Abstract
Circulating C-reactive protein (CRP) plays an important role in mediating extra-pulmonary complications of chronic obstructive pulmonary disease (COPD). The aim of this study was to investigate the relationship between changes in high sensitivity (hs)-CRP levels and the resolution of airway inflammatory markers and clinical health status during the recovery period after an acute exacerbation of COPD (AECOPD). Consecutive patients hospitalized for AECOPD were recruited. Serum hs-CRP, airway inflammatory markers, and COPD Assessment Test (CAT) score were evaluated at admission prior to treatment and at days 4, 7, and 14. Adverse outcomes were recorded. The relationship between changes in airway inflammatory markers, CAT score, and hs-CRP during the recovery period was studied. A total of 135 patients were enrolled. Serum hs-CRP levels at admission of patients with adverse outcomes were marginally higher than those without an adverse outcome (7.6 [4.8, 16.7] vs. 6.6 [4.7, 9.3], p = 0.061). Compared with patients without cardiovascular complications, patients with cardiovascular complications had higher serum hs-CRP levels at admission (11.6 [6.7, 16.7] vs. 6.6 [4.4, 10.0], p = 0.001). Sputum neutrophils were positively correlated to hs-CRP at admission (r = 0.474, p < 0.001). A decreasing hs-CRP level was positively related to decreasing sputum neutrophils at day 4 and 7 (r = 0.455, p < 0.001; r = 0.504, p < 0.001, respectively). Significant correlations between decreasing hs-CRP and CAT at all time-points were noted. Hs-CRP may be useful in monitoring airway inflammation resolution and improvement of health status during AECOPD treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality throughout the world. Acute exacerbation of COPD (AECOPD) is an acute event characterized by worsening of respiratory symptoms that is beyond normal day-to-day variations, and leads to a change in medication. Hospitalization for AECOPD is associated with a high risk of morbidity and mortality. Airway inflammation is a prominent feature of COPD, and is amplified during exacerbations [1].
Recent evidence has shown that COPD is a systemic disorder associated with increased inflammatory proteins in the circulation [2]. Serum C-reactive protein (CRP) levels are easily and rapidly measured, and recent studies show that they may play an important role in mediating the extra-pulmonary complications of COPD (such as cardiovascular diseases), and may contribute substantially to the overall morbidity and mortality of COPD patients [3, 4]. Compared to stable patients, those AECOPD have higher circulating CRP levels [5–8]. Furthermore, following treatment for AECOPD, hs-CRP levels decrease significantly compared with admission values [9]. However, there have been few studies examining whether a decrease of CRP level correlates with clinical improvement during recovery from AECOPD. In addition, no studies have examined the relationship between dynamic changes of CRP and sputum inflammatory markers during a COPD exacerbation. Therefore, in order to explore the relationship between dynamic changes of hs-CRP and airway inflammation and short-term outcomes, we designed a prospective study and utilized a well-characterized cohort in which airway inflammation and serum hs-CRP were assessed at admission prior to treatment and throughout the recovery period on days 4, 7, and 14. Furthermore, the relationship between changes in airway inflammatory markers, clinical indices, and health status and hs-CRP was studied during the recovery period.
Methods
Patient enrollment
Consecutive patients with AECOPD admitted to the Respiratory Department in Peking University Third Hospital from April 2009 to September 2011 were enrolled. For patients admitted more than once during the study period, only the first admission was included. Inclusion criteria were a diagnosis of COPD and an acute exacerbation that met the Global Initiative of Chronic Obstructive Lung Disease definition [10]. Post-bronchodilator spirometric measurements obtained when patients were stable that were in the medical records were used to diagnose COPD. Patients were only included if they were hospitalized for more than 24 h. Exclusion criteria were: asthma, bronchiectasis, pneumonia, sleep apnea syndrome, lung cancer, or other active pulmonary disease; hospitalization due to acute coronary syndrome or heart failure; the need for intubation or admission to the intensive care unit (ICU); length of stay (LOS) longer than 30 days; long-term glucocorticoid therapy (more than 3 months on ≥7.5 mg per day prednisone or equivalent); the patient had received systemic glucocorticoids for an exacerbation more than 48 h before presentation. Written informed consent forms were obtained from all patients, and the Ethics Committee of the hospital approved the study protocol.
Clinical data collection
Demographic data, spirometric measurements during the stable phase of the disease, comorbid conditions, tobacco exposure, exacerbation frequency in the previous year, and admission symptoms were collected. Frequent exacerbation was defined as an exacerbation frequency ≥2 in the previous year, and non-frequent as <2 exacerbations in the previous year [11]. On the day of admission, the Anthonisen type of AECOPD was determined according to symptoms before starting treatment [12].
Therapy strategy
Patients were treated with nebulized salbutamol, ipratropium bromide, and budesonide, and intravenous prednisolone with an initial dose of 30–40 mg daily. On day 4, patients were switched to oral prednisolone. The duration of prednisolone administration was about 2 weeks. Antibiotics were administered if a bacterial infection was suspected (patient-reported sputum purulence), and adjusted according to anti-microbial susceptibilities when available.
Mechanical ventilation (non-invasive, whenever possible) was instituted for indications such as respiratory arrest, deterioration in consciousness, or increasing partial pressure of arterial carbon dioxide (PaCO2) despite maximal pharmacological treatment. Decisions regarding admission or transfer to ICU were made by the treating physician. The criteria for discharge from the hospital after the index exacerbation and the management of stable COPD followed the standard protocol utilized at our hospital.
Follow-up
After a baseline assessment at admission, patients were assessed on days 4, 7, and 14. At each time-point, dyspnea was assessed using the 5-grade Medical Research Council dyspnea scale [13]. The COPD Assessment Test (CAT) was completed in the morning. Peak expiratory flow (PEF) was measured with a mini-Wright peak flow meter (Clement Clarke, Harlow, UK) three times in the morning before treatment. The highest of the three measurements was used for the analysis.
Clinical outcome
Adverse clinical outcomes (death from any cause in the hospital or within 8 weeks after discharge, need for mechanical ventilation after the second hospital day, recurrent exacerbation within 8 weeks after discharge) and cardiovascular complications (acute coronary syndrome, heart failure, dysrhythmia, sudden cardiac death) in hospital or within 8 weeks after discharge were recorded. A recurrent exacerbation was defined as a second exacerbation, fulfilling the present criteria, occurring within 8 weeks of discharge. Symptoms from the first exacerbation must have recovered to pre-exacerbation levels.
Sample processing
Sputum and serum samples were collected at admission prior to initial treatment, and on days 4, 7, and 14. Spontaneous sputum was primarily used, but induced sputum was collected when necessary (of the sputum samples, 97 % were spontaneous). Each sample was confirmed to be a valid sputum sample if there were >25 leukocytes and <10 squamous cells present on Gram’s stain with low power (×100) magnification [14]. Samples were processed within 2 h with phosphate buffered saline (PBS). Neither dithioerythritol nor dithiothreitol was used for sputum sample processing. Cytospins were prepared, and cell-free supernatant was collected and stored at −80 °C pending analyses of soluble mediators. Differential cell counts were counted on May Grünwald Giemsa stained cytospins in a blinded fashion. Peripheral venous blood (7 mL) was collected into a Vacutainer tube (BD Diagnostics, NJ, USA) and centrifuged at 6716×g for 10 min at 4 °C. Serum was then separated and stored at −80 °C for further analysis.
Sputum interleukin (IL)-8 and myeloperoxidase (MPO) were quantified using commercial sandwich ELISA kits (R&D Systems, Abingdon, UK). Serum high sensitivity C-reactive protein (hs-CRP) was measured by a latex agglutination test, using an Olympus AU5400 automatic biochemical analyzer. All the samples from each patient were measured in the same assay to reduce inter-assay variability. The limit of detection was 7.5 pg/mL for sputum IL-8, 0.062 ng/mL for sputum MPO, and 0.1 mg/L for serum hs-CRP.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0. Data were not normally distributed, and nonparametric analyses were used. Categorical variables were presented as number (%), and continuous variables as median with interquartile range (IQR: 25th, 75th percentiles). Mann–Whitney U test was used for comparisons between two independent groups. Receiver operating characteristic (ROC) curve was used to analyze the predictive usefulness of serum hs-CRP for clinical adverse events. Correlations between change in systemic inflammation markers and change in airway inflammation and health status were analyzed using Spearman’s correlation analysis. Results were considered statistically significant at p < 0.05.
Results
Patient characteristics
A total of 173 eligible patients were initially included. However, 38 patients were withdrawn due to pneumonia (n = 6); heart failure at admission (n = 5); pulmonary embolism (n = 3); need for intubation at admission (n = 14); LOS longer than 30 days (n = 5); received systemic corticosteroids for more than 48 h before admission (n = 5). Therefore, 135 patients were enrolled in the final analysis and their characteristics are summarized in Table 1.
Relationship between clinical outcomes and serum hs-CRP at admission
During the study period, a total of 42 (31.1 %) adverse events including death during hospitalization and within 8 weeks after discharge, need for mechanical ventilation, and recurrent exacerbation after discharge occurred (Table 2). Serum hs-CRP levels at admission of patients with adverse outcomes were marginally higher than those without adverse outcomes (7.6 [4.8, 16.7] vs. 6.6 [4.7, 9.3], p = 0.061).
Relationship between cardiovascular complications and serum hs-CRP at admission
During the study period, a total of 27 (20 %) cardiovascular adverse events in hospital or within 8 weeks after discharge were observed including acute coronary syndrome, heart failure, dysrhythmia, and sudden cardiac death (Table 3). Compared with those without cardiovascular complications, patients with cardiovascular complications had significantly higher levels of serum hs-CRP at admission (11.6 [6.7, 16.7] vs. 6.6 [4.4, 10.0], p = 0.001).
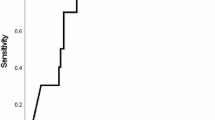
A ROC curve was created to evaluate the predictive usefulness of serum hs-CRP for cardiovascular complications. As shown in Fig. 1, the area under the ROC curve was 0.729 (95 % confidence interval 0.626–0.832, p < 0.001). Using a cutoff value of 7 mg/L, the sensitivity, specificity, positive predictive value and negative predictive value of hs-CRP were 67.9, 68.2, 35.8 and 89.0 %, respectively, to identify a patient with cardiovascular complications.
Relationship between airway inflammatory markers and serum hs-CRP at admission
At admission, there was a significant relationship between sputum leukocyte count and serum hs-CRP (r = 0.474, p < 0.001) (Table 4). However, neither IL-8 nor MPO in sputum were statistically related to serum hs-CRP (both, p > 0.05).
Correlation between changes in serum hs-CRP and airway inflammatory markers
The 42 patients who had adverse clinical outcomes were excluded; thus, 93 patients were included in the analysis. The correlations between decreasing hs-CRP levels and decreasing levels of airway inflammatory markers at days 4, 7, and 14 are shown in Table 5. There is a significant correlation between the decrease in hs-CRP and decrease of both sputum neutrophils and MPO on day 4 (r = 0.455, p < 0.001; r = 0.211, p = 0.043, respectively), and between hs-CRP and sputum neutrophils on day 7 (r = 0.504, p < 0.001). No significant correlations between change in hs-CRP level and either sputum neutrophils or MPO on day 14 are noted. There is no significant correlation between change in hs-CRP and change in IL-8.
Correlation between serum hs-CRP change and clinical indices
The correlations between hs-CRP level and clinical indices on days 4, 7, and 14 are shown in Table 5. There are positive correlations between decreased of hs-CRP level and decrease CAT score at all the time-points (day 4: r = 0.273, p = 0.008; day 7: r = 0.254, p = 0.014; day 14: r = 0.268, p = 0.009).
Discussion
There are a number of new and principal findings of the current study. (1) Monitoring CRP levels may be clinically important, as there is a relationship between serum hs-CRP and short-term adverse outcomes and cardiovascular complications in AECOPD recovery period. (2) A direct relationship is identified between decreasing serum hs-CRP level and the response of airway neutrophil inflammation to therapy, but this relationship is only seen during the first week of treatment. (3) It is demonstrated for the first time that a decreasing serum hs-CRP level may reflect an improvement of health status during treatment of AECOPD.
Several studies assessed the role of CRP in AECOPD, but the results have been inconsistent. Stolz et al. [5] analyzed 167 patients presenting to the emergency department due to AECOPD. Of 3 biomarkers that were assessed (copeptin, C-reactive protein, and procalcitonin), only copeptin on admission is associated with a prolonged hospital stay and long-term clinical failure (occurrence of an exacerbation of COPD requiring hospitalization or death from any cause up to 6 months). A review assessed the role of CRP as a prognostic predictor in COPD exacerbations, and concludes that CRP alone is neither sensitive nor specific in predicting clinical severity or outcome [7]. Another study shows that although it is neither sensitive nor specific, plasma CRP in combination with other variables obtained at admission shows good predictive ability to identify an adverse outcome [15]. A recent study finds that serum hs-CRP at admission is a predictor of adverse outcome (death in hospital or within 30 days after discharge; transfer to the intensive care unit; intubation and mechanical ventilation) in AECOPD [6]. Another recent study finds that high CRP levels are a prognostic factor of AECOPD severity, and are associated with an increased mortality risk [8]. In the current study, serum hs-CRP levels at admission of patients who had an adverse outcome are higher than those without an adverse outcome, though the difference only attained borderline significance. All the aforementioned studies, including ours, are observational studies. Further interventional studies are needed to clarify the exact role of CRP in predicting the prognosis of AECOPD.
Biomarkers such as CRP, IL-6, and tumor necrosis factor (TNF)-α are generally considered markers of endothelial dysfunction, and in particular CRP is one of the strongest independent predictors of vascular morbidity and mortality [16, 17]. Recent studies show that in stable COPD patients, baseline CRP levels can predict the incidence of cardiovascular comorbidities, and are associated with cardiovascular mortality [18, 19]. A novel finding of the current study is that a higher serum hs-CRP at admission is significantly related to the occurrence of cardiovascular complications during an exacerbation of COPD. Cardiovascular disease is a primary cause of death in patients previously admitted for AECOPD [20]. Statins and inhaled corticosteroids have been investigated as potential therapeutic interventions in COPD for lowering cardiovascular risk [21]. The results of this study suggest that serum hs-CRP obtained at admission may help to identify patients at high risk for cardiovascular events.
Consistent with previous studies, recovery from the exacerbation results in a significant decrease in both serum hs-CRP and markers of airway inflammation [22]. One previous study examined the relationships between sputum and systemic inflammatory markers at exacerbation, and finds that the degree of systemic inflammation correlates with the neutrophil count in the lower airway; however, the study only evaluated a single time-point (onset of AECOPD) [23]. The current study, demonstrates that a dynamic decrease in serum hs-CRP reflects a resolution of neutrophilic lower airway inflammation in AECOPD. Previous studies show that elevation of both airway neutrophil inflammation and CRP levels in peripheral blood are correlated to bacterial infection in AECOPD [23, 24]. Viral or bacterial infection is found to be present in 78 % of AECOPD requiring hospitalization [25]. We speculate that clearance of the pathogen after anti-microbial therapy may be part of the reason for the resolution of both the serum CRP and sputum neutrophil inflammation.
The current study does not find that a decrease of serum hs-CRP correlates with improvement of dyspnea or PEF, but finds that a decrease in serum hs-CRP is related to improvement of health status as determined by the CAT score. Studies on stable COPD patients show there is a significant relationship between blood CRP levels and St George’s Hospital Respiratory Questionnaire (SGRQ) score [26, 27]. Another study shows that an increase in CAT score from baseline to exacerbation onset, is significantly related to increase in CRP level [28].
There are limitations to this study. First, we only included patients with severe AECOPD who required hospitalization, therefore the results are probably not generally applicable to all populations of COPD patients. Second, the airway inflammatory response in AECOPD is complex, and some studies report eosinophilia occurring in the airway [29–31], whereas others document increased neutrophilic inflammation [32–34]. The current study focused only on neutrophilic inflammation-related biomarkers because these were primarily investigated by previous studies of severe AECOPD. Serum hs-CRP was chosen because of its association with cardiovascular morbidity, and its widespread availability. Lastly, COPD phenotyping was not involved in our study. The phenotype of COPD is related to clinical symptoms, rate of FEV1 decreasing, imaging manifestations, frequency of acute exacerbation, systemic inflammation, complications, etc. [35]. However, the methods of COPD phenotyping are not very clear now, and is relatively complicated, so it is not considered in our study. But this is the future of research direction of COPD.
In conclusion, the results of this study demonstrate a relationship between hs-CRP and airway inflammation and health status in patients with an exacerbation of COPD. The levels of hs-CRP may be useful for monitoring resolution and response to therapy of patients with AECOPD. In addition, it might help to identify patients with AECOPD at high risk of adverse cardiovascular complications.
References
Sapey E, Stockley RA (2006) COPD exacerbations. 2: aetiology. Thorax 61(3):250–258
Joppa P, Petrasova D, Stancak B, Tkacova R (2006) Systemic inflammation in patients with COPD and pulmonary hypertension. Chest 130(2):326–333
Miniati M, Monti S, Bottai M, Cocci F, Fornai E, Lubrano V (2011) Prognostic value of C-reactive protein in chronic obstructive pulmonary disease. Intern Emerg Med 6(5):423–430
Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG (2007) C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175(3):250–255
Stolz D, Christ-Crain M, Morgenthaler NG et al (2007) Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest 131(4):1058–1067
Tofan F, Rahimi-Rad MH, Rasmi Y, Rahimirad S (2012) High sensitive C-reactive protein for prediction of adverse outcome in acute exacerbation of chronic obstructive pulmonary disease. Pneumologia 61(3):160–162
Antonescu-Turcu AL, Tomic R (2009) C-reactive protein and copeptin: prognostic predictors in chronic obstructive pulmonary disease exacerbations. Curr Opin Pulm Med 15(2):120–125
Karadeniz G, Polat G, Senol G, Buyuksirin M (2013) C-reactive protein measurements as a marker of the severity of chronic obstructive pulmonary disease exacerbations. Inflammation 36(4):948–953
Koutsokera A, Kiropoulos TS, Nikoulis DJ et al (2009) Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir Med 103(6):919–926
Rabe KF, Hurd S, Anzueto A et al (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176(6):532–555
Hurst JR, Vestbo J, Anzueto A et al (2010) Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 363(12):1128–1138
Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA (1987) Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 106(2):196–204
Mahler DA, Wells CK (1988) Evaluation of clinical methods for rating dyspnea. Chest 93(3):580–586
Gleckman R, DeVita J, Hibert D, Pelletier C, Martin R (1988) Sputum gram stain assessment in community-acquired bacteremic pneumonia. J Clin Microbiol 26(5):846–849
Ruiz-Gonzalez A, Lacasta D, Ibarz M, Martinez-Alonso M, Falguera M, Porcel JM (2008) C-reactive protein and other predictors of poor outcome in patients hospitalized with exacerbations of chronic obstructive pulmonary disease. Respirology 13(7):1028–1033
Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S (2003) New markers of inflammation and endothelial cell activation: part I. Circulation 108(16):1917–1923
Szmitko PE, Wang CH, Weisel RD, Jeffries GA, Anderson TJ, Verma S (2003) Biomarkers of vascular disease linking inflammation to endothelial activation: part II. Circulation 108(17):2041–2048
Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG (2012) Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186(10):982–988
Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD (2006) C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 61(10):849–853
Gudmundsson G, Ulrik CS, Gislason T et al (2012) Long-term survival in patients hospitalized for chronic obstructive pulmonary disease: a prospective observational study in the Nordic countries. Int J Chron Obstruct Pulmon Dis 7:571–576
Ghoorah K, De Soyza A, Kunadian V (2013) Increased cardiovascular risk in patients with chronic obstructive pulmonary disease and the potential mechanisms linking the two conditions: a review. Cardiol Rev 21(4):196–202
Valipour A, Schreder M, Wolzt M et al (2008) Circulating vascular endothelial growth factor and systemic inflammatory markers in patients with stable and exacerbated chronic obstructive pulmonary disease. Clin Sci (Lond) 115(7):225–232
Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA (2006) Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173(1):71–78
Gompertz S, O’Brien C, Bayley DL, Hill SL, Stockley RA (2001) Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur Respir J 17(6):1112–1119
Papi A, Bellettato CM, Braccioni F et al (2006) Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 173(10):1114–1121
Garrod R, Marshall J, Barley E, Fredericks S, Hagan G (2007) The relationship between inflammatory markers and disability in chronic obstructive pulmonary disease (COPD). Prim Care Respir J. 16(4):236–240
Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM (2006) Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 61(1):17–22
Mackay AJ, Donaldson GC, Patel AR, Jones PW, Hurst JR, Wedzicha JA (2012) Usefulness of the chronic obstructive pulmonary disease assessment test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med 185(11):1218–1224
Maestrelli P, Saetta M, Di SA et al (1995) Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am J Respir Crit Care Med 152(6 Pt 1):1926–1931
Zhu J, Qiu YS, Majumdar S et al (2001) Exacerbations of Bronchitis: bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractants. Am J Respir Crit Care Med 164(1):109–116
Fujimoto K, Yasuo M, Urushibata K, Hanaoka M, Koizumi T, Kubo K (2005) Airway inflammation during stable and acutely exacerbated chronic obstructive pulmonary disease. Eur Respir J 25(4):640–646
Aaron SD, Angel JB, Lunau M et al (2001) Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163(2):349–355
Sethi S, Muscarella K, Evans N, Klingman KL, Grant BJ, Murphy TF (2000) Airway inflammation and etiology of acute exacerbations of chronic bronchitis. Chest 118(6):1557–1565
Qiu Y, Zhu J, Bandi V et al (2003) Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168(8):968–975
Han MK, Agusti A, Calverley PM et al (2010) Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 182(5):598–604
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (81400017).
Conflict of interest
None.
Ethical Standard
This study was reviewed by Peking University Third Hospital Medical Science Research Ethics Committee and arbitrated as not systematic research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, Y., Chang, C., Zhu, H. et al. Correlation between decrease of CRP and resolution of airway inflammatory response, improvement of health status, and clinical outcomes during severe acute exacerbation of chronic obstructive pulmonary disease. Intern Emerg Med 10, 685–691 (2015). https://doi.org/10.1007/s11739-015-1228-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-015-1228-2