Abstract
Procalcitonin (PCT) levels can distinguish between infectious and non-infectious systemic inflammatory response. However, there are some differences between Gram-negative (G−), Gram-positive (G+), and fungal bloodstream infections, particularly in different cytokine profiles, severity and mortality. The aim of current study was to examine whether PCT levels can serve as a distinguishing mark between G+, G−, and fungal sepsis as well. One hundred and sixty-six septic patients with positive blood cultures were examined on C-reactive protein (CRP) and PCT on the same date of blood culture evaluation. The median (interquartile range, IQR) of CRP and PCT in G+, G−, and fungal cohorts and comparison of measured values between groups were made using the Kruskal–Wallis test with subsequent Bonferroni’s corrections, with p < 0.05. In 83/166 (50 %) of blood cultures, G+ microbes, 78/166 (47 %) G− rods, and 5/166 (3 %) fungi were detected. PCT concentrations (ng/ml) were significantly higher in G− compared to other cohorts: 8.90 (1.88; 32.60) in G−, 0.73 (0.22; 3.40) in G+, and 0.58 (0.35; 0.73) in fungi (p < 0.00001). CRP concentrations did not differ significantly in groups. Significantly higher PCT levels could differentiate G− sepsis from G+ and fungemia. In contrast to CRP, PCT is a good discriminative biomarker in different bloodstream infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis, a systemic inflammatory response to noxious infection, is a relatively frequent and serious complication in critically ill patients. Mean sepsis incidence of around 30–40 % in intensive care units (ICU) patients is commonly reported: for instance, the SOAP study (Sepsis Occurrence in Acutely Ill Patients), which evaluated data from 3147 ICU patients from 198 centers, reported sepsis during hospitalization in 38 % of patients, whereas mortality of these septic patients was 27 % compared to 14 % in non-septic ICU cohort [1]. Early and accurate diagnostics of sepsis with rapid initiation of adequate therapy is crucial for patients’ surviving. However, microbiological testing of sepsis and accurate identification of etiological agents are time-consuming; moreover, failure of capture agents in blood cultures could occur even in apparently septic individuals, and clinical signs could not be always clear promptly. Diagnostic criteria for sepsis [2, 3], therefore, include (1) clinical symptoms such as changes in body temperature, breathing, and heart rate, and (2) laboratory markers of inflammation. To identify sepsis and to determine septic patients’ prognosis, more than one hundred different biomarkers were reported until today. However, a biomarker with 100 % sensitivity and specificity for sepsis was not described yet [4]. The combination of commercially well-available markers C-reactive protein (CRP), procalcitonin (PCT), and leukocyte count is used for evaluation in most cases. CRP is less sensitive (75 and 88 %, respectively) and less specific (67 and 81 %, respectively) than PCT for differentiating bacterial from non-infective causes of inflammation [5]. The leukocyte count also has significant limitations, especially in immunosuppressed patients. PCT has the highest sensitivity and specificity for predicting systemic bacterial inflammation; moreover, high PCT concentrations have a positive predictive value for severe sepsis and septic shock, and correlate with the severity of inflammation [6–8] and distinguish between viral and bacterial infections. [5, 6] Plasma PCT levels can be influenced by multiple factors such as individual genetically determined immune alert as well as the degree and type of microbial aggression or the extent of inflammation.
In our current study, we have evaluated PCT and CRP levels in septic patients with positive blood cultures. The main goal of the work was to examine whether PCT and CRP levels could serve as a distinguishing marker between Gram-negative (G−), Gram-positive (G+), and fungal sepsis.
Materials and methods
Samples
We have performed a retrospective study of plasma PCT and CRP levels in ICU patients with positive blood cultures during the six-month period. One hundred and sixty-six (166) patients with positive blood cultures were assessed. The clinical status of patients was classified according to the Sepsis Definition Conference criteria [3]. The majority of patients from our cohort were from hematological ICUs, and as there is no routine Sequential Organ Failure Assessment (SOFA) performed on these patients, this score was not included in our study. Plasma PCT and CRP values were analyzed from the day of positive blood culture realization. The proven hemoculture-positive individuals were divided into three groups according to detected infectious agents: (1) G− bacteremia, (2) G+ bacteremia, and (3) fungal infection groups.
Laboratory examinations
Serum PCT concentrations were measured by sandwich electrochemiluminescence immunoassay (Cobas ECLIA, Japan). Analyses were performed according to manufacturer‘s recommendations. The concentration of 0.5 ng/mL was chosen as the cutoff value. CRP was measured by a turbidimetry Modular SWA (Roche Diagnostic, Switzerland). The cutoff value of greater than 5.0 mg/l was adopted. One set of blood cultures (aerobic, anaerobic) was taken by sterile venipuncture and processed using the Bactec 9420 system (Becton–Dickinson, Heidelberg, Germany). Bacteremia was defined by microbial growth in one blood culture bottle, and only for coagulase-negative Staphylococcus species were two positive blood culture bottles from different venipunctures required; otherwise, the results were assessed as contamination of blood during venipuncture.
Statistical analysis
Statistical analysis was performed using the software Statistica CZ 9.0 (StatSoft Inc, USA). Different groups were compared using the two-sided Kruskal–Wallis non-parametric test. Follow-up tests were conducted by using the Bonferroni approach. The threshold for significance was set at p < 0.05.
Results
Basic demographic characteristics of analyzed cohorts are included in Table 1.
In our cohorts, 75 cases of G+ cocci were found in blood cultures, G+ rods in 8 individuals, G− cocci in 2 patients, G− rods in 76 patients, and in 5 patients fungal infection was found. One hundred and forty-seven (89 %) positive blood cultures were aerobic and 19 (11 %) anaerobic.
Median (interquartile range, IQR) of CRP and PCT in different cohorts and comparison of measured values are included in Table 2.
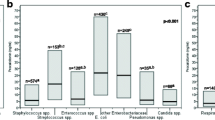
PCT concentrations were significantly higher in G− compared to other cohorts, see Fig. 1.
PCT concentrations in different cohorts according to hemoculture results. PCT procalcitonin, G+ Gram-positive, G− Gram-negative. The box plot represents the lower and upper quartiles, the horizontal line represents the median, and the whiskers represent the sample minimum and maximum. Bonferroni adjustment was used in multiple comparison procedures
Moreover, significantly higher PCT levels were found in patients with Escherichia, Klebsiella, and Pseudomonas in blood cultures. Candida, Streptococcus, and Staphylococcus were linked with the mild PCT levels elevation.
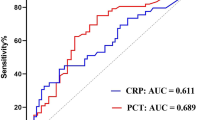
The ROC plots and respective AUCs for PCT and CRP in the diagnosis of G− bacteremia are shown in Fig. 2. Sensitivities of 75.10 and 61.20 and specificities of 87.80 and 54.30 % for the G− bacteremia were achieved with a PCT cutoff value of 15 pg/mL and a CRP cutoff value of 86.2 mg/L. The AUC for PCT was 0.871, which was significantly higher than that for CRP (0.705; p = 0.002).
The ROC curves of PCT (AUC 0.871) and CRP (AUC 0.705) for the diagnosis of G− bacteremia. PCT for cutoff 15 pg/mL. % sensitivity: 75.10 %. % specificity: 87.80. CRP for cutoff 86.2 mg/L. % sensitivity: 61.20 %. % specificity: 54.30 %. PCT procalcitonin, CRP C-reactive protein, AUC area under the curve
For cases with G− bacteremia, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of different PCT concentrations were calculated, see Table 3.
In summary, significantly higher PCT levels were observed in G− bacteremia compared to G + bacteremia and fungal infection. No such significant differences were found for CRP.
Discussion
In the current study, we have focused on the rapid diagnosis of sepsis. We have assessed PCT and CRP as most commonly used markers and have looked for possible relationships with the nature of a causal microbial agent. PCT levels in our G− septic cohort were significantly higher than those of G+ and fungal sepsis. The difference in PCT levels between G+ and fungal agents was not statistically significant. Plasma CRP levels between different types of microbial agents do not differ statistically as well.
G+ bloodstream infections slightly dominated in our cohort (G+ in 50 % cases and G− in 46 % cases). Unambiguous fungal infection was identified in only 3 % of individuals, which was in accordance with other observations [4] of formidable detection of fungal bloodstream infections.
Staphylococci were identified as the most frequent causal pathogens in our cohort (33 %), followed by E. coli (16 %) and Klebsiella (14 %). In general, Staphylococci (i.e., Staphylococcus aureus) and Gram-negative rods from family Enterobacteriacea are the most common causative agents of sepsis. On the other hand, isolation of coagulase-negative staphylococci is frequently a consequence of contamination of blood samples. In patients with Escherichia coli, Klebsiella spp., and Pseudomonas spp. in blood cultures, the highest PCT values were found; on the other side, Candida spp, Streptococcus spp., and Staphylococcus spp. were associated mainly with the mild elevation of PCT. In CRP, we did not find any significant differences from this point of view.
Inflammatory cascades in septicemia are complex processes. In vitro studies have clearly described the functional differences of G−, G+, and fungal agents invading the human host organism. These differences reflect the different pathways of activation and initiation of inflammatory cascades. At the beginning of these defensive cascades, the detection of the pathogen or its parts (pathogen-associated molecular patterns, PAMPs) through Toll-like receptors (TLRs) takes part. Superficial TLR1, TLR12, TLR14, TLR15, TLR16, and TLR110 are primarily intended for recognizing bacterial products, while intracellular TLR3, TLR7, TLR8, and TLR9 recognize nucleic acids that are produced during the replication of viruses. There is evidence that TLR4 recognizes lipopolysaccharide (LPS) patterns of G− bacteria, while TLR2 identifies lipoteichoic acid of G+. After the TLRs activation, different inflammatory cascades are triggered, in particular, via nuclear factor kB and other mediators leading to the synthesis of proinflammatory cytokines and acute-phase proteins [9]. G− infections probably increase the production of TNF-alpha more compared to G+ microbes [10], and differences were found also in plasma levels of IL-1, IL-6, IL-10, and IL-8 [11, 12]. Interestingly, similar differences have been recently described also for PCT [13]. Mohamed et al. [12] have demonstrated significantly lower in vitro production of IL-6, IL-8, TNF-alpha, and IL-1b in staphylococcal infections compared to the production of these proinflammatory cytokines in E. coli. Bjerre et al. [11] reported higher TNF-alpha, IL-10, and IFN-gamma production in G− sepsis, and in particular, Gram-negative meningococcal septicemia. The differences in the levels of IL-6 and IL-18 as well as PCT between G+ and G− infections were observed also by Feezor et al. [10]. Abe et al. [14] affirmed that G− bacteremia induces greater magnitude of inflammatory response than G+ bacteremia. And this may be the answer for a higher elevation levels of PCT in G− bacteremia, as described by Feezor and Charles [10, 13]. Charles also concluded that G− bacteremia could be associated with higher PCT values than those found in G+ bacteremia, regardless of the severity of the disease [13]. But why CRP is not higher at the same time in G− is hard to say. To clarify this clearly, more studies have to be undertaken. However, our results are in line with greater magnitude of plasma PCT level in G− sepsis in comparison with G+ sepsis as in above-mentioned studies. But we did not compare SOFA in our cohort, so we could not confirm the dependence of the severity of disease.
To clarify exactly the reason for the different elevation of PCT and its independence on severity of disease, more studies should be conducted.
Martini et al. [15] have suggested setting the PCT boundary value at 2 ng/mL, whereas values under this value had negative predictive value of 94 % for the bacterial septicemia, in comparing bacteremia versus fungemia. In our study, we clearly demonstrated better diagnostic value for G- sepsis of PCT by using ROC. The ROC plots and respective AUCs for PCT and CRP in the diagnosis of G− bacteremia shown sensitivities of 75.10 and 61.20 %, and specificities of 87.80 and 54.30 % for the G− bacteremia were achieved with a PCT cutoff value of 15 pg/mL and a CRP cutoff value of 86.2 mg/L. The AUC for PCT was 0.871, which was significantly higher than that for CRP (0.705; p = 0.002). Similar conclusions were reported by Charles et al. [13], no significant differences in CRP but definite divergence in PCT levels (mean PCT concentrations of 39.0 ng/mL in G− and 5.42 ng/mL in G+, p = 0.003); however, this study does not include subjects with fungal sepsis. Significantly higher PCT levels in G− septicemia compared to G + and mycotic sepsis (p = 0.0001) were noticeably expressed in our patients with hematological malignancies and patients after bone marrow transplantation. Hence, PCT levels could serve as a simple utility for confirmation or exclusion of G− bacterial septicemia in these groups of patients. Our findings are in line with observations of Montagna et al. [16] In their study of neutropenic septic patients, the low PCT levels often showed fungal infection (values around 1.0 ng/mL for Candida spp. and 1.91 ng/mL for Aspergillus spp.); however, a small number of patients limited this study’s results. CRP levels did not differ significantly in our three cohorts. It affirms the role of CRP as a highly sensitive but poorly specific marker of infection, which is not able to distinguish the type of infection. The finding the combination of high plasmatic level of CRP (more than 100) and low level of PCT (less than 2.0) is highly suspect to mycotic affection.
In contrast to CRP, PCT could distinguish the severity of clinical condition (sepsis, severe sepsis, septic shock, multi-organ dysfunction syndrome/MODS/degrees) as well as inform us about the type of microbial agent [6, 17, 18]. Generally, PCT is more specific marker for infection than CRP (ROC analysis), and CRP has good sensitivity but less specificity [6, 13, 16–18]. Also the physiological role of both markers is not the same. We actually do not know how does it work exactly. Each marker is slightly different. We use to combine both markers together with other markers, such as WBC and IL-6, and with clinical signs. Thus, we improve the diagnostic effect.
In conclusion, plasma PCT level is an additional marker to both clinical and microbiological criteria of sepsis, providing the possibility to estimate the type of microbe as well as the seriousness and severity of infection and consequently to consider first-choice antibiotic treatment. We definitely do not want to reduce the role of microbiological diagnosis with the identification of microbe and assessment of sensitivity to different antibiotics. This part of laboratory examination is an absolutely unexceptionable part of sepsis management. However, the time needed for this testing in manifold is larger compared to the few minutes required for PCT measurements. PCT could be considered as a good discriminative biomarker in different bloodstream infections.
References
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H et al (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353
ACCP-SCCM Consensus Conference (1992) Definitions of sepsis and multiple organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992(20):864–874
(2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis DefinitionsConference. Crit Care Med 31:4
Pierrakos CH, Vincent JL (2010) Sepsis biomarkers: a review. Cri Care 14(1):R15
Simon L, Gauvin F, Saint Louis P, Lacroix J (2004) Serum procalcitonin and C-reactive levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 39:206–217
Reinhart K, Meisner M (2011) Biomarkers in the critically ill patient: procalcitonin. Crit Care Clin 27:253–263
Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R (2000) Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Med 26:S148–S152
Reinhart K, Meisner M (2006) Markers for sepsis diagnosis: what is useful? Crit Care Clin 22:503–519
Gao H, Evans TW, Finney SJ (2008) Bench-to-bedside review: sepsis, severe sepsis and septic shock—does the nature of the infecting organism matter? Crit Care 12(3):213
Feezor RJ, Oberholzer C, Baker HV, Novick D, Rubinstein M, Moldawer LL et al (2003) Molecular characterization of the acute inflammatory response to infections with gram-negative versus gram-positive bacteria. Infect immune 7:5803–5813
Bjere A, Brusletto B, Holby EA, Kjerulf P, Brandtzaeg P (2004) Plasma Interferon gamma and interleukin 10 concentration in systemic meningococcal disease compared with severe systemic Gram-positive septic shock. Crit Care Med 32:433–438
Mohamed MA, Cunningham-Rundles S, Dean CR, Hammad TA, Nesin M (2007) Levels of proinflammatory cytokines produced from cord blood in vitro are pathogen dependent and increased in comparison to adult controls. Cytokine 39:171–177
Charles PE, Ladoire S, Aho S, Quenot JP, Doise JM, Prin S (2008) Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either gram negative or gram positive bacteria. BMC Infect Dis 8:38
Abe R, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Tateishi Y (2010) Gram-negative bacteremia induces greater magnitude of inflammatory response than gram-positive bacteremia. Crit Care 14:R27
Martini A, Gottin L, Menestrina N, Schweiger V, Simion D, Vincent JL (2010) Procalcitonin levels in surgical patients at risk of candidemia. J Infect 60:425–430
Montagna MT, Coretti C, Caggiano P (2011) Procalcitonin: a possible marker of invasive fungal infection in high risk patients? J Prev Hyg 52:38–39
Vincent JL, Donadello K, Schmidt X (2011) Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clin 27:241–251
Delevaux I, Andre M, Colombier M, Albuisson E, Meylheuc F, Bégueet RJ (2003) Can procalcitonin measurement help in differentiating between bacterial infection and other kinds of inflammatory processes? Rheum Dis 62:337–340
Acknowledgments
This work was supported by the Research Program P25 of Charles University in Prague.
Conflict of interest
Authors certify that they have no affiliation with or financial involvement with any organization or entity with a direct financial or any other interest in the subject matter or materials discussed in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brodská, H., Malíčková, K., Adámková, V. et al. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin Exp Med 13, 165–170 (2013). https://doi.org/10.1007/s10238-012-0191-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-012-0191-8