Abstract
Lichens are phototrophic organisms tolerant to adverse environmental conditions. However, the mechanisms underlying their stress tolerance are not fully understood. For photosynthetic organisms depending on solar radiation, UV-B radiation (280–320 nm) acts as a stress factor. We studied the pro-/antioxidant and respiratory metabolism of Peltigera aphthosa to identify adaptive responses of lichen to a physiological dose of UV-B radiation (14 kJ day−1 for 10 days). A browning of the upper cortex, the appearance of dark spots in the medulla layer of treated thalli, and an increase in the browning reflectance index indicated the synthesis of protective UV screening pigments. UV-B treatment did not cause significant changes in the photosynthetic activity of thalli and isolated algal cells. More intense lipid peroxidation activity and transient changes in H2O2 content accompanied the acclimation process. Higher superoxide dismutase and catalase isoenzyme levels and activity were noted 4 days following the termination of the UV-B treatment. Increased alternative respiration capacity (AP) and a contribution of this energy-dissipating respiratory pathway of up to 45% of the total respiration rate were noted in treated thalli, but not in isolated algal cells. These data demonstrate the UV-B effect on the Peltigera aphthosa respiratory metabolism to be higher due to reactions of the mycobiont than those of the photobiont. We suggest that the activation of the energy-dissipating AP in mycobiont mitochondria may be associated with the synthesis of protective pigments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lichens are symbiotic associations between fungi (mycobionts) and certain groups of unicellular green algae and/or cyanobacteria (photobionts). This symbiotic association has been essential for the colonisation of terrestrial habitats. The symbiotic nature and poikilohydric properties of lichens provide them with strong resistance to negative environmental influences (Kranner et al. 2005).
UV-B radiation (280–320 nm) is an omnipresent stress factor for living organisms, especially for photoautotrophs, which depend on solar radiation. UV-B rays can penetrate cells and damage DNA as well as other important biomolecules through the induction of oxidative stress (Frohnmeyer and Staiger 2003).
Lichens are capable of withstanding the exposure to UV-B and even UV-C (λ < 290 nm) radiation for extended periods in the air-dried state (Sánchez et al. 2014). However, in wet thalli, UV-B suppresses growth rate and induces programmed cell death (Ünal and Uyanikgi̇l 2011; Chowdhury et al. 2017). This raises the question of how lichens are able to maintain viability and what adaptive mechanisms are induced in lichens under the exposure to physiological doses of UV-B radiation. Lichens from forest habitats are of particular interest in this regard. While lichens in open habitats can be insusceptible to extended exposure to increased solar radiation levels, the shade-adapted lichens exhibit low levels of resistance to intense irradiation (Solhaug et al. 2003; Beckett et al. 2021). Therefore, the anthropogenic disturbance of forest habitats can cause oxidative stress as a result of increased UV-B exposure. Peltigera aphthosa is a convenient species for studying the issues posed. This lichen can be found on moss, soil, and plant debris in sites with shaded and wet conditions (Thomson 1984) and is a typical representative species of the boreal lichen biota. Even a modest high light level is extremely stressful to P. aphthosa (Kershaw and Macfarlane 1980).
It is a well-known fact that higher plants’ response to UV-B radiation stress is implemented through efficient damage repair systems. For example, they accumulate UV-screening phenolic substances (Caldwell et al. 2007; Jenkins 2009, 2014; Garmash et al. 2020). UV-B receptor (UVR8) was first discovered in higher plant cells (Kliebenstein et al. 2002; Rizzini et al. 2011); however, it has now been established that UVR8 evolved for the first time in green algae and is present in all representatives of the Viridiplantae group (Fernández et al. 2016). In addition, fungi also have photoreceptors that can perceive different regions of the light spectrum and induce specific responses (Fuller et al. 2015). It has been shown that photoreceptors of the white-collar complex can perceive the UV region of light in fungal hyphae and induce specific protective reactions. Reception of UV in lichens has not yet been studied; however, the presence of UVR8 in free-living green algal cells and specific photoreceptors in non-lichenized fungi indicates that UV-B radiation is perceived and can cause specific responses in lichen photo- and mycobiont cells. It is known that lichens also produce protective compounds that act as UV screens. The synthesis of these multifunctional, mainly aromatic compounds occurs in the upper cortex of thalli, and they either absorb or reflect excessive visible light and UV radiation, thereby shielding the underlying layers of cells (Nguyen et al. 2013). Further, UV-B causes the synthesis of lichen metabolites such as parietin and melanins in a number of lichen species (Solhaug et al. 2003; Nybakken et al. 2004; Solhaug and Gauslaa 2012; Mafole et al. 2019b). The maximal absorption levels of parietin are detected in the UV-B and blue regions of the light spectrum. It has been demonstrated that parietin in lichen thalli is primarily responsible for the absorption of high-energy blue light photons as well as the protection of the algal layer from excess light and photoinhibition (Gauslaa and Ustvedt 2003; Solhaug and Gauslaa 2012). Melanins have a maximum absorption in the UV region. In addition to the ability to screen lichen thalli cells from harmful UV-B radiation, melanins take part in protecting lichens from the adverse impacts of a range of environmental factors (temperature stress, desiccation, heavy metal exposure), as well as from pathogenic infections and herbivores (Mafole et al. 2019b). The reflectance spectra could be used as a sensitive, efficient, and non-destructive tool to fulfil quantitative assessment of the synthesis of melanin-like pigments that accompany the browning process (Chivkunova et al. 2001).
The respiration of photoautotrophs oxidises the products of photosynthesis, releasing energy represented by ATP and forming reducing equivalents, metabolites, and intermediates. These products are necessary for cellular biosynthesis and the maintenance of cellular structures. It is known that alongside with the cytochrome respiratory pathway (CP), which is associated with ATP synthesis, the mitochondrial electron transport chain (ETC) of plants, fungi, algae, and some other organisms also have an alternative oxidase (AOX) and an alternative (energy-dissipating) pathway (AP) of respiration (McDonald and Vanlerberghe 2006). With regard to plants, the respiratory rate and electron distribution into the two respiratory pathways are determined by environmental conditions (Lambers and Ribas-Carbo 2005). The induction of the AP in plants is seen to be one of the key physiological attributes of stress (van Dongen et al. 2011). It has been determined that AP affects stress tolerance in different plant species (Umbach et al. 2009; Kornfeld et al. 2013; Del-Saz et al. 2018). Little has been known about the activity of the respiration pathways in lichens until recently (Shelyakin et al. 2018; Shelyakin et al. 2020, 2021a, b).
Thus, in the present work, our aim was to study the effect of UV-B radiation on pro-/antioxidant and respiratory metabolism in Peltigera aphthosa thalli as well as isolated photobiont cells.
Materials and methods
Lichens
Peltigera aphthosa (L.) Willd. is a foliose lichen occurring in circumpolar regions. It grows in the Arctic, boreal, and temperate zones (Thomson 1984). P. aphthosa’s main phycobiont is the green algae of the genus Pseudococcomyxa. Cyanoprokaryotes from the genus Nostoc develop in the cephalodia on the thallus surface.
P. aphthosa thalli were obtained from a pine forest with a mixture of spruce and deciduous trees (in the taiga zone of the Komi Republic, Russia), particularly from shaded and moist places, in July 2019 and 2020. The maximal photosynthetic photon flux density (PPFD) under the canopy did not exceed 300 μmol quanta m−2 s−1, and the intensity of UV (A + B) radiation was 1.5 W m−2 (20 times lower than in an open space). PPFD was measured using an LI-1400 data logger equipped with light sensor (LI-COR, USA), and UV (A + B) radiation was measured with a UV radiometer (TKA-PKM 12, Russia). Substrate residues were removed from the thalli and the latter were transported to the laboratory. The desiccated thalli were stored in the dark at the temperature of 4 °C.
Experiment design
All thalli were hydrated and preconditioned at a PPFD of 120 μmol quanta m−2 s−1 (10/14 h photoperiod) under fluorescent lamps (Philips TL-D Aquarelle) for 3 days at 25 °C. Thereafter, the thalli were treated with UV by means of lamps (LER 40, Russia) for 2 h day−1 over 10 days. The maximum emission spectrum of the lamps reached 315 nm, and the UV-B radiation intensity amounted to 2 W m−2. Consequently, the lichen thalli exposed to approximately 14 kJ day−1 of UV-B. The dose was environmentally realistic, as it coordinates with the daily dose of UV-B radiation reaching open soil surfaces in this region on a sunny day in summer. The treated thalli were analysed immediately after exposure to UV-B on the first, third, and tenth days. The total doses of UV-B radiation for these days were 14, 43, and 144 kJ, respectively. The aftereffects of UV-B radiation were evaluated 4 days following the end of UV treatment. The control thalli were not exposed to UV. Functional parameters of the control samples were measured on the first, third, and tenth days, and biochemical parameters were determined on the first and tenth days of the experiment. The results of one-way analysis of variance (ANOVA; Duncan's test) showed that the duration of the experiment did not have a statistically significant effect on the studied parameters of the control lichens (Online Resource 1). Therefore, we combined the data obtained for the control samples on different days of the experiment and presented them as one average value.
The reflectance spectra of the lichen upper cortex were recorded in a range of 400–800 nm using a spectroradiometer (FieldSpec HandHeld 2, Analytical Spectral Devices Inc., Boulder, CO, USA) equipped with a focusing lens. The hydrated thalli were lightened by the external halogen lamp angled at 60° from the nadir direction. The reflectance spectra were collected at a 60° angle from the nadir direction and documented against barium sulphate as a standard with a spectral resolution of 3 nm. The browning reflectance index (BRI) and chlorophyll normalised difference vegetation index (ChlNDI) were obtained as: BRI = (1/R550 − 1/R700)/R750 (Chivkunova et al. 2001) and ChlNDI = (R750 − R705)/(R750 + R705) (Gitelson and Merzlyak 1994), where R550, R700, R750, and R705 are the reflectance at 550, 700, 705, and 750 nm, respectively.
The colour change (darkening) of the lichen upper cortical layer was visualised on cross sections of thalli under an Amplival microscope (Carl Zeiss, Jena, Germany).
Isolation of lichen photobionts
The algal cells were isolated from P. aphthosa thalli according to the method described by Kotlova (2000). The lichens were washed with distilled water. The fragments of thalli were homogenised in 0.1 M Na-phosphate buffer (pH 7.2). The homogenate was filtered through nylon 4–6 times. The filtrate was centrifuged in a T23 centrifuge with a bucket rotor (Janetzki, Czech Republic) at 3000g for 15 min. The precipitate was resuspended in 1 ml of phosphate buffer. The suspension was then centrifuged at 1500g for 10 min in a gradient of sucrose solutions with mass fractions of 65, 50, and 30%, prepared using phosphate buffer.
A clear green fraction enriched with photobiont cells was selected at the boundary of 30 and 50% sucrose solutions. The algal suspension was washed from sucrose by centrifugation in phosphate buffer at 1000g for 15 min. The precipitate was then resuspended in a small volume of phosphate buffer and used to measure physiological parameters. The purity of the algal suspension was evaluated using an Amplival light microscope (Carl Zeiss Jena, Germany). Microscopic observation showed the resulting suspension to contain mainly intact algal cells and an insignificant amount of broken fungal hyphae fragments with, however, no lichen fragments (Online Resource 2). Algal cells had all the features characteristic of the Pseudococcomyxa genus. To detect the dry mass of photobiont cells, an aliquot of the sample was taken three times, dried lypophilically in FreeZone® Dry Systems (Labconco, USA), and then weighed.
The fluorescence of photosystem II chlorophyll a (PSII Chla) in control, UV-B-treated thalli, and isolated photobiont cells was estimated using a PAM-2100 fluorometer (Walz, Germany). The leaf-clip holder 2030-B (Walz, Germany) was applied to obtain chlorophyll fluorescence of the thalli. For the isolated photobiont cells, 200 µl of suspension was placed in a thin-walled polypropylene cap, which was fixed in a holder with a leaf clip. The minimum (F0) and maximal (Fm) fluorescence yields were determined after 30–40 min for thalli and after 5 min of dark adaptation for isolated photobiont cells. The steady-state (Ft), background (F0′), and peak (Fm′) fluorescence values were measured in the samples that have been placed under actinic light for 10–15 min at an intensity of 150 μmol quanta m−2 s−1. The potential (Fv/Fm) and effective (ΦPSII) quantum yields of PSII were calculated with the help of the following equations (Jensen 2002): Fv/Fm = (Fm − F0)/Fm′ and ΦPSII = (Fm′ − Ft)/Fm′.
The rate of CO2 uptake (Pn) in control and treated thalli was measured at 20 °C using the IR gas analyser Li-7000 (Li-Cor, USA) at PPFD 150 μmol quanta m−2 s−1.
The respiration rate was obtained through estimation of the O2 uptake via polarography by means of an Oxytherm System Clark electrode (Hansatech Instruments, Pentney, UK). The data were exhibited as nmol O2 (g dry weight min)−1. The marginal regions of lichen thalli were cut into 2–3 mm2 strips. Samples(15–20 mg) were put into a 4 ml reaction vessel with 1.5 ml 50 mM HEPES buffer (pH 7.2) (Helicon, Moscow, Russia) and were regularly mixed throughout the measurements. The respiration rate of the lichen photobiont was measured based on the O2 uptake recorded for 200 µl of the cell suspension added to a reaction vessel containing 1.5 ml 0.1 M phosphate buffer (pH 7.2). All respiration measurements were performed at 20 °C.
The capacities of CP and AP were obtained with the help of specific inhibitors (Bahr and Bonner 1973). The optimal concentrations of inhibitors for the isolated photobiont cells and thalli were determined in preliminary experiments as per the method described by Møller et al. (1988). The AOX activity in the thalli and photobiont cells was inhibited with 6 and 1 mM benzhydroxamic acid (BHAM) (Lancaster, UK), respectively. 2 mM KCN (Sigma Aldrich, St. Louis, MO, USA) were used to inhibit the activity of cytochrome oxidase (COX). The inhibitors were added following the measurement of the total O2 uptake rate. The duration of the measurement cycle of each sample was 30 min. In preliminary experiments showed that the total O2 uptake rate by samples demonstrated stability over the whole period. The rates of each respiratory pathway were estimated in absolute (nmol O2) and relative (% of total respiration) values.
The rate of O2 uptake was calculated as follows: Vt = Valt + Vcyt + Vres, where Vt is total respiration, Valt is alternative respiration reduced by an AOX inhibitor, Vcyt is cyanide-sensitive (cytochrome) respiration, and Vres is residual respiration documented in the presence of CP and AP inhibitors.
Valt was estimated on the basis of the O2 consumption rate under the exposure to CP inhibitor KCN. Vcyt was calculated based on the rate of O2 consumption in the presence of the AOX pathway inhibitor, BHAM. Vres was obtained as the rate of oxygen consumption following the admixture of both the AOX and COX inhibitors.
Lipid peroxidation activity in lichen thalli was assessed based on the concentration of thiobarbituric acid-reactive substances (TBARS) (Heath and Packer 1968). The concentration of H2O2 was determined with the use of the xylenol orange assay (Bellincampi et al. 2000).
Activity of antioxidant enzymes
Superoxide dismutase (SOD) activity was measured based on the capacity to suppress the photochemical reduction of nitroblue tetrazolium (NBT) (Beauchamp and Fridovich 1971). Catalase (CAT) activity was estimated based on the amount of decomposed H2O2 per unit time (Aebi 1984). The soluble protein content was obtained through the Bradford (1976) method, with bovine serum albumin used as a standard. All absorbance measurements were performed using a UV-1700 spectrophotometer (Shimazu, Japan).
The SOD and CAT isoenzyme composition was assessed using native electrophoresis in 12.5% polyacrylamide gel. Samples of lichen thalli (0.5 g fresh mass) were homogenised in liquid nitrogen by adding of an ice-cold extraction buffer (pH 8.0) containing 100 mM Tricine, 3 mM MgSO4, 1 mM DTT, 3 mM EGTA, 0.5 g 100 ml−1 polyvinylpyrrolidone, and 20% glycerol. A protease inhibitor was added to the buffer. The homogenate was centrifuged for 12 min at 10,000g and a temperature of 4 °C. Electrophoresis was performed as described in Libik et al. (2005), without the addition of sodium dodecyl sulphate. Protein (20 µg) was added to each gel pocket.
To determine the activity of SOD isoforms, the gel was incubated in a 0.5 M phosphate buffer (pH 7.8), containing 10 mM Na-EDTA, 28 mM N,N,N′,N′-tetramethylethylenediamine, 22 mM riboflavin, and 0.25 mM NBT for 30 min in complete darkness at room temperature. The gel was then kept under the exposure to the light until light stripes emerged on a purple background. Inhibitory analysis was used to identify SOD isoforms (Beauchamp and Fridovich 1971; Miszalski et al. 1998). To inhibit Cu/Zn-SOD and Fe-SOD, 5 mM H2O2 was added to the staining buffer. The selective inhibition of Cu/Zn-SOD occurred during the incubation of gels in a buffer with 3 mM KCN. A new gel with separated proteins was obtained for each inhibitory analysis.
CAT isoform activity was detected on the gel using the method of Woodbury et al. (1971), with modifications (Pezzoni et al. 2018). The gel was held in a 4 mM H2O2 solution for 10 min at room temperature, rinsed with distilled water twice to remove excess H2O2 and stained with 1 g 100 ml−1 solution of ferric chloride and potassium ferricyanide (1:1 proportion) for 4–5 min. The gels were washed with distilled water as soon as they began to turn blue-green.
Gels were scanned using a ChemiDoc XRS. Signal intensities of the bands were calculated by densitometry by means of Quantity One® 1-D Analysis Software, Version 4.6.9 (Bio-Rad, USA).
Statistical analysis
Data analysis was carried out with Statistica 10.0 software (StatSoft Inc., Tulsa, OK, USA). The results are presented as the mean ± standard error. The Shapiro–Wilk test provided evidence for the normal distribution. Means were verified by means of one-way ANOVA and Duncan's test. A value of p ≤ 0.05 was considered statistically significant.
Results
Reflection spectra
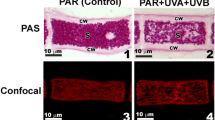
No colour changes were observed in control thalli of P. aphthosa (Fig. 1a). The fungal hyphae of the cortical and medullary layers remained colourless. The algal layer had a bright green colour. UV-B treatment induced browning of the thalli upper cortex and medullary layer (Fig. 1b). Reflection spectra of UV-B-treated thalli showed a decrease in the ChlNDI value by 20–25% and an increase in the BRI value by 50% (Table 1).
Photosynthetic activity
UV-B treatment displayed no considerable effect on the photosynthetic activity of lichens (Table 2). The values of the maximum quantum yield of PSII in the control and treated thalli were similar and averaged 0.7 rel. units. 13% lower Fv/Fm was noted four days after the termination of UV-B treatment. The values of the effective quantum yield of PSII measured at PPFD 150 μmol quanta m−2 s−1 in the control and treated thalli were similar and averaged 0.5 rel. units. A non-significant decrease in ΦPSII was noted 4 days after the end of UV-B treatment. UV-B had no effect on the rate of CO2 uptake. Pn in both control and treated thalli was about 1 µmol CO2 m−2 s−1.
Photobiont cells isolated from control and treated thalli were functionally active. However, they had lower fluorescence parameters compared to intact thalli. The values of the Fv/Fm and ΦPSII in the isolated algal cells were on average 13–15% lower than in thalli. The UV-B treatment of thalli demonstrated no considerable effect on the photochemical activity of isolated photobiont cells.
Lipid peroxidation and antioxidant enzyme activity
UV-B treatment increased the level of lipid peroxidation products in P. aphthosa thalli (Table 3). The content of TBARS, which are lipid peroxidation products, such as free malondialdehyde (MDA), increased as total doses of UV-B rose. The TBARS content was 19% higher in treated than in control lichens after the first exposure to UV-B radiation. On day 10, the content of TBARS was 42% higher and continued to increase for 4 days after the termination of UV-B treatment.
The content of H2O2 in P. aphthosa thalli ranged from 30 to 50 µmol g−1 dry mass (DM). After 3 days, UV-B treatment increased the H2O2 concentration of thalli by 20% compared with the control. By 10 day, when the total dose of UV-B amounted to 144 kJ, the content of H2O2 in thalli decreased and was 25% lower than that of the control. The concentration of H2O2 in the treated thalli did not deviate from the control at four days after UV-B treatment termination.
No significant changes in SOD and CAT activity in P. aphthosa occurred during the 10 days of UV-B treatment (Table 4). However, more intense activity of these antioxidant enzymes was noted 4 days after the UV-B treatment had been completed. SOD activity increased by 18%, and CAT activity increased more than four times in the UV-treated thalli compared to the control. We found four to six active SOD isoforms in control and UV-exposed P. aphthosa thalli (Fig. 2a). The addition of KCN (an inhibitor of the Cu/Zn-SOD isoform) to the gels did not lead to a decrease in the activity of the identified isoforms. After exposure to H2O2 (an inhibitor of the Fe- and Cu/Zn-SOD isoforms), the activity of more labile SOD isoforms was suppressed (Online Resource 3). Thus, only Mn-SOD and Fe-SOD isoforms were active in P. aphthosa thalli. In control thalli, two active Mn- and two Fe-SOD isoenzymes were identified. The number of active Fe-SOD isoforms increased to four after 3 days of UV-B treatment and remained stable thereafter.
Effect of UV-B radiation on the superoxide dismutase (a, b) and catalase (c, d) isoenzymes activity in Peltigera aphthosa thalli. Electropherograms of the different antioxidant isoenzymes are on the left and their relative activity are on the right. 1 and 2—Mn- and Fe-containing SOD’s isoenzymes and two CAT's isoenzymes, respectively. Different isoenzymes relative activity was calculated as average bands density minus gels background. Data are shown as the mean ± SE (n = 3–4 for each parameters). Different letters at each column indicate significant differences between control and UV-B treated thalli as well as the effect of UV-B treatment duration (ANOVA, Duncan’s test, p ≤ 0.05). C—control thalli were not treated with UV-B; the arrows indicate the days of the beginning and end of daily exposure to UV-B, measurements were made on days 1, 3 and 10 of treatment and on day 4 after the end of UV-B treatment
The total relative activity of Fe-SOD isoforms increased 4 days after the termination of UV-B treatment, and it was 1.6 times higher than in the control lichens (Fig. 2b). UV-B treatment had no effect on the levels and relative activity of Mn-SOD.
One isoform of CAT was identified in the control and treated thalli (Fig. 2c). UV-B radiation did show no substantial effect on the relative activity of this isoform. However, we noted the appearance of the second active CAT isoform 4 days upon termination of UV-B treatment, following which the total relative activity of the enzyme isoforms increased three times (Fig. 2d).
Respiration of intact thalli and photobiont cells
The total O2 uptake rate of P. aphthosa thalli was on average 950 nmol g DM min−1 (Fig. 3a, b). The contribution of CP and AP to the total respiration rate of control thalli was 60% and 25%, respectively. UV-B treatment had a significant effect on the total O2 consumption rate, activities, and ratio of respiratory pathways. Total rate of O2 uptake and CP capacity (Vcyt) decreased 1.3 and 1.5 times, respectively, while AP capacity (Valt) increased 1.5 times after first exposure of the thalli to UV-B. The total respiration rate increased by 25% after three days of UV-B treatment, when the total dose of UV-B reached 43 kJ. The Vcyt activity did not change significantly, and its proportion was about 40%. Valt activity increased 1.5 times, and its proportion was 45% after 3 days of UV-B treatment. Then respiration decreased again, and total O2 uptake rate was 25% lower in comparison to the control on the tenth day of treatment. The activity of Vcyt was more than two times lower, and Valt was 1.5 times higher compared to control at this time. The contribution of respiratory pathways in total respiration rate remained the same as on day 3 of UV-B treatment. After the end of UV-B treatment total O2 uptake increased, and an activation of the Vcyt respiratory pathway in thalli occurred. The proportion of CP respiration increased from 40 to 55%, and AP respiration decreased from 45 to 30% in treated thalli on day 4 upon termination of UV-B treatment.
Effect of UV-B radiation on total respiration rate, respiratory pathway rates and the relative contribution of each respiratory pathway to total O2 uptake in Peltigera aphthosa (a, b) and photobiont cells extracted from the thalli (c, d). The total respiration rate (open circle) and each respiratory pathway: cytochrome respiration (filled circle), alternative respiration (filled square), and residual respiration (filled triangle). The contributions of the cytochrome (black) and alternative pathways (grey) as well as residual respiration (white) to total respiration. Data are presented as the mean ± SE (n = 6–25 for each parameter). Different letters at each data point and column indicate significant differences between control and treated thalli as well as the effect of UV-B treatment duration (ANOVA, Duncan’s test, p ≤ 0.05). C—control thalli were not treated with UV-B; the arrows indicate the days of the beginning and end of daily exposure to UV-B, measurements were made on days 1, 3 and 10 of treatment and on day 4 after the end of UV-B treatment
There was no substantial impact of UV-B on the residual respiration rate in treated thalli. The proportion of residual respiration not suppressed by inhibitors of mitochondrial oxidases varied from 10 to 15% of the total respiration rate.
The total O2 uptake rate in the photobiont cells, isolated from the control and UV-B-treated thalli, did not significantly differ from that of intact lichens (Fig. 3c, d). The activity of the individual respiration pathways changed similarly to that of the total respiration rate. Vcyt was 1.7 times higher than Valt. The contributions of CP and AP were 55 and 30% of the total respiration rate, respectively. Residual respiration accounted for 12–15% of the total O2 consumption rate.
Discussion
UV-B treatment of plants has been found to decrease the light-saturated photosynthetic rate and maximum quantum yield of PSII (Yang et al. 2007). It has also been suggested that reduction in CO2 assimilation resulting from UV-B exposure can induce the production of excessive reactive oxygen species (ROS) and oxidative damage in plants. Plants’ response to UV-B radiation stress occurs through upregulating antioxidant defence systems and the activity of damage repair processes (Jansen et al. 1998; Hideg and Strid 2017). Lichens can withstand high doses of UV (Brandt et al. 2015). For example, dry thalli of lichens restored their functional and photosynthetic activity even after 10 days in outer space (Raggio et al. 2011). UV-C irradiation can strongly suppress the photochemical activity of wet thalli (Sánchez et al. 2014), although activity recovered 3 days after exposure. Therefore, we did not expect to observe prominent negative outcomes of physiological UV-B doses on the photochemical activity of P. aphthosa collected from a shaded habitat. Furthermore, we identified a range of likely tolerance mechanisms induced by UV-B stress in thalli. Some of them, such as the accumulation of melanins and the mobilization of an energy-inefficient alternative respiratory pathway, are related to the lichen mycobiont adaptation to UV-B exposure.
UV-B treatment induced browning of the upper cortex and the formation of brown areas in the medullary layer of the thalli, apparently due to melanisation (Fig. 1a, b). The value of BRI increased by more than 45% (Table 1). It is likely that the change in BRI value indicates accumulation of the melanin-like pigments in the lichen thalli. The accumulation of melanin pigments in lichens following treatment with UV radiation and high insolation has been observed in a number of studies (Gauslaa and Solhaug 2001; Solhaug et al. 2003; Solhaug and Gauslaa 2012; Matee et al. 2016; Mafole et al. 2017). The results of the studies clearly exhibit that the main trigger of melanin accumulation in lichens is UV-B exposure. Various lichens, including P. aphthosa, are capable of accumulating melanin pigments (Mafole et al. 2019a). Melanised P. aphthosa thalli were found in natural conditions in open, well-lit areas. Some lichen species, such as Lobaria pulmonaria, are characterised by a cortical type of melanisation (McEvoy et al. 2007). We noted the browning of hyphae of the upper cortex layer as well as in the medullar layer of UV-B-treated P. aphthosa thalli. Our data may indicate that the UV-induced accumulation of melanins not only protects cells of the algal layer, but also prevents UV penetration into cells of the mycobiont (Gauslaa et al. 2017; Mafole et al. 2019b).
We also found that the ChlNDI in treated thalli fell by 25% after 10 days of being exposed to UV-B. A linear relationship between changes in ChlNDI values and chlorophyll content was shown for the leaves of higher plants (Gitelson and Merzlyak 1994). In addition, we noted a slight but significant decrease in Fv/Fm, as well as an non-significant decrease in ΦPSII and Pn in the thalli 4 days after ending the UV-B treatment (Table 2). These data indicate a possible negative aftereffect of UV-B irradiation on the lichen photobiont. However, it is unclear what causes these changes. Grounds are available to prove that ecologically relevant levels of UV-B can suppress the growth of lichen thalli (Chowdhury et al. 2017). It was also shown that a seasonal increase in the level of insolation and the UV dose can lower the quantity of photobiont cells in the thalli (Tretiach et al. 2013). We found that UV-B caused an increase in the BRI index, which could result in a decrease in the amount of photosynthetically active radiation (PAR), reaching the algal layer. A decrease in the rate of CO2 uptake was shown in melanised thalli of L. pulmonaria compared with pale samples (Mafole et al. 2017). A decrease in photosynthetic activity and growth rate of thalli may be the “cost” of lichen melanisation to save against the negative consequences of UV-B (Mafole et al. 2017, 2019b). These facts indicate the multiplicity of UV-B effects. At the same time the other authors have not noted a decrease in the maximal PSII quantum yield in melanised thalli under the influence of high light or UV radiation (Chowdhury et al. 2017; Gauslaa et al. 2017; Mafole et al. 2017). Therefore, the direct negative aftereffect of UV-B on photobiont functional state cannot be denied. More detailed studies of the pigment complex and chlorophyll fluorescence parameters in P. aphthosa thalli are necessary.
An increase in lipid peroxidation activity in the UV-B-treated thalli of P. aphthosa (Table 3) indicated that UV increases ROS production and the resulting oxidative stress. The content of TBARS was 1.5 higher on day 10 and continued to increase for 4 days following the end of UV-B treatment, indicating that physiological doses of UV-B can produce lasting effects on lichens. On the other hand, the accumulation of lipid peroxidation products may reflect the processes of acclimation. For instance, it is known that MDA activates regulatory genes that are involved in protecting cells under oxidative stress (Morales and Munné-Bosch 2019).
H2O2 is a slightly reactive and relatively lasting molecule with a half-life of 1 ms. As H2O2 is the only ROS that can diffuse through some membrane aquaporins, known as peroxyporins, and over longer distances within cells, it has aroused exceptional interest as a signal molecule participating in regulating specific biological processes and triggering tolerance against a variety of environmental stresses (Smirnoff and Arnaud 2019). We found that UV-B treatment caused transient changes in H2O2 content. A 20% increase in H2O2 concentration on day 3 and a 1.7-fold decrease on day 10 of UV-B exposure were noted. Further, the H2O2 content in the treated thalli did not differ from the control 4 days after UV-B treatment termination.
H2O2 content is controlled by two antioxidant enzymes, namely SOD and CAT. SOD catalyses the dismutation of the superoxide anion radical (O2·−) to H2O2, and CAT catalyses the decomposition of two H2O2 molecules into water and oxygen. Peroxidases are also important in regulating the concentration of hydrogen peroxide in the cells of living organisms, including most lichens. However, it was shown that in representatives of the Peltigera genus peroxidase activity was very low or absent (Beckett et al. 2013).
We did not observe any significant changes in SOD and CAT activity in P. aphthosa during 10 d of UV-B treatment. However, increases in their activities were noted four days after the cessation of UV-B treatment. As a result, the content of H2O2 in treated thalli did not differ from the control. Available information displays that the reaction of lichen antioxidant enzymes to different stress may vary. For example, during desiccation SOD and CAT activities drop and remain very low during the first hours of rehydration (at the time when stress-induced ROS formation is highest) (Mayaba and Beckett 2001). However, an increase in the activity of antioxidant enzymes in lichens was noted in conditions of industrial pollution (Weissman et al. 2006; Golovko et al. 2018).
Four active isoforms of SOD (two Mn- and two Fe-containing SOD) and one isoform of CAT (Fig. 2a, c) were identified in the control thalli of P. aphthosa. Activity of the Cu/Zn-SOD isoform, which is typical for plants, was absent. UV-B treatment activated Fe-SOD isoenzymes and induced the expression of new isoforms after exposure. An increase in the overall relative activity and the appearance of another CAT isoform were noted on the fourth day after halting UV-B exposure. Six active SOD isoforms (three Fe-, two Mn-, and one Cu/Zn-SOD) were identified in Pseudevernia furfuracea and Hypogymnia physodes (Schlee et al. 1995). The lichens Xanthoria parietina and Ramalina duriaei had three Mn- and Fe-containing isoforms of SOD (Silberstein et al. 1996). Five Mn-, four Fe-, and one Cu/Zn-SOD isoforms were identified in Ramalina lacera (Weissman et al. 2005). Apparently, the number and composition of SOD isoforms in lichen thalli depend on the species and habitat conditions. Limited data are available on the importance of enzymatic antioxidants for lichen ROS scavenging under strong light exposure (Beckett et al. 2021), especially under UV-induced stress. It is clear that individual SOD and CAT isoforms take part in the regulation of the pro-/antioxidant state of P. aphthosa during repair after UV-B treatment. The increase in activity and the appearance of new SOD and CAT isoforms after the end of UV-B exposure may indicate participation of these antioxidant enzymes in protection against subsequent UV irradiation. Further investigation into the roles of other antioxidants in protecting lichens from direct UV-B exposure is therefore warranted.
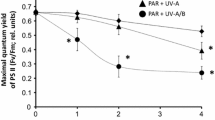
Respiration is a global process and the foundation of life in all cells. In the present study, UV-B treatment had significant effects on total O2 consumption rates, individual respiration pathway activities, and their ratios. The ratio of CP to AP was halved after the first exposure of thalli to UV-B (Fig. 4a). This mainly resulted from a decrease in the CP activity rather than an increase in AP activity. AP activation continued until the third day of treatment, which led to a further decrease in the Vcyt/Valt ratio. Upon termination of UV-B treatment a partial restoration of respiratory parameters occurred.
Effect of UV-B radiation on cytochrome (Vcyt) and alternative (Valt) respiratory pathway capacities ratio in Peltigera aphthosa thalli (a) and photobiont cells extracted from the thalli (b). Data are shown as the mean ± SE (n = 6–25 for each parameter). Different letters at each data point and column indicate significant differences between control and treated thalli and the effect of the duration of UV-B treatment (ANOVA, Duncan’s test, p < 0.05). C—control thalli were not treated with UV-B; the arrows indicate the days of the beginning and end of daily exposure to UV-B, measurements were made on days 1, 3, and 10 of treatment and on day 4 after the end of UV-B exposure
We previously reported that adverse conditions, including elevated UV, can increase in alternative respiration capacity and change the ratios of the respiration pathways in boreal and Antarctic lichens (Shelyakin et al. 2018; Shelyakin et al. 2020, 2021a, b). AP, which provides weaker ATP synthesis, diverges from the main respiratory pathway at ubiquinone. The overreduction of ubiquinone is a major source of mitochondrial ROS, which are detrimental to living cells. As a terminal oxidase, AOX can accept electrons from ubiquinone and reduce molecular oxygen to water, preventing overreduction of the ubiquinone pool and lowering ROS formation. The role of AP as an element of the ROS-scavenging system in lichens was considered previously (Beckett et al. 2008). It has also been shown that H2O2 induces the activity and accumulation of AOX in higher plant leaves (Zhao et al. 2007). We found an increase in H2O2 content and alternative respiration activity in thalli on the third day of UV-B treatment.
In the lichen association, the mass of the mycobiont was at least 10 times the mass of the photobiont. Therefore, it is believed that most respiration in intact thalli derives from the mycobiont (Palmqvist 2000). To identify the possible impact of UV radiation on the P. aphthosa photobiont, we obtained a suspension of well-preserved algal cells from control and UV-B-treated thalli. UV-B treatment had a minor effect on the total O2 uptake rate of photobiont cells (Fig. 3c, d). The activity of respiration pathways changed similarly to the total respiration rate, and the Vcyt/Valt ratio was near 2 (Fig. 4b). Taken together, the data obtained demonstrate that the effect of UV-B on the respiration of P. aphthosa was more due to reactions of the mycobiont than the photobiont.
The most pronounced reactions of the mycobiont to UV-B exposure were the browning of the upper cortex resulting in an increase in BRI and the appearance of dark spots in the medulla. These changes were indicative of the synthesis of protective pigments. Respiration provides the energy required for cellular biosynthesis, reducing equivalents, and metabolites. Acetyl CoA is the precursor for the synthesis of secondary lichen compounds via the mevalonic acid and acetyl-polymalonyl pathways (Elix and Stocker-Wörgötter 2008), and also acts as a precursor for melanin synthesis via the dihydroxynaphthalene pathway, found in most fungi (Eisenman and Casadevall 2012). Acetyl CoA is formed during the decarboxylation of pyruvate as well as the oxidation of fatty acids and proteins. Further, it participates in the tricarboxylic acid cycle. The engagement of AOX can promote TCA cycle turnover without overreduction and risk of damage to the mitochondrial ETC. The interconnection of AOX with secondary metabolism has been established in plants (Zhang et al. 2012; Sircar et al. 2012). However, it is worth mentioning that in the L. pulmonaria thalli l-dopa melanin is accumulated in high light conditions, which is synthesized in a different way from l-dopa or tyrosine (Matee et al. 2016). Nevertheless, further research is needed to identify the secondary metabolites that accumulate in lichens under the influence of UV-B and mechanisms underlying the connection between alternative respiration and secondary biosynthesis in lichens, to know whether activation of the AOX is essential for the synthesis of secondary compounds.
Conclusion
In this study, we studied the response of the foliose lichen Peltigera aphthosa to an environmentally realistic UV-B radiation dose and revealed that UV-B treatment caused changes in respiration and pro-/antioxidant metabolism. Treatment also induced increased lipid peroxidation rate and transient changes in the H2O2 content. Higher total activities of SOD and CAT, caused by the upregulation of Fe-SOD and CAT isoenzymes, occurred 4 days following the end of UV-B treatment. An increase in alternative respiration capacity and its input to the total respiration rate were noted in treated thalli, but not in algal cells. Our data show that the effect of UV-B on the respiration of P. aphthosa was more due to the reactions of the mycobiont than the those of the photobiont. We suggest that the activation of the energy-dissipating AP in mycobiont mitochondria may be associated with the synthesis of protective pigments. Respiration plays a vital role in the growth and adaptation of lichens to environmental conditions determines, and as a result there is a need to continue research into the effects of UV-B on this vital process at all levels. Further studies will benefit from -omics type studies to study the tolerance mechanisms, and how this stress may affect the interactions between the symbionts.
Author contribution statement
All authors facilitated the study conception and design. Material preparation, data collection, and analysis were implemented by MS, IZ, RM, and ES. The first draft of the manuscript was prepared by MS and TG. All authors provided their comments on previous versions of the manuscript. All authors read and approved the final manuscript.
References
Aebi H (1984) Catalase in vitro. Methods in enzymology. Academic Press, Cambridge, pp 121–126
Bahr JT, Bonner WD (1973) Cyanide-insensitive respiration. I. The steady states of skunk cabbage spadix and bean hypocotyl mitochondria. J Biol Chem 248:3441–3445
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Beckett RP, Kranner I, Minibayeva FV (2008) Stress physiology and the symbiosis. In: Nash TH (ed) Lichen biology, 2nd edn. Cambridge University Press, Cambridge, pp 134–151
Beckett RP, Minibayeva FV, Liers C (2013) On the occurrence of peroxidase and laccase activity in lichens. Lichenologist 45:277–283. https://doi.org/10.1017/S0024282912000771
Beckett RP, Minibayeva F, Solhaug KA, Roach T (2021) Photoprotection in lichens: adaptations of photobionts to high light. Lichenologist 53:21–33. https://doi.org/10.1017/S0024282920000535
Bellincampi D, Dipierro N, Salvi G et al (2000) Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol 122:1379–1386
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brandt A, de Vera J-P, Onofri S, Ott S (2015) Viability of the lichen Xanthoria elegans and its symbionts after 18 months of space exposure and simulated Mars conditions on the ISS. Int J Astrobiol 14:411–425. https://doi.org/10.1017/S1473550414000214
Caldwell MM, Bornman JF, Ballaré CL et al (2007) Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem Photobiol Sci 6:252–266. https://doi.org/10.1039/B700019G
Chivkunova O, Solovchenko A, Sokolova S et al (2001) Reflectance spectral features and detection of superficial scald–induced browning in storing apple fruit. J Russ Phytopathol Soc 2:73–77
Chowdhury DP, Solhaug KA, Gauslaa Y (2017) Ultraviolet radiation reduces lichen growth rates. Symbiosis 73:27–34. https://doi.org/10.1007/s13199-016-0468-x
Del-Saz NF, Ribas-Carbo M, McDonald AE et al (2018) An in vivo perspective of the role(s) of the alternative oxidase pathway. Trends Plant Sci 23:206–219. https://doi.org/10.1016/j.tplants.2017.11.006
Eisenman HC, Casadevall A (2012) Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol 93:931–940. https://doi.org/10.1007/s00253-011-3777-2
Elix JA, Stocker-Wörgötter E (2008) Biochemistry and secondary metabolites. In: Nash TH (ed) Lichen biology, 2nd edn. Cambridge University Press, Cambridge, pp 104–133
Fernández MB, Tossi V, Lamattina L, Cassia R (2016) A comprehensive phylogeny reveals functional conservation of the UV-B photoreceptor UVR8 from green algae to higher plants. Front Plant Sci 7:1–6. https://doi.org/10.3389/fpls.2016.01698
Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-mediated responses in plants. Balancing Damage and Protection Plant Physiol 133:1420–1428. https://doi.org/10.1104/pp.103.030049
Garmash EV, Velegzhaninov IO, Ermolina KV et al (2020) Altered levels of AOX1a expression result in changes in metabolic pathways in Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation. Plant Sci 291:110332. https://doi.org/10.1016/j.plantsci.2019.110332
Gauslaa Y, Solhaug KA (2001) Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia 126:462–471. https://doi.org/10.1007/s004420000541
Gauslaa Y, Ustvedt EM (2003) Is parietin a UV-B or a blue-light screening pigment in the lichen Xanthoria parietina? Photochem Photobiol Sci 2:424–432. https://doi.org/10.1039/B212532C
Gauslaa Y, Alam MdA, Lucas P-L et al (2017) Fungal tissue per se is stronger as a UV-B screen than secondary fungal extrolites in Lobaria pulmonaria. Fungal Ecol 26:109–113. https://doi.org/10.1016/j.funeco.2017.01.005
Gitelson A, Merzlyak MN (1994) Quantitative estimation of chlorophyll-a using reflectance spectra: experiments with autumn chestnut and maple leaves. J Photochem Photobiol B 22:247–252. https://doi.org/10.1016/1011-1344(93)06963-4
Golovko TK, Shelyakin MA, Zakhozhiy IG et al (2018) The response of lichens to the environmental pollution under the bauxite mining in the taiga zone. Theor Appl Ecol. https://doi.org/10.25750/1995-4301-2018-2-044/2-053/1 (in Russian)
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hideg É, Strid Å (2017) The effects of UV-B on the biochemistry and metabolism of plants. In: Jordan BR (ed) UV-B radiation and plant life: molecular biology to ecology. CABI, Wallingford, pp 90–110
Jansen MAK, Gaba V, Greenberg BM (1998) Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci 3:131–135. https://doi.org/10.1016/S1360-1385(98)01215-1
Jenkins GI (2009) Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol 60:407–431. https://doi.org/10.1146/annurev.arplant.59.032607.092953
Jenkins GI (2014) The UV-B photoreceptor UVR8: from structure to physiology. Plant Cell 26:21–37. https://doi.org/10.1105/tpc.113.119446
Jensen M (2002) Measurement of chlorophyll fluorescence in lichens. In: Kranner IC, Beckett RP, Varma AK (eds) Protocols in lichenology: culturing, biochemistry, ecophysiology and use in biomonitoring. Springer, Berlin, pp 135–151
Kershaw KA, Macfarlane JD (1980) Physiological-environmental interactions in lichens X. Light as an ecological factor. New Phytol 84:687–702. https://doi.org/10.1111/j.1469-8137.1980.tb04781.x
Kliebenstein DJ, Lim JE, Landry LG, Last RL (2002) Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to Human regulator of chromatin condensation 1. Plant Physiol 130:234–243. https://doi.org/10.1104/pp.005041
Kornfeld A, Heskel M, Atkin OK et al (2013) Respiratory flexibility and efficiency are affected by simulated global change in Arctic plants. New Phytol 197:1161–1172. https://doi.org/10.1111/nph.12083
Kotlova ER (2000) Possible role of carotenoids in green alga and cyanobacteria adaptation to the lichen symbiosis condition. Botanicheskii Zhurnal 85:103–114 (in Russian)
Kranner I, Cram WJ, Zorn M et al (2005) Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. PNAS 102:3141–3146. https://doi.org/10.1073/pnas.0407716102
Lambers H, Ribas-Carbo M (eds) (2005) Plant respiration: from cell to ecosystem. Springer, Dordrecht
Libik M, Konieczny R, Surowka E, Miszalski Z (2005) Superoxide dismutase activity in organs of Mesembryanthemum crystallinum L. at different stages of CAM development. Acta Biol Crac Ser Bot 47:199–204
Mafole TC, Chiang C, Solhaug KA, Beckett RP (2017) Melanisation in the old forest lichen Lobaria pulmonaria reduces the efficiency of photosynthesis. Fungal Ecol 29:103–110. https://doi.org/10.1016/j.funeco.2017.07.004
Mafole TC, Solhaug KA, Minibayeva FV, Beckett RP (2019a) Tolerance to photoinhibition within lichen species is higher in melanised thalli. Photosyntica 57:96–102. https://doi.org/10.32615/ps.2019.008
Mafole TC, Solhaug KA, Minibayeva FV, Beckett RP (2019b) Occurrence and possible roles of melanic pigments in lichenized ascomycetes. Fungal Biol Rev 33:159–165. https://doi.org/10.1016/j.fbr.2018.10.002
Matee LP, Beckett RP, Solhaug KA, Minibayeva FV (2016) Characterization and role of tyrosinases in the lichen Lobaria pulmonaria (L.) Hoffm. Lichenologist 48:311–322. https://doi.org/10.1017/S0024282916000293
Mayaba N, Beckett R (2001) The effect of desiccation on the activities of antioxidant enzymes in lichens from habitats of contrasting water status. Symbiosis 31:113–121
McDonald AE, Vanlerberghe GC (2006) Origins, evolutionary history, and taxonomic distribution of alternative oxidase and plastoquinol terminal oxidase. Comp Biochem Physiol Part D Genomics Proteomics 1:357–364. https://doi.org/10.1016/j.cbd.2006.08.001
McEvoy M, Gauslaa Y, Solhaug KA (2007) Changes in pools of depsidones and melanins, and their function, during growth and acclimation under contrasting natural light in the lichen Lobaria pulmonaria. New Phytol 175:271–282. https://doi.org/10.1111/j.1469-8137.2007.02096.x
Miszalski Z, Slesak I, Niewiadomska E et al (1998) Subcellular localization and stress responses of superoxide dismutase isoforms from leaves in the C3-CAM intermediate halophyte Mesembryanthemum crystallinum L. Plant Cell Environ 21:169–179. https://doi.org/10.1046/j.1365-3040.1998.00266.x
Møller IM, Bérczi A, van der Plas LHW, Lambers H (1988) Measurement of the activity and capacity of the alternative pathway in intact plant tissues: identification of problems and possible solutions. Physiol Plant 72:642–649. https://doi.org/10.1111/j.1399-3054.1988.tb09176.x
Morales M, Munné-Bosch S (2019) Malondialdehyde: facts and artifacts. Plant Physiol 180:1246–1250. https://doi.org/10.1104/pp.19.00405
Nguyen K-H, Chollet-Krugler M, Gouault N, Tomasi S (2013) UV-protectant metabolites from lichens and their symbiotic partners. Nat Prod Rep 30:1490–1508. https://doi.org/10.1039/C3NP70064J
Nybakken L, Solhaug KA, Bilger W, Gauslaa Y (2004) The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats. Oecologia 140:211–216. https://doi.org/10.1007/s00442-004-1583-6
Palmqvist K (2000) Carbon economy in lichens. New Phytol 148:11–36
Pezzoni M, Pizarro RA, Costa CS (2018) Detection of catalase activity by polyacrylamide gel electrophoresis (PAGE) in cell extracts from Pseudomonas aeruginosa. Bio Protoc. https://doi.org/10.21769/BioProtoc.2869
Raggio J, Pintado A, Ascaso C et al (2011) Whole lichen thalli survive exposure to space conditions: results of lithopanspermia experiment with Aspicilia fruticulosa. Astrobiology 11:281–292. https://doi.org/10.1089/ast.2010.0588
Rizzini L, Favory J-J, Cloix C et al (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332:103–106. https://doi.org/10.1126/science.1200660
Sánchez FJ, Meeßen J, Ruiz MDC et al (2014) UV-C tolerance of symbiotic Trebouxia sp. in the space-tested lichen species Rhizocarpon geographicum and Circinaria gyrosa: role of the hydration state and cortex/screening substances. Int J Astrobiol 13:1–18. https://doi.org/10.1017/S147355041300027X
Schlee D, Kandzia R, Tintemann H, Türk R (1995) Activity of superoxide dismutase and malondialdehyde content in lichens along an altitude profile. Phyton 35:233–242
Shelyakin MA, Zakhozhiy IG, Golovko TK (2018) Changes of total respiration and respiratory pathways ratio in lichens adaptation to UV-B radiation. Izvestia Ufimskogo Nauchnogo Tsentra RAN 3:100–104. https://doi.org/10.31040/2222-8349-2018-5-3-100-104 (in Russian)
Shelyakin M, Zakhozhiy I, Golovko T (2020) The effect of temperature on Antarctic lichen cytochrome and alternative respiratory pathway rates. Polar Biol 43:2003–2010. https://doi.org/10.1007/s00300-020-02758-4
Shelyakin MA, Silina EV, Golovko TK (2021a) The effect of UV-B radiation on the antioxidant system in the Peltigera aphthosa and Peltigera rufescens lichens. J Sib Fed Biol 14:328–338. https://doi.org/10.17516/1997-1389-0359
Shelyakin MA, Zakhozhiy IG, Dalke IV et al (2021b) Photosynthetic and respiratory capacity of foliose lichen Lobaria pulmonaria throughout the annual cycle. Russ J Plant Physiol 68:1048–1058. https://doi.org/10.1134/S1021443721060182
Silberstein L, Siegel BZ, Siegel SM et al (1996) Comparative studies on Xanthoria Parietina, a pollution resistant lichen, and Ramalina Duriaei, a sensitive species. I. Effects of air pollution on physiological processes. Lichenologist 28:355–365. https://doi.org/10.1017/S0024282996000461
Sircar D, Cardoso HG, Mukherjee C et al (2012) Alternative oxidase (AOX) and phenolic metabolism in methyl jasmonate-treated hairy root cultures of Daucus carota L. J Plant Physiol 169:657–663. https://doi.org/10.1016/j.jplph.2011.11.019
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221:1197–1214. https://doi.org/10.1111/nph.15488
Solhaug KA, Gauslaa Y (2012) Secondary lichen compounds as protection against excess solar radiation and herbivores. In: Lüttge U, Beyschlag W, Büdel B, Francis D (eds) Progress in botany. Springer, Berlin, pp 283–304
Solhaug KA, Gauslaa Y, Nybakken L, Bilger W (2003) UV-induction of sun-screening pigments in lichens. New Phytol 158:91–100. https://doi.org/10.1046/j.1469-8137.2003.00708.x
Thomson J (1984) American arctic lichens. I. The macrolichens. Columbia University Press, New York
Tretiach M, Bertuzzi S, Candotto Carniel F, Virgilio D (2013) Seasonal acclimation in the epiphytic lichen Parmelia sulcata is influenced by change in photobiont population density. Oecologia 173:649–663. https://doi.org/10.1007/s00442-013-2654-3
Umbach AL, Lacey EP, Richter SJ (2009) Temperature-sensitive alternative oxidase protein content and its relationship to floral reflectance in natural Plantago lanceolata populations. New Phytol 181:662–671. https://doi.org/10.1111/j.1469-8137.2008.02683.x
Ünal D, Uyanikgi̇L Y, (2011) UV-B induces cell death in the lichen Physcia semipinnata (J.F.Gmel). Turk J Biol 35:137–144. https://doi.org/10.3906/biy-0901-5
van Dongen JT, Gupta KJ, Ramírez-Aguilar SJ et al (2011) Regulation of respiration in plants: a role for alternative metabolic pathways. J Plant Physiol 168:1434–1443. https://doi.org/10.1016/j.jplph.2010.11.004
Weissman L, Garty J, Hochman A (2005) Characterization of enzymatic antioxidants in the lichen Ramalina lacera and their response to rehydration. Appl Environ Microbiol 71:6508–6514. https://doi.org/10.1128/AEM.71.11.6508-6514.2005
Weissman L, Fraiberg M, Shine L et al (2006) Responses of antioxidants in the lichen Ramalina lacera may serve as an early-warning bioindicator system for the detection of air pollution stress. FEMS Microbiol Ecol 58:41–53. https://doi.org/10.1111/j.1574-6941.2006.00138.x
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305. https://doi.org/10.1016/0003-2697(71)90375-7
Yang S-H, Wang L-J, Li S-H et al (2007) The effects of UV-B radiation on photosynthesis in relation to Photosystem II photochemistry, thermal dissipation and antioxidant defenses in winter wheat (Triticum aestivum L.) seedlings at different growth temperatures. Funct Plant Biol 34:907–917
Zhang L, Oh Y, Li H et al (2012) Alternative oxidase in resistance to biotic stresses: Nicotiana attenuata AOX contributes to resistance to a pathogen and a piercing-sucking insect but not Manduca sexta larvae. Plant Physiol 160:1453–1467. https://doi.org/10.1104/pp.112.200865
Zhao M-G, Liu Y-G, Zhang L-X et al (2007) Effects of enhanced UV-B radiation on the activity and expression of alternative oxidase in red kidney bean leaves. J Integr Plant Biol 49:1320–1326. https://doi.org/10.1111/j.1744-7909.2007.00484.x
Fuller KK, Loros JJ, Dunlap JC (2015) Fungal photobiology: visible light as a signal for stress, space and time. Curr Genet 61:275–288. https://doi.org/10.1007/s00294-014-0451-0
Acknowledgements
This work was carried out as part of the “Photosynthesis, respiration and bioenergetics of plants and phototrophic organisms (physiological, biochemical, molecular, genetic and ecological aspects)” project (No. 122040600021-4). We thank Irina Novakovskaya (Department of Flora and Vegetation of the North of the Institute of Biology of the Komi Science Center of the Ural Branch of the Russian Academy of Sciences) for her help in identifying photobiont cells isolated from thalli. Furthermore, we would like to express our gratitude to Editage (www.editage.com) for competent English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by K. Jan Strzałka.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shelyakin, M., Malyshev, R., Silina, E. et al. UV-B induced changes in respiration and antioxidant enzyme activity in the foliose lichen Peltigera aphthosa (L.) Willd.. Acta Physiol Plant 44, 116 (2022). https://doi.org/10.1007/s11738-022-03457-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03457-9