Abstract
Tree peony is considered as an important ornamental and medicinal plant in China, and 60Co gamma radiation breeding can be potentially used to improve its quality. In this study, we have analyzed the biochemical characteristics and transcriptomic effects induced by different doses of 60Co gamma radiation on the grafted seedlings to explore the possible mutagenic effects of 60Co gamma radiation on the tree peony. The higher radiation doses were found to be harmful to the seedlings, and LD50 (50% of the lethal value) was 40.30 Gy. 60Co gamma radiation treatment obviously affected the physiological and biochemical states of tree peony. The activity of antioxidant enzymes including superoxide dismutase, peroxidase, and catalase gradually increased after radiation exposure, with maximal at 40 Gy. The total soluble protein content gradually decreased, while the content of proline and malondialdehyde was significantly increased. A total of 77,699 unigenes were identified, of which 399, 1447 and 1928 identified were differentially expressed genes (DEGs) in H0 (control) vs H1 (10 Gy), H0 vs H5 (50 Gy), H1 vs H5, respectively. GO and KEGG results showed that the DEGs were closely related to phenylalanine metabolism, lipid metabolism, carbohydrate metabolism pathways, plant hormone signal transduction and secondary biosynthesis metabolism. A total of 768 different expressed transcription factors were identified, which were mainly distributed in MYB, WRKY, NAC and other families related to plant stress regulation which was up-regulated upon exposure to 50 Gy. This is the first transcriptome-based study to demonstrate significant variations in the expression of the various genes in response to 60Co gamma radiation. The findings of this study could be useful in the development of a mutation breeding program for tree peony.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been found that exposure to gamma radiation can result in an increase of plant variability and has been successfully employed for cultivar breeding of ornamental plants, among which 60Co gamma rays are one of the most commonly used as radiation sources. It has been successfully used in the breeding of Trifolium repens (Song et al. 2009), Malus spectabilis (Liu et al. 2010), Dianthus caryophyllus (Deshmukh and Malode 2018), and Chrysanthemum × morifolium (Wu et al. 2020). Besides phenotypic variations, excellent characteristics such as salt tolerance, drought resistance, higher yield and disease resistance have been found in the plants exposed to radiation (Beyaz 2019; Dawar et al. 2010; Sarker et al. 2014). Gamma rays could be used to increase the genetic variability in various plants, and the mutagenic effects on the physiological, cellular as well as molecular levels could be elicited in the plants by gamma radiation. The effects of mutagens on plants have been found to be species specific (Dawar et al. 2010).
Low doses of gamma radiation can stimulate growth which is known as ‘hormesis’, and induce different metabolic and physiological variations (Parchin et al. 2019; Mariadoss et al. 2020). However, higher doses of gamma radiation have been reported to have deleterious effects on plants such as growth inhibition, the loss of pigmentation, chromosomal aberrations, increased production of reactive oxygen species (ROS), decrease in protein content, and deletions of DNA nucleotide sequence which may cause reading-frame shifts, thereby leading to the production of inactive or faulty protein products (Brown 2013; Sheppard and Evenden 1986). Thus high radiation doses often results in infertility and individual death in some plants. The determination of optimal radiation dose and the investigation of response to gamma radiation could be useful in the development of a mutation breeding program.
Tree peony (Paeonia suffruticosa Andrews) is a well-known ornamental and medicinal plant which is native to China (Zhou et al. 2014). It has been cultivated for more than 2000 years, and formed cultivars rich in flower color and flower type. 60Co radiation mutagenic breeding has been applied to improve tree peony cultivars and mainly used seeds of P. ostti ‘Fengdanbai’ used as materials (Su et al. 2012; Yan et al. 2017). For woody plants, the branches in vitro and seedlings have been used to make the breeding more effective. The potential effects of radiation on the seedlings of Ginkgo biloba (Liang et al. 2001), Morus alba (Ramesh et al. 2012) and Rhododendron decorum (Zhao et al. 2012) were examined. However, there are still no reports on the seedlings of 60Co gamma radiation in tree peony, and information is limited on the possible changes that can be induced at the biochemical and molecular levels, as well as the genetic damage and the mutagenesis mechanisms in the tree peony after exposure to radiation.
In the present study, we exposed grafted seedlings of P. suffruticosa ‘Okan’ to 60Co gamma radiation. The annual grafted seedlings were subjected to different radiation doses to obtain the optimal radiation dose for tree peony. Thereafter biochemical indexes were determined and transcriptome sequencing was performed on the irradiated materials to explore the potential mutagenic effects of 60Co gamma radiation on the grafted seedlings of tree peony. These findings enriched our understanding of the use of gamma radiation for breeding purposes in the tree peony.
Materials and methods
Materials and treatment
The annual grafted seedlings of ‘Okan’ with uniform size were treated with six different doses of gamma radiation (10, 20, 30, 40, 50 and 60 Gy), with a dose rate of 7 Gy min−1. Non-irradiated grafted seedlings were used as the control. Gamma radiation was given using 60Co gamma source at the Radiation Center for the Institute of Agro-Food Sciences and Technology, Shandong Academy of Agricultural Science (Jinan, China). Twenty annual grafted seedlings per treatment group were exposed to the gamma rays according to assigned doses. After exposure, the irradiated and non-irradiated grafted seedlings were planted under the same cultivation management conditions on the experimental farm of Qingdao Agricultural University (Qingdao, China). The radiated grafted seedlings were dormant in autumn and winter after the planting but sprouted in March during the following spring. The survival rate was investigated in April to calculate LD50 value (50% of the lethal dose) based on probit analysis (Surakshitha et al. 2017). The mature leaves of each treatment group were collected for freezing in liquid nitrogen and stored at – 80 ℃ for the measurement of biochemical index and extraction of total RNA. The samples exposed to 0 Gy, 10 Gy, 50 Gy (H0, H1, H5) were collected for RNA-Seq to obtain transcriptome data.
Measurement of biochemical index and antioxidant enzyme activity
Biochemical indexes and antioxidant enzyme activity assays were conducted according to the previously reported methods (Liu et al. 2017). The activities of SOD, POD and CAT were measured by nitro blue tetrazolium (NBT) photochemical reduction, guaiacol colorimetric and ultraviolet absorption, respectively. The contents of MDA, proline and soluble protein were measured by thiobarbituric acid method, ninhydrin method and coomassie brilliant blue staining method, respectively.
RNA isolation extraction, database construction and illumina sequencing
Total RNA was extracted using the RNA prep Pure Plant Kit (Tiangen Biotech, CO. Ltd, Beijing, China) as per the manufacturer’s instructions. The purified mRNA was fragmented, and the fragment was used as the template for reverse transcription into cDNA. Following the end repair, and then were ligated with adapters, and the resulting cDNA was enriched by PCR amplification, poly(A) was added, and then sequenced on the Illumina HiSeq™ 2000 platform (I) in Majorbio (Shanghai, China).
De novo assembly
The raw reads were filtered by removing adapter sequences, low-quality sequences containing ambiguous bases ‘N’, and low-quality reads with more than 10% Q < 20 bases. Then clean reads were used for de novo assembly using Trinity software. The filtered and assembled unigenes sequences were compared by BLAST (E value < 10E−5) to the NCBI non-redundant protein database (NR), Swiss-Prot protein database, Protein family (Pfam), Gene Ontology (GO), Clusters of Orthologous Groups of proteins (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG). GO annotations of the unigenes were obtained by the BLAST2GO program (Conesa et al. 2005). The unigenes sequences were compared with the COG database by Diamond software to predict and classify their possible functions (Wu et al. 2014). The KEGG pathways were assigned to the unigenes sequences using online software (Nakatsuka et al. 2007).
Differential expression analysis and annotation of DEGs
The FPKM (Fragments Per Kilobase of transcriptome per Million mapped reads) values were used to compare the gene expression differences between the various sample groups, and DEGSeq was employed to analyze the DEGs (differentially expressed genes). |log2 (fold change)| ≥ 1 and false discovery rate (FDR) ≤ 0.001 were used as the screening criteria for DEGs (Ji et al. 2016), p adjust is the adjusted p value as determined by BH (Benjamini Hochberg) multiple times. The functional classifications of DEGs in six databases were performed using MapMan software (Thimm et al. 2010), Goatools were used for GO enrichment analysis of DEGs, when the corrected p value < 0.05, DEGs were defined as significant enrichment (Klopfenstein et al. 2018). KOBAS was used for KEGG pathway enrichment analysis of DEGs, and DEGs were considered to be significantly enriched when the adjusted p value was < 0.05 (Xie et al. 2011).
qRT-PCR verification
The DEGs screened according to the analysis results were also verified by qRT-PCR. The template was the single-stranded cDNA synthesized from 1 μl total RNA using the FastQuant RT Kit (Vazyme, China). The fluorescent dye was SYBR Green PCR Master Mix (Vazyme, China). The primers were designed with Primer 5.0 software (Supplementary file 1). The reactions were carried out in a 20 μl volume containing 2 μl cDNA sample, 10 μl SYBR qPCR Master Mix, and 0.4 μl each primer under the following conditions: 95 °C for 30 s, followed by 40 cycles of 95 °C for 60 s and 60 °C for 30 s. Three replicates were set, and the tublin gene was used as an internal control. The relative expression levels were calculated using the 2−ΔΔCt method (Hao et al. 2016).
Statistical analysis
The LD50 values for gamma radiation was measured by probit analysis. LD50 was estimated through the simple linear regression model by fitting the straight line equation y = a + bx, where y is the response variable (survival rate), x is the independent variable (radiation dose), while a and b present the slope and constant, respectively (Surakshitha et al. 2017). The different obtained values in this study were expressed as the mean ± SE (standard error) and tested with a one-way analysis of variance (ANOVA) with Duncan’s multiple comparisons test using the SPSS software package for windows (version 19.0).
Results
Determination of the LD50
The survival rate was found to gradually decrease as the radiation dose was increased, and no surviving plants were found when the radiation dose applied was 60 Gy. When the seedlings were subjected to radiation dose of only 10 Gy, they appeared to be well sprouted, and the survival rate was the same to the non-treated ones (100%). LD50 of ‘Okan’ was determined by probit analysis. The survival rate (y) and radiation dose (x) were negatively correlated (y = − 1.88 x + 126, R2 = 0.94). Where y = 50, x = 40.30, that was, the LD50 of 60Co gamma radiated ‘Okan’ was identified at 40.30 Gy (Fig. 1).
Physiological and biochemical responses to radiation
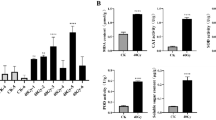
It was found that activities of SOD, POD and CAT increased after exposure to radiation, and exhibited a maximum value at 40 Gy. Thereafter, a gradual decline was noted and these alterations were significantly different from the control (p < 0.05). The MDA and proline levels significantly increased (p < 0.05) in the seedlings exposed to doses from 20 to 50 Gy, except for a radiation dose of 10 Gy. The highest MDA and proline concentration was observed after radiation of 50 Gy, and increased by 333% and 345% that of the controls. The soluble protein content decreased significantly (p < 0.05) as a radiation dose increased and with a minimum at 50 Gy (Fig. 2). These results indicated that radiation treatment markedly affected the biochemical state of the plants.
Effect of the gamma radiation dose on the activities of the antioxidant enzymes SOD, POD and CAT, and the contents of MDA, proline and soluble protein in leaves. SOD superoxide dismutase, POD peroxidase, CAT catalase, MDA malondialdehyde. Values are presented as the means ± standard error from three replicates. The bars represent standard error. Different letters above the columns represent statistically significant differences between mean values at p < 0.05 level
Sequencing and de novo assembly
Transcriptome of the leaves exposed to the radiation doses of 0, 10 and 50 Gy was obtained and a total of 59,125,012, 58,502,474, and 53,894,556 reads were generated, respectively. After filtering out low-quality data, 169,858,552 clean reads were finally obtained, and the percentages of Q20 as well as Q30 bases were both greater than 97.65% and 93.25% in each sample, respectively. The N50 value of unigenes was 1328 bp and the average percentage of GC content was approximately around 45%. The total number of unigenes assembled by trinity software was 77,629, and the total length as well as the mean length was 64,216,007 bp and 827.22 bp, respectively (Supplementary file 2). There were 39,593 (51%) of the unigenes between 201 and 500 bp, 18,065 (23%) were between 501 and 1000 bp, 13,067 (17%) were between 1001 and 2000 bp, and 6904 (8.9%) > 2000 bp in length (Supplementary file 3).
Functional annotation and classification
All unigenes obtained by transcriptome sequence were compared with six different databases to obtain the annotation information in each specific database. 31,051 (41.16%), 26,657 (35.33%), 26,136 (34.64%), 22,256 (29.50%), 21,648 (28.69%) and 13,285 (17.61%) unigenes were annotated to NR, GO, COG, Swiss-Prot, Pfam, and KEGG database, respectively (Supplementary file 4, 5).
The statistical analysis of the e-value distribution of NR annotation was run in the terms of species statistics, which demonstrated that 11.97%, 9.83% and 6.08% were annotated to Vitis vinifera, Nyssa sinensis and Vitis riparia, respectively (Fig. 3A). 47.37% unigenes were not found in the known species, which might be possibly specific to the plant after exposure to radiation.
The unigenes were divided into 50 functional subgroups and three major GO groups, “cellular components” (36.33%), “biological processes” (32.88%) and “molecular functions” (30.80%) (Fig. 3B). In the “cellular component” category, the assignments were mainly given to “cellular parts” and the “membrane parts”. In the “biological processes”, unigenes participating in the various “cellular processes” and “metabolic processes”. The binding and catalytic activities were the most dominant categories among the “molecular function” classification.
The metabolic pathway analysis conducted using the KEGG database revealed that 13,285 unigenes were related to 20 different KEGG pathways (Fig. 3C). The total number of unigenes was further divided into six separate categories. Most unigenes were associated with “metabolism” (4413, 49.97%), followed by those regulating “genetic information processing” (2,904, 32.88%), “environmental information processing” (556, 6.30%), “cellular processes” (552, 6.25%), organic systems (387, 4.38%), and human diseases (20, 0.23%).
Analysis of DEGs
Analysis of DEGs was performed between every two treatments (H0 vs H1, H0 vs H5, and H1 vs H5) based on FPKM with thresholds FDR) ≤ 0.001 and |log2 (fold change)| ≥ 1 (Fig. 4). In total, there were 1934 up-regulated and 794 down-regulated DEGs among the three comparison groups (Fig. 4A). A total of 399 DEGs were identified in H0 vs H1, among which 148 DEGs were up-regulated whereas 251 were down-regulated. There were 1447 DEGs (1027 up- and 420 down-regulated) between H0 and H5 libraries, and 1928 DEGs (1546 up- and 382 down-regulated) obtained from H1 vs H5 (Fig. 4B). It was found that with an increase in radiation dose, the number of up-regulated DEGs was also increased proportionately, and the number of up-regulated DEGs in H0 vs H5 was about 6.94 times that in H0 vs H1, thereby indicating that high dose radiation can promote the expression of large numbers of related genes in plants and the influence of 50 Gy dose radiation on the tree peony transcriptome was more pronounced as compared to 10 Gy dose radiation. There were 14 DEGs that displayed a significant up-regulation and down-regulation in three comparison groups, thus revealing that these genes may be involved in the response to 60Co gamma radiation (Fig. 4A).
Statistics of differentially expressed genes (DEGs) and Venn diagrams. H0, H1 and H5 indicate the plants under the radiation doses of 0 Gy, 10 Gy and 50 Gy, respectively. A Venn diagram of up-regulated and down-regulated DEGs. The |log2 (fold change)| ≥ 1 and false discovery rate (FDR) ≤ 0.001 were the threshold for determining significant DEGs. B Number of up-regulated and down-regulated DEGs in the pairwise group
GO enrichment analysis of the DEGs
To obtain a more precise understanding of the functions of various DEGs in gamma radiated leaves, Go term enrichment analysis was conducted (Fig. 5). The DEGs were annotated into three main categories of Go, that were, cellular components, biological processes and molecular functions, which were similar with that in All-unigenes. The up- and down-regulated transcripts were assessed based on these three distinct categories. The up-regulated DEGs were more abundant than the down-regulated for H0 vs H5, H1 vs H5, except for H0 vs H1. The DEGs in H1 vs H5 were most abundant, followed by H0 vs H5, H0 vs H1. For the “cellular components”, DEGs were found to relate primarily to the “cell part”, there were 121, 380 and 473 DEGs expressed in H0–H1, H0–H5 and H1–H5, respectively, whereas in the “membrane part”, there were 74, 362 and 419 DEGs, respectively. Moreover, within the “biological process” category, DEGs identified were mainly involved in the regulation of “cellular” and “metabolic processes”. There were 141, 310 and 490 DEGs expressed in H0–H1, H0–H5 and H1–H5 of “cellular process”, respectively. Additionally, 135, 285 and 430 DEGs were noted to be involved in “metabolic process”, respectively. In “molecular function” analysis, “catalytic activity” was the most abundant, 138, 525 and 668 DEGs expressed in H0–H1, H0–H5 and H1–H5, respectively. This was followed by “binding”, and 143, 444, 656 DEGs were expressed, respectively. The results indicated the potential existence of an exuberant metabolic activity in the transition period after exposure to 60Co gamma radiation.
KEGG pathway analysis of the DEGs
DEGs were also subjected to KEGG pathways analysis, to ascertain the specific biological pathways that various DEGs may be involved in (Supplementary file 6). H0 vs H5 and H1 vs H5 were divided into six DEG gene sets, while H0 vs H1 were five which was without “Human diseases”. “Metabolism” and “genetic information process” were the main processes among the DEG gene sets. For H0 vs H1, “translation in genetic information processing” was found to be associated with the most DEGs number (39), which were not rich in H0 vs H5 and H1 vs H5. While “carbohydrate metabolism” in the processing of metabolism exhibited the greatest DEGs number in H0 vs H5 (55) and H1 vs H5 (54). Furthermore, the KEGG enrichment analysis was performed to identify the functional features. As shown in Fig. 6, the “Ribosome”, “Oxidative phosphorylation” and “Glutathione metabolism” were over-represented among the DEGs of H0 vs H1, while H0 vs H5 had significant enrichments in “Phenylpropanoid biosynthesis”, “Monoterpenoid biosynthesis” and “Plant hormone signal transduction”, and H1 vs H5 were in “Phenylpropanoid biosynthesis”, “Glutathione metabolism” and “Monoterpenoid biosynthesis”. The results indicated that the seedlings displayed a significant response to the gamma radiation, the pathways of “Ribosome” and “Glutathione metabolism” were closely linked to lower radiation dose, while “Phenylpropanoid biosynthesis” and “Monoterpenoid biosynthesis” were closely linked to higher radiation dose.
KEGG enrichment analysis diagram of DEGs between H0 vs H1, H0 vs H5, and H1 vs H5 leaves, respectively. Q values correspond to p-values corrected for multiple hypothesis testing for KEGG analyses, with values from 0 to 1, with 0 indicating more significant enrichment. KEGG Kyoto Encyclopedia of Genes and Genomes
Transcription factor analysis
To understand the complex signaling pathway of tree peony upon exposure to gamma radiation, we performed transcription factor (TF) analysis on 77,629 unigenes that were assembled to study the alterations in the expression dynamics of TFs in tree peony. A total of 768 TFs belonging to 34 different TF families were identified (Fig. 7). MYB (v-myb avian myeloblastosis viral oncogene homolog) family (15.76%) accounted for the highest proportion, and mainly up-regulated genes (59.60%, 77.12% and 78.15% in H0 vs H1, H0 vs H5, H1 vs H5, respectively). This was followed by AP2/ERF (APETALA2/ethylene-responsive factor) (10.03%), C2C2 (7.81%), bHLH (Basic helix-loop-helix) (7.16%), FAR1 (6.64%), WRKY (5.86%) and NAC (NAM, ATAF1/2 and CUC) (5.73%) families. These families of TFs generally play an important regulatory roles in plant secondary metabolism, growth and development, biosynthesis, and response to stress (Khan et al. 2017). The up-regulated genes were more abundant than the down-regulated for H0 vs H5, H1 vs H5, except for H0 vs H1 of the TFs genes.
Gene expression validation by qRT-PCR
To verity the RNA-seq data, 16 DEGs were selected in the RNA sequence data were confirmed by the qRT-PCR analysis (Fig. 8). As a result, the DEGs that were upregulated included Unigene61701, Unigene343301, Unigene9251, Unigene452, Unigene24236, Unigene4843, Unigene48733, Unigene54881, and downregulated in Unigene9517, Unigene9514, Unigene2048, Unigene76103, Unigene91463, Unigene95104, Unigene93001, Unigene210431. These results were consistent with the RNA-seq results, thus confirming both the accuracy and validity of the RNA-seq analysis.
Discussion
Determination of optimal radiation dose of seedlings for tree peony
Low doses of radiation can accelerate the metabolic activity in plants, and may not cause any mutations or induce minor mutations, that may not be very effective (Nazir et al. 1998). While, high doses can make the plants susceptible to mutations, which may be fatal and also cause direct negative effects on the tissues (Ramesh et al. 2012). Therefore, selecting the optimal dose is essential to induce a higher mutation rate on target traits without affecting other ones (Surakshitha et al. 2017). The LD50 is generally considered to be the appropriate radiation dose, which can produce effective mutagenic effects. The study of 60Co radiation breeding on tree peony was primarily focused on seeds of the tree peony (P. ostti ‘Fengdanbai’), however, LD50 value was obtained in a previously published study. Shi et al. (2010) reported that the LD50 value was 4.97 Gy used dry seeds, and Yan et al. (2017) found that the LD50 value was18.94 Gy and 9.51 Gy for dry seeds and wet seeds, respectively. The germination rate of seeds could be affected by different sowing conditions and seed moisture to impact the LD50 value. The seed moisture can also affect the radiation sensitivity of the seeds. In this study, we tried to use the grafted seedlings as the radiation materials for the first time to the best of our knowledge. According to this study, the LD50 of grafted seedlings of tree peony (‘Okan’) was 40.30 Gy, which was significantly higher than the reported LD50 dose of seeds. The grafted seedlings of tree peony were found to be more resistant to the radiation as compared to the seeds.
Physiological and biochemical response of tree peony by radiation
Gamma radiation can induce physiological responses in a plant (Beyaz et al. 2016). One of the major effects of gamma radiation is increased production of ROS. To protect themselves against ROS, plant cells generally employ various antioxidant defense systems. SOD and POD are two important antioxidant enzymes, which can remove excessive ROS produced in the plants and repair the damage within the affected cells (Shah et al. 2001). Proline is a scavenger of ROS. It can stabilize the structure and function of different macromolecules such as DNA, proteins and membranes (Akshatha et al. 2013). CAT can catalyze the decomposition of H2O2 into H2O and O2, thus removing H2O2 to maintain the stability of the plant cell membrane (Alam et al. 2019). Exposure to gamma radiation can lead to increases in the activities of SOD and CAT (Beyaz 2019). MDA is the major mutagenic product generated by lipid peroxidation (Huang et al. 2018). In this study, the content of proline and MDA as well as the activities of SOD, POD and CAT were all found to be significantly increased in irradiated tree peony, which indicated that the radiation dose used in this study could lead to the membrane lipid peroxidation, damage the structures of the cell membranes, and thus the various cellular antioxidant defense mechanisms were activated to protect against the damage caused by the radiation. These results suggested that gamma radiation treatment could affect both the physiological and biochemical states of the plants.
Characterization of the tree peony transcriptome
Transcriptome sequencing technology (RNA-seq) has been widely used in biological research related to tree peony to study the low-temperature dormancy in the buds (Gai et al. 2012), anthocyanin biosynthesis in the petals and leaves (Zhang et al. 2014, 2015), fatty acids present in the seeds (Li et al. 2015), and effect of the drought resistance (Zhao et al. 2012). However, there are no existing reports describing the transcriptome sequencing study in 60Co gamma radiation breeding of the tree peony. Transcriptomic sequence comparative analysis on radiation-treated tree peony was performed to broaden our understanding of radiation breeding. Approximately 75,444 (97.18%) unigenes were found to match accurately to the known proteins in these databases, thereby suggesting that the non-annotated unigenes may be unique to peony after radiation. We also found that the cell part, membrane part, binding, catalytic activity, cellular process and metabolic process were the most represented parameters in GO database. The result was similar to the gene ontology analysis obtained in the study of petal development transcriptome in P. suffruticosa (Li et al. 2017) as well as Capsicum annuum (Lu et al. 2011). The metabolism and genetic-related information processing accounted for a large proportion of KEGG database, and similar results were obtained in transcriptome comparative studies of Chrysanthemum indicum (Han et al. 2018).
The response of biological processes caused by radiation exposure in tree peony
There were hundreds of DEGs that were found to be mainly involved in carbohydrate metabolism, secondary compound biosynthesis, plant hormone signal transduction and lipid metabolism which were similar to the enrichment pathways of tree peony under drought stress (Zhao et al. 2012). In carbohydrate metabolism, many DEGs that were closely associated with starch and sucrose metabolism were discovered in our study. Under stress conditions, sucrose may have a direct protective function, by providing easily accessible energy sources for the plants, and thus serving as an optimal osmotic regulator and for signaling purposes (Ducat et al. 2012).
Gamma radiation has been considered a rapid and reliable method for inducing variations in a plant’s metabolic as well as physiological conditions, and as a novel technique for the enhanced production of secondary metabolites (Parchin et al. 2019; Mariadoss et al. 2020). 60Co gamma radiation has been proved to be an effective inducer of oxidative stress, which can promote the production of several secondary metabolites in plants (Kapare et al. 2017). Radiation-induced stress could significantly increase linolenic acid levels and stimulate the activation of plant defense mechanisms (Zhang et al. 2017). More DEGs involved in metabolic processes were found in higher radiation dose than in lower dose in our study. In lipid metabolism, many DEGs associated with linoleic acid, alpha-linolenic acid metabolism, etc. were discovered, which indicated that exposure to the high dose radiation could induce the regulation of linolenic acid synthesis genes, stimulate the initiation of defense mechanisms and improve the overall resistance of the pants. Tree peony is an important woody oil crop rich in alpha-linolenic acid, and thus DEGs associated with alpha-linolenic acid metabolism obtained in this study might contribute to the quality improvement of oil peony. 60Co gamma radiation breeding could be used as a novel strategy for oil tree peony breeding. Hormones can initiate diverse response mechanisms in plant biotic and abiotic stress processes (Mendy et al. 2017). Some DEGs related to hormone signal transduction were found in the tree peony leaves exposure to high doses of radiation. The failure of some plants to grow normally after radiation may be due to the destruction of auxin in the hormone (Smith and Kersten 1942).
In addition, a total of 768 TFs were identified, belonging to 34 TF families, and most families were involved in the stress control process. It has been reported that TFs such as bZIP, MYB, NAC and WRKY could play an important regulatory role in plant stress response (Liu et al. 2018; López-Galiano et al. 2018). TFs also can modulate the plant response to adversity stress by affecting ROS levels (Kong et al. 2018), for example, the dominant inhibition of Arabidopsis thaliana TF MYB44 could cause oxidative damage and lead to the accumulation of ROS (Persak and Pitzschke 2014), and the TF NAC55 as well as NAC56 in Brassica campestris could induce the expression of the various anti-oxidant genes, reduce the accumulation of ROS, and thus improve abiotic stress tolerance (Chen et al. 2017).
Conclusions
Our study has shown that exposure to 60Co gamma radiation in the grafted seedlings could cause significant biochemical and transcriptome response. The higher radiation doses were found to be harmful to the tree peony, and LD50 was 40.30 Gy. There was a certain tolerance in tree peony seedlings to 60Co gamma radiation dose based on the results of biochemical indexes. The content of MDA and the activity of SOD, POD and CAT increased gradually after exposure to radiation, while the total soluble protein content decreased. To our knowledge, this is the first transcriptome-based study that demonstrates variations in the expression of various genes in response to 60Co gamma radiation. The DEGs number, Go enrichment, KEGG pathway and TF number in H0 vs H5, H1 vs H5 were more than in H0 vs H1, which showed distinct transcriptome response to gamma radiation. This study will be potentially helpful to understand the pleiotropic mutagenic effects of 60Co gamma radiation in tree peony to further accelerate germplasm innovation.
Author contribution statement
QH designed the study and revised the manuscript. PW carried out plants germination, leaves collection, qRT-PCR analysis and wrote the manuscript. DG attended the date collection, biochemical index measurement and data analyses. SS conducted the transcriptomes data analyses. ZW carried out the 60Co gamma radiation. KW revised the manuscript.
References
Akshatha CKR, Somashekarappa HM, Souframanien J (2013) Effffect of gamma irradiation on germination, growth, and biochemical parameters of Terminalia arjuna Roxb. Radiat Prot Environ 36:38–44
Alam MZ, McGee R, Hoque MA, Ahammed GJ, Carpenter-Boggs L (2019) Effect of Arbuscular mycorrhizal fungi, selenium and biochar on photosynthetic pigments and antioxidant enzyme activity under arsenic stress in Mung Bean (Vigna radiata). Front Physiol 10:193
Beyaz R (2019) Impact of gamma irradiation pretreatment on the growth of common vetch (Vicia sativa L.) seedlings grown under salt and drought stress. Int J Radiat Biol 96:257–266
Beyaz R, Sancak C, Yildiz CÇ, Kuşvuran SŞ, Yildiz M (2016) Physiological responses of the M-1 sainfoin (Onobrychis viciifolia Scop) plants to gamma radiation. Appl Radiat Isotopes 118:73–79
Brown N (2013) Mutagenesis: institution of plant breeding, genetics and genomics. University of Georgia, Athens
Chen QQ, Niu FF, Yan JL, Chen BS, Wu FF, Guo XH, Yang B, Jiang YQ (2017) Oilseed rape NAC56 transcription factor modulates reactive oxygen species accumulation and hypersensitive response-like cell death. Physiol Plant 160:209–221
Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676
Dawar S, Zaki MJ, Ikram N, Tariq M (2010) Gamma radiation (60Co) exposure and application of antagonists for the suppression of root rot diseases in Sunflower (Helianthus annus L.) and Mung Bean (Vigna radiata (L.) R. Wilczek). Our Nature 8:26–33
Deshmukh PD, Malode SN (2018) Effects of gamma radiation on seed germination, plant survival and growth characteristics in Dianthus caryophyllus var. Chabaud. J Glob Biosci 7:5403–5410
Ducat DC, Avelar-Rivas JA, Way JC, Silver PA (2012) Rerouting carbon flux to enhance photosynthetic productivity. Appl Environ Microbiol 78:2660–2668
Gai SP, Zhang YX, Mu P, Liu CY, Liu S, Dong L, Zheng GS (2012) Transcriptome analysis of tree peony during chilling requirement fulfillment: assembling, annotation and markers discovering. Gene 497:256–262
Han ZZ, Ma XY, Wei M, Zhao T, Zhan RT, Chen WW (2018) SSR marker development and intraspecific genetic divergence exploration of Chrysanthemum indicum based on transcriptome analysis. BMC Genom 19:291
Hao Q, Ren HX, Jin Z, Wang LS, Huang SC, Liu ZA, Gao ZM, Shu QY (2016) Overexpression of PSK1, a SKP1-like gene homologue, from Paeonia suffruticosa, confers salinity tolerance in Arabidopsis. Plant Cell Rep 36:151–162
Huang CP, Qin NN, Sun L, Yu MY, Hu WZ, Qi ZY (2018) Selenium improves physiological parameters and alleviates oxidative stress in strawberry seedlings under low-temperature stress. Int J Mol Sci 19:1913
Ji ZJ, Zeng YX, Liang Y, Qian Q, Yang CD (2016) Transcriptomic dissection of the rice-fusarium fujikuroi interaction by RNA-Seq. Euphytica 211:123–137
Kapare V, Satdive R, Fulzele DP, Malpathak N (2017) Impact of gamma irradiation induced variation in cell growth and phytoecdysteroid production in Sesuvium portulacastrum. J Plant Growth Regul 36:919–930
Khan ZH, Kumar B, Dhatterwal P, Mehrotra S, Mehrotra R (2017) Transcriptional regulatory network of cis-regulatory elements (Cres) and transcription factors (Tfs) In plants during abiotic stress. Int J Plant Biol Res 5:1064
Klopfenstein DV, Zhang L, Pedersen BS, Fidel R, Alex WV, Aurélien N, Mungall CJ, Yunes JM, Olga B, Mark W (2018) GOATOOLS: a python library for Gene Ontology analyses. Sci Rep 8:10872
Kong X, Tian H, Yu Q, Zhang F, Wang R, Gao S, Xu W, Liu J, Sha NE, Fu C (2018) PHB3 maintains root stem cell niche identity through ROS-responsive AP2/ERF transcription factors in arabidopsis. Cell Rep 22:1350–1363
Li SS, Wang LS, Shu QY, Wu J, Chen LG, Shao S, Yin DD (2015) Fatty acid composition of developing tree peony (Paeonia section Moutan DC.) seeds and transcriptome analysis during seed development. BMC Genom 16:208
Li Y, Lu JX, Chang YH, Tang WL, Yang QS (2017) Comparative analysis of tree peony petal development by transcriptome sequencing. Acta Physiol Plant 39:216
Liang H, Liu SH, Ou LY (2001) Preliminary study on radiation tolerance of Ginkgo biloba seedlings. Acta Agric Nucl Sin 15:219–223
Liu LQ, Liu JL, Zhang J, Geng H, Zhang YP, Shen HX, Gao XH, Yao YC (2010) Mutagenic effects on tissue culture plants of ornamental crabapple induced by 60Co-γ irradiation. Sci Agric Sin 43:4255–4264
Liu YJ, Yu XF, Feng YM, Zhang C, Wang C, Zeng J, Huang Z, Kang HY, Fan X, Sha LN, Zhang HQ, Zhou YH, Gao SP, Chen QB (2017) Physiological and transcriptome response to cadmium in cosmos (Cosmos bipinnatus Cav.) seedlings. Sci Rep 7:14691
Liu C, Sun QH, Zhao L, Li ZX, Zheng HP, Zhang JR (2018) Heterologous expression of the transcription factor EsNAC1 in arabidopsis enhances abiotic stress resistance and retards growth by regulating the expression of different target genes. Front Plant Sci 9:1495
López-Galiano MJ, González-Hernández AI, Crespo-Salvador O, Rausell C, Real MD, Escamilla M, Camañes G, García-Agustín P, González-Bosch C, García-Robles I (2018) Epigenetic regulation of the expression of WRKY75 transcription factor in response to biotic and abiotic stresses in Solanaceae plants. Plant Cell Rep 37:167–176
Lu FH, Yoon MY, Cho YI, Chung JW, Kim KT, Cho MC, Cheong SR, Park YJ (2011) Transcriptome analysis and SNP/SSR marker information of red pepper variety YCM334 and Taean. Sci Hortic 129:38–45
Mariadoss A, Satdive R, Fulzele DP, Ramamoorthy S, Zayed H, Younes S, Rajasekaran C (2020) Enhanced production of anthraquinones by gamma-irradiated cell cultures of Rubia cordifolia in a bioreactor. Ind Crops Prod 145:111987
Mendy B, Ombe MWW, Radakovic ZS, Holbein J, Siddique S (2017) Arabidopsis leucine-rich repeat receptor-like kinase NILR1 is required for induction of innate immunity to parasitic nema todes. PLoS Pathog 13:e1006284
Nakatsuka T, Abe Y, Kakizaki Y, Yamamura S, Nishihara M (2007) Production of red-flowered plants by genetic engineering of multiple flavonoid biosynthetic genes. Plant Cell Rep 26:1951–1959
Nazir MB, Mohamad O, Affida AA, Sakinah A (1998) Research highlights on the use of induced mutations for plant improvement in Malaysia. Malay Inst Nucl Technol Res (MINT), Bangi
Parchin RA, Ghomi AAN, Badi HN, Eskandari A, Navabpour S, Mehrafarin A (2019) Growth characteristics and phytochemical responses of Iranian fenugreek (Trigonella foenum-graecum L.) exposed to gamma irradiation. Ind Crops Prod 139:111593
Persak H, Pitzschke A (2014) Dominant repression by arabidopsis transcription factor MYB44 causes oxidative damage and hypersensitivity to abiotic stress. Int J Mol Sci 15:2517
Ramesh HL, Murthy Y, Munirajappa VN (2012) Effect of gamma radiation on morphological and growth parameters of Mulberry variety M5. Int J Sci Nat 3:447–452
Sarker S, Rahman MS, Islam MR, Hasna S, Islam MS (2014) Effect of gamma radiation on morpho-physiological characters of soybean. J Environ Sci Nat Resour 7:25–30
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Sheppard SC, Evenden WG (1986) Factors controlling the response of fifield crops to very low doses of gamma irradiation of the seed. Can J Plant Sci 66:431–441
Shi J, Dong PH, Zhang SL, Huo ZP, Yang JX (2010) Effect of 60Co-radiation on seed radiation dose of peony and herbaceous peony. J Henan Univ Sci Technol Nat Sci 3:72–74
Smith GF, Kersten H (1942) Auxin and calines in seedlings from x-rayed seeds. Am J Bot 29:785–819
Song IJ, Kang HG, Kang JY, Kim HD, Bae TW, Kang SY, Lim PO, Adachi T, Lee HY (2009) Breeding of four-leaf white clover (Trifolium repens L.) through 60Co gamma-ray irradiation. Plant Biotechnol Rep 3:191–197
Su MH, Zhao LY, Fang T (2012) Effect of 60Co-radiation on germination rate and seedling growth of peony seed. J Agron 3:18–20
Surakshitha NC, Soorianathasundaram K, Meenakshi ganesan N (2017) Determination of mutagenic sensitivity of hardwood cuttings of grapes ‘Red Globe’ and ‘Muscat’ (Vitis vinifera L.) to gamma rays. Sci Hortic 226:152–156
Thimm O, Blsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M (2010) Mapman: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37:914–939
Wu HB, Gong H, Liu P, He XL, Luo SB, Zheng XM, Zhang CY, He XM, Luo JN (2014) Large-scale development of EST-SSR markers in sponge gourd via transcriptome sequencing. Mol Breed 34:1903–1915
Wu JH, Zhang J, Lan F, Fan WF, Li W (2020) Morphological, cytological, and molecular variations induced by gamma rays in ground-grown chrysanthemum ‘Pinkling.’ Can J Plant Sci 100:68–77
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY, Wei L (2011) KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39:316–322
Yan YH, Zhu YW, Wei WQ, Xu YC (2017) Effects of different doses of 60Co-radiation on rooting, seedling emergence and seedling growth of Paeonia suffruticosa ‘Fengdan’ seeds. J Jiangsu Fores Sci Technol 44:17–21
Zhang C, Wang WN, Wang YJ, Gao SL, Du DN, Fu JX, Dong L (2014) Anthocyanin biosynthesis and accumulation in developing flowers of tree peony (Paeonia suffruticosa) ‘Luoyang Hong’. Postharvest Biol Technol 97:11–22
Zhang YZ, Cheng YW, Ya HY, Xu SZ, Han JM (2015) Transcriptome sequencing of purple petal spot region in tree peony reveals differentially expressed anthocyanin structural genes. Front Plant Sci 6:964
Zhang J, Kevin K, Bryan UJ, John E, Gregory MD (2017) Changes of leaf membrane fatty acid composition and saturation level of warm-season turfgrass during drought stress. Crop Sci 57:2843
Zhao YL, Li CC, Chen X (2012) Effect of 60Co-γ radiation on the dwarfing mechanism of Rhododendron. N Gard 9:48–50
Zhou S, Zou X, Zhou Z, Liu J, Xu C, Yu J et al (2014) Multiple species of wild tree peonies gave rise to the ‘king of flflowers’, Paeonia suffruticosa Andrews. Proc Biol Sci 281:1687–1694
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDA23080601), National Natural Science Foundation of China (no. 31601789) and the Foundation of High-level Talents of Qingdao Agricultural University (6631114306).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by R. Beckett.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Geng, D., Song, S. et al. Transcriptome and biochemical response to 60Co gamma radiation exposure on the grafted seedlings of Paeonia suffruticosa. Acta Physiol Plant 44, 91 (2022). https://doi.org/10.1007/s11738-022-03426-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03426-2