reduce mortality [6, 30, 31]. However, ultimate diagnosis that relies on results of blood cultures needs time (up to 4 days, depending on Candida species), and waiting can delay starting of antifungal therapy with a negative impact on outcome. The term pre-emptive therapy has been introduced to promptly start antifungal therapy whenever supported by the clinical appearance and the positivity of rapid non cultural microbiological tests, such as β-1,3-d-glucan, mannan antigen in association with anti-mannan antibodies, and Candida albicans germ tube (CAGT) antibodies [8]. However, these tests show limitations, mainly due to the high rate of false positive results, lack of specificity, turnaround time, and more importantly the limited availability in most hospitals. At the moment of the writing of this manuscript, β-1,3-d-glucan test (the most accepted and diffuse test) is available in no more than ten hospitals in our country.
Indeed, in septic patients admitted to internal medicine units, and a suggestive risk profile for Candida infection, a PCT value lower than 2.5 ng/mL should raise a high probability of candidemia, and justify the prompt initiation of antifungal therapy, pending the results of microbiological tests and indirect tests such as β-1,3-d-glucan.
Taken together, the potential values of these findings are somewhat limited by the retrospective nature of this study: firstly, it limits the exportability of the results as compared to a prospective study; and secondly, it prevents further exploration of patient cases given the impossibility of gathering serial measurements of PCT that could have provided a complete picture on PCT temporal trend for Candida. On the other hand, due to the low prevalence of fungal infections, as for other studies in the ICU setting (where most of the literature is available), a prospective study would have hardly been feasible.
In conclusion, this is a study exploring the role of procalcitonin in differentiating Candida and bacterial blood stream infections in critically ill septic patients admitted to internal medicine units. Patients with candidemia have significantly lower values of procalcitonin compared to patients with bacteremia. A procalcitonin value of 2.5 ng/mL gives the most accurate cut-off to differentiate Candida from bacterial blood stream infection. A PCT level ≥2.5 ng/mL has a 98% negative predictive value and a 15% positive predictive value for candidemia-related sepsis. On the other hand, a value lower than that threshold is an independent predictor of candidemia determining a eightfold increase in the probability of finding Candida in blood cultures. Thus, in septic patients at risk for a Candida infection admitted to internal medicine units, a PCT value lower than 2.5 ng/mL should raise the probability of fungal infection, adding value for considering prompt initiation of antifungal therapy.
References
HAI-Net PPS interactive database: Healthcare-associated infections and antimicrobial use in acute care hospitals. http://ecdc.europa.eu/en/healthtopics/Healthcare-associated_infections/database/Pages/database.asp. Accessed 25 July 2016
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39(3):309–317
Arendrup MC (2010) Epidemiology of invasive candidiasis. Curr Opin Crit Care 16(5):445–452
Bassetti M, Trecarichi EM, Righi E, Sanguinetti M, Bisio F, Posteraro B et al (2007) Incidence, risk factors, and predictors of outcome of candidemia. Survey in 2 Italian university hospitals. Diagn Microbiol Infect Dis 58(3):325–331 (Elsevier)
Falagas ME, Apostolou KE, Pappas VD (2006) Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur J Clin Microbiol Infect Dis 25(7):419–425
Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS et al (2006) Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43(1):25–31
Clancy CJ, Nguyen MH (2013) Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis 56(9):1284–1292
Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O et al (2012) ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):19–37
Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE et al (2009) Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48(5):503–535
Held J, Kohlberger I, Rappold E, Busse Grawitz A, Häcker G (2013) Comparison of (1->3)-β-d-glucan, mannan/anti-mannan antibodies, and Cand-Tec Candida antigen as serum biomarkers for candidemia. J Clin Microbiol 51(4):1158–1164
Digby J, Kalbfleisch J, Glenn A, Larsen A, Browder W, Williams D (2003) Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin Diagn Lab Immunol 10(5):882–885
Gilbert DN (2010) Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol 48(7):2325–2329
Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet (London, England) 341(8844):515–518 (internet)
Brodská H, Malíčková K, Adámková V, Benáková H, Šťastná MM, Zima T (2013) Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin Exp Med 13(3):165–170
Guo SY, Zhou Y, Hu QF, Yao J, Wang H (2015) Procalcitonin is a marker of Gram-negative bacteremia in patients with sepsis. Am J Med Sci 349(6):499–504
Christ-Crain M, Müller B (2005) Procalcitonin in bacterial infections—hype, hope, more or less? Swiss Med Wkly 135(31–32):451–460
Pieralli F, Vannucchi V, Mancini A, Antonielli E, Luise F, Sammicheli L et al (2015) Procalcitonin kinetics in the first 72 hours predicts 30-day mortality in severely ill septic patients admitted to an intermediate care unit. J Clin Med Res 7(9):706–713
Jain S, Sinha S, Sharma SK, Samantaray JC, Aggrawal P, Vikram NK et al (2014) Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC Res Notes 7:458
Martini A, Gottin L, Menestrina N, Schweiger V, Simion D, Vincent J-L (2010) Procalcitonin levels in surgical patients at risk of candidemia. J Infect 60(6):425–430
Charles PE, Dalle F, Aho S, Quenot J-P, Doise J-M, Aube H et al (2006) Serum procalcitonin measurement contribution to the early diagnosis of candidemia in critically ill patients. Intensive Care Med 32(10):1577–1583
Montagna MT, Coretti C, Caggiano G (2011) Procalcitonin: a possible marker of invasive fungal infection in high risk patients? J Prev Med Hyg. 52(1):38–39
Cortegiani A, Russotto V, Montalto F, Foresta G, Accurso G, Palmeri C et al (2014) Procalcitonin as a marker of Candida species detection by blood culture and polymerase chain reaction in septic patients. BMC Anesthesiol 14:9
Jin M, Khan AI (2010) Procalcitonin: uses in the clinical laboratory for the diagnosis of sepsis. Lab Med 41(3):173–177 (The Oxford University Press)
Frank M, Sivagnanaratnam A, Bernstein J (2015) Nutritional assessment in elderly care: a MUST!. BMJ Qual Improv Reports 4(1):1–3
Tavares E, Maldonado R, Ojeda ML, Miñano FJ (2005) Circulating inflammatory mediators during start of fever in differential diagnosis of Gram-negative and Gram-positive infections in leukopenic rats. Clin Diagn Lab Immunol 12(9):1085–1093
Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Müller B (2004) Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med 32(8):1715–1721
Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH et al (2011) Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306(23):2594–2605
Molter GP, Soltész S, Kottke R, Wilhelm W, Biedler A, Silomon M (2003) Procalcitonin plasma concentrations and systemic inflammatory response following different types of surgery. Anaesthesist 52(3):210–217
Bassetti M, Merelli M, Righi E, Diaz-Martin A, Rosello EM, Luzzati R et al (2013) Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol 51(12):4167–4172
Morrell M, Fraser VJ, Kollef MH (2005) Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49(9):3640–3645
De Rosa FG, Corcione S, Filippini C, Raviolo S, Fossati L, Montrucchio C et al (2015) The Effect on mortality of fluconazole or echinocandins treatment in candidemia in internal medicine wards. PLoS One 10(5):1–9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
The study was performed in agreement with the principles set in the Declaration of Helsinki on human research ethics.
Informed consent
Informed consent was not needed in accordance to current regulatory laws on retrospective observational studies in our country. Local ethics committee approval was obtained (SepsiMed Study protocol number 0018329).
Rights and permissions
About this article
Cite this article
Pieralli, F., Corbo, L., Torrigiani, A. et al. Usefulness of procalcitonin in differentiating Candida and bacterial blood stream infections in critically ill septic patients outside the intensive care unit. Intern Emerg Med 12, 629–635 (2017). https://doi.org/10.1007/s11739-017-1627-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-017-1627-7