Abstract
Mosses are considered highly resistant against desiccation because they maintain photosynthetic activity even when severely dehydrated. In our study, we investigated changes in the photochemical processes of photosynthesis, as well as the spectral reflectance parameters during controlled rehydration and desiccation in two Antarctic species, i.e. Brachythecium austro-glareosum and Bryum pseudotriquetrum. Changes in primary photochemical processes were evaluated by chlorophyll fluorescence, i.e. slow Kautsky kinetics supplemented with saturation light pulses. In desiccating thalli, an effective quantum yield of photosynthetic processes in PS II (ΦPSII) remained unchanged within low to moderate desiccation, i.e. with a relative water content (RWC) decrease from fully wet (100%) to semidry (30%). Below 20% RWC, ΦPSII showed a species-specific decline, as well as steady-state fluorescence (FS). A half-decrease in ΦPSII was reached at an RWC of 12.6% (B. austro-glareosum) and 9.8% (B. pseudotriquetrum). Rapid light–response curves showed a strong limitation of photosynthetic electron transport (ETR) at an RWC below 20% in both species. The ΦPSII and ETR data suggested that both species were desiccation-tolerant and well adapted to harsh Antarctic environments. However, B. pseudotriquetrum was more resistant in a state of severe dehydration (RWC below 20%) than B. austro-glareosum. Spectral reflectance indices responded to desiccation either (a) similarly in the two species (normalised difference vegetation index [NDVI]), (b) with similar trends but different values (modified chlorophyll absorption in reflectance index [MCARI] and greenness index [GI]) and (c) species-specifically (photochemical reflectance index [PRI]).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to lichens, mosses are dominant components of non-vascular flora in the Polar Regions, based on their ability to cope with a variety of harsh conditions. Due to their poikilohydric character, mosses are well adapted to repeated dehydration/rehydration and long periods spent in a dry state, which may be quite frequent events in Polar Regions.
Most moss species are highly resistant to desiccation. Some genera, however, are considered sensitive to drought stress (Jassey and Signarbieux 2019; Rastogi et al. 2020). The desiccation/rehydration cycles can be repeated several times without causing major changes in the functioning of the moss (Stoklasa-Wojtasz 2012). It has been established that mosses can withstand considerable desiccation (water content of 5% ± 10% of their dry weight), followed by recovery to normal vitality upon rehydration (Alpert 2000). This ability is a common property of bryophytes and a high number of mosses have been experimentally verified to be desiccation-tolerant (Wood 2007). Bryophytes are frequently subjected to cyclical desiccation–rehydration events and have evolved remarkable constitutive and inducible mechanisms of desiccation tolerance to survive in arid desert environments (Li et al. 2014). Studies conducted by Bewley (1979), Bewley and Krochko (1982), Proctor (1990), Oliver et al. (1993), Tuba et al. (1996) and Hu et al. (2016) focused on desiccation tolerance-evaluated photosynthesis at different thallus hydration levels ranging from a fully wet to dry state. These studies used gas exchange and/or chlorophyll fluorescence (ChlF) measurements to evaluate hydration-dependent changes in photosynthesis.
The exploitation of ChlF is beneficial in photosynthetic studies because methods based on ChlF can detect the impact of environmental stressors. Stress-induced changes in the functioning of photosynthetic systems can be sensed without any damage to leaves. Within the past number of decades, several aspects of photosynthetic processes have been studied using ChlF, such as reactivation of photosynthetic activity in moss and lichen thalli after rewetting (Schlensog and Schroeter 2001). The idiosyncrasies of several ChlF techniques and their proper application were reviewed by Kalaji et al. (2014). In mosses, ChlF was used to evaluate the effects of different stressors. Baxter et al. (1991) used ChlF to study moss response to different concentrations of bisulphite ions. Photosynthetic response to freezing was determined in mosses by Rütten and Santarius (1992) by measuring the freezing-induced decrease in ChlF ratio (FV/FM), the results indicating an increased sensitivity to frost damage in young and old tissue. Similarly, Deltoro et al. (1999) reported a freezing-induced decrease in photosynthetic CO2 fixation, accompanied by an increase in non-photochemical quenching of ChlF. The effect of photoinhibition on photosynthesis and the growth of arctic moss was studied under field and laboratory conditions by Murray et al. (1993). The study reported a moderate light-induced decrease in FV/FM in Sphagnum sp., indicating sensitivity to photoinhibition in open habitats of the Alaskan tundra. Since the 1990s, chlorophyll fluorescence has been used in the measurement of moss photosynthetic processes under different degrees of thallus desiccation. Seel et al. (1992a) studied changes in the activity of PS II during drying and gradual rehydration in Tortula ruralis ssp. ruraliformis and Dicranella palustris mosses. The results showed differences in PS II activity between the mosses rehydrated immediately after reaching a dry state and those that remained in a dry state for one week before rehydration. Changes in ChlF parameters during drying and consequent rewetting were highlighted in a study by Deltoro et al. (1998a), who found species-specific responses, e.g. the capability of mosses from xeric but not hydric and mesic habitats to restore photochemical activity after rewetting from a dry state. Recently, several detailed studies focused on the underlying mechanisms of moss photosynthesis resistance to dehydration, e.g. the glassy state of thalli in rapidly desiccating mosses and the activity of violaxanthin de-epoxidase (Fernández-Marín et al. 2011), protective mechanisms based on energy dissipation during moss desiccation (Hamerlynck et al. 2000), the rate of mRNA loss during rapid drying and its synthesis during rehydration, as well as a turnover of mRNAs stored in the dried state (Oliver et al. 2000). Gene expression analysis suggested that jasmonic acid signalling, proteosomal activation and alternative splicing are components of desiccation tolerance mechanisms in mosses (Tortula ruralis in Oliver et al. 2009). Pressel and Duckett (2010) reported that in the de- and repolymerisation of microtubules during a drying–rewetting cycle in a moss protonemata plays a role during fast and slow desiccation.
For a better understanding of the mechanisms of moss resistance to desiccation, an integrated approach can be used. Typically, such studies combine different approaches that include special ChlF techniques. Tuba et al. (1996) analysed the RFd parameter measured in parallel with gas-exchange during a drying-rehydration cycle (Tortula ruralis ssp. ruralis). Detailed analysis of ChlF parameters, their interspecific differences, particularly in response to drying and remoistening, was conducted by Csintalan et al. (1999) for three species (Rhytidiadelphus loreus, Anomodon viticulosus and Grimmia pulvinate) with different degrees of desiccation tolerance. Bartošková et al. (1999) and Nabe et al. (2007) showed that dehydration-induced changes in chlorophyll emission and the absorption spectra of Rhizomnium punctatum indicated the grouping of chlorophyll molecules, as well as the reabsorption of ChlF. The study explored time relations of the recovery of photosynthetic activity and CO2 uptake following rehydration of a desiccation-tolerant moss. In other studies such as e.g. Zhang (2016), changes in ChlF parameters, based on different water content were underscored.
Although many of the protective mechanisms of bryophytes are common within vascular plants, there are fundamental interspecific differences in their response to desiccation. Marschall et al. (2018) reported desiccation-tolerant (Porella platyphylla) and desiccation-sensitive (Sphagnum angustifolium) mosses. Recently, moss tolerance to desiccation and underlying physiological mechanisms were studied using a wide range of methods and approaches. These included measurements of (1) the ionic conductivity of cellular membranes (Šinžar-Sekulić et al. 2005), (2) the accumulation of osmoprotective compounds, (3) the activation of ROS scavengers, protection against ROS-induced damage, and the repair of ROS-induced damage (Hu et al. 2016), (4) the protection of membrane integrity by restructuring the cell membrane and the synthesis of osmolytes following disruption of the membrane (Mahajan and Tuteja 2005) and, most recently, (5) a genetic approach focused on transcriptomes during the hydration–dehydration–rehydration cycle (Bryum argenteum in Gao et al. 2015).
Spectral characteristics of thalli may change in poikilohydric autotrophs such as lichens (Barták et al. 2018) and mosses (Van Gaalen et al. 2007) during desiccation. In the case of mosses, however, only a small number of physiological studies have addressed spectral reflectance change during desiccation. Lovelock and Robinson (2002) studied Antarctic mosses (Bryum pseudotriquetrum, Ceratodon purpureus, Grimmia Antarctica), spectral reflectance parameters in relation to pigment composition, photosynthetic performance and water content. The results showed the strongest correlation with spectral reflectance parameters as being with intrathalline water content. Similarly, Van Gaalen et al. (2007) showed that the photochemical reflectance index (PRI) correlated with the rate of net photosynthesis, as well as with the xanthophyll-cycle pigment content and non-photochemical quenching (NPQ). Harris (2008) used parallel ChlF measurements and spectral reflectance parameters during the desiccation of Sphagnum. Here, desiccation led to a significant reduction in ФPSII, which weakly correlated with the PRI. Moreover, a strong negative correlation was found between changes in PRI and NPQ. The NDVI indicated a change in thallus dehydration. These data suggest that NDVI and PRI are good proxies for short-term hydration/dehydration-induced changes in the photosynthetic activity of mosses, similarly to what was reported for lichens (Barták et al. 2015a). Therefore, simultaneous measurements of photosynthetic processes by ChlF and spectral properties during the dehydration of moss species is beneficial for analysing their physiological responses activated by wetting.
For Antarctic mosses, only a small number of studies have been devoted to photosynthetic performance during partial dehydration and their desiccation tolerance in the field and under laboratory conditions. Davey (1997) studied rehydration times by carbon exchange rate in Antarctic bryophytes using an infra-red gas analysis system. The study found fast respiration bursts and slow restoration of net photosynthesis; for Antarctic species, this required more time compared to non-polar species. Robinson et al. (2000) focused on the physiological tolerance to desiccation measured by ChlF of Antarctic moss during natural drying in a laboratory. More recent studies addressed several aspects of moss photosynthesis in response to their hydration status. These have investigated the metabolic recovery of continental Antarctic cryptogams following a dry winter season, e.g. Schlensog et al. (2004), where FV/FM and the effective quantum yield of PS II (ΦPSII) were employed to show the rapid and slow phases of recovery of photosynthetic processes after rewetting. Wasley et al. (2006) documented the species-specific responses of mosses from differently hydrated niches in Antarctic terrestrial ecosystems. The role of desiccation in the UV-B tolerance of mosses, as well as stress assessment using imaging spectroscopy data, were studied by Malenovský (2015), who found a satisfactory relationship between chlorophyll content, ChlF parameters and spectral image data of the Antarctic moss carpet.
In our study, we focused on primary photosynthetic processes and spectral reflectance, their responses to controlled desiccation, in two moss species from the James Ross Island of Antarctica. Although both of the studied mosses occurred within similar habitats, we hypothesised that their drought tolerance would be species-specific. Concerning the anatomical–morphological characteristics of each species, we assumed that Brachythecium austro-glareosum’s photosynthetic processes would show lower resistance to a strong degree of drying compared with Bryum pseudotriquetrum. Finally, we analysed the protective quenching mechanism activated in the two species by dehydration.
Materials and methods
Collection and site characteristics
Samples of Brachythecium austro-glareosum and Bryum pseudotriquetrum were collected from the James Ross Island (Antarctica). The collection site was N-facing slope (63° 48ʹ 12ʹʹ S, 57° 51ʹ 02ʹʹ W) and fed by several snowfields located on the northern foothills of the Berry Hill mesa. The site is denoted as Area 2 in the list of vegetation oases of the James Ross Island (Barták et al. 2015b). The area is rich in mosses, carpets of which form irregular patches related to individual water streams rich in cyanobacterial and algal species. Moss vegetation is dominated by Bryum pseudotriquetrum and Hypnum revolutum. Apart from mosses, several lichen species contribute to the vegetation oasis community. Climatic characteristics of the site of collection have been described (Láska et al. 2011). For austral summer season (September to February), mean air temperature of − 4.6 °C and mean air RH of 81% are reported. At the collection site, S and W winds prevails (both in 25% of time) with majority of speeds found within the range of 5–10 m s−1 in Austral summer season (Bohuslavová et al. 2018).
The collected samples of B. austro-glareosum and B. pseudotriquetrum were dried under natural (field) conditions and then transferred to a laboratory in Brno (Czech Republic) where the below-specified ecophysiological measurements were performed.
Species characteristics
Brachythecium austro-glareosum (C. Muell.) Kindb. This species forms relatively thin carpets covering wet post-glacial lowlands. In Antarctica, it has been reported for several locations in the South Shetland Islands, Argentine Islands and the Danco Coast (the west coast of the Antarctic peninsula) (Putzke and Pereira 2001). It prefers wet areas, such as regions in which the drainage of meltwater occurs. The mosses are rarely cespitose, bright, brownish or yellowish-green above and generally somewhat brown below. The stems are 1.0–6.5 cm high, prostrate or ascending, irregularly or subpinnately branched, with brown rhizoids in groups under the leaf insertion. The leaf margins are intermittently recurved throughout, with costa ending near the mid-leaf. Photosynthetic performance of B. austro-glareosum has not yet been investigated in either the field or a laboratory setting.
Bryum pseudotriquetrum (Hedw.) Gaertn., Mayer and Scherb forms dense tufts of dark-green to reddish colour, approximately 1.5–5.0 cm high. The stems are simple or forked, typically red and somewhat tomentose below. The leaves are more crowded at the top. When dry, the leaves are contorted but change to erect spreading when wet; leaves are variable in shape but range generally from oblong–lanceolate to ovate–lanceolate, with an obtuse, acute or acuminate apex. The margins of the leaves are revolute or not and entirely or variably serrulate near the apex; costa red below, percurrent or ending near the apex. In Antarctica, B. pseudotriquetrum is quite abundant. Field studies using gas exchange measurements revealed a net photosynthetic rate of 4.0 µmol m–2 s–1 (Ino et al. 1990; Pannewitz et al. 2005). Photosynthetic processes of the species that are dependent on desiccation were studied by ChlF (Robinson et al. 2000). However, only the potential yield of photochemical processes in photosystem II (FV/FM) was analysed.
Evaluation of constitutive amounts of pigments
From each sample, 100 mg of biomass (dry matter) was taken to determine pigment content. After homogenisation, ethanol extracts were used for spectrophotometric evaluation of chlorophyll (Chl a/Chl b) and carotenoids (Car) according to Lichtenthaler (1983), using a Specord 205 (Analytik, Jena, Germany) spectrophotometer. The absorbance measured at 649 and 665 nm was used for Chl and 470 nm for Car evaluation.
Rehydration and activation of photosynthetic processes
Restoration of photosynthetic processes upon rehydration was studied by ChlF. Dry samples of B. austro-glareosum and B. pseudotriquetrum were rehydrated in Petri dishes by regular spraying for 48 h. The rehydration was done at a temperature of 5 °C and under low light (10 μmol m–2 s–1 of photosynthetically active radiation [PAR]). Activation of primary photosynthetic processes was evaluated by slow Kautsky kinetic measurements with quenching mechanism analysis (typically at 1 h intervals). The kinetics were measured by a FluorCam HFC-010 fluorometer (Photon Systems Instruments, Drásov, Czech Republic) using the method described elsewhere (Trnková and Barták 2017). From the ChlF measurements, the following parameters were calculated and expressed as dependent on the time of rehydration: FV/FM (potential yield of photosynthetic processes in PS II), ΦPSII (effective quantum yields from the photosynthetic processes of PS II), FS (steady-state fluorescence) and RFd (relative fluorescence decline ratio, vitality index).
Desiccation and relative water content determination
For desiccation experiments, the moss samples were handled as follows: Before conducting experiments, moss clusters approximately 1 cm in diameter were rehydrated in the laboratory for 48 h at 10 °C. Then, excess surface water was removed by blotting the samples on a paper towel. The samples were placed into a plastic tube of the same diameter and with numerous perforations across its surface, allowing for desiccation of the moss cluster from the side. Then, the samples were allowed to desiccate naturally at room temperature (23.5 °C) and in dim light (20 µmol (photons) m–2 s–1 of photosynthetically active radiation). During desiccation, the change in water content of each sample was determined gravimetrically using a Mettler AE 100 scale (Germany). The relative water content (RWC) was calculated using the formula:
During the dehydration from a fully wet to dry state, changes in the water potential (WP) of moss clusters were also measured. For a single WP measurement, a sample of thallus was left in a chamber of a dew point water potential meter (WP4T, Decagon Devices, Pullman, USA) for 10–20 min until an equilibrium was reached. Then the WP reading was taken using a method described by Jupa et al. (2012). Finally, the relation between WP and RWC was evaluated for particular moss species.
Chlorophyll fluorescence parameters during desiccation
During desiccation from a fully wet (RWC = 100%, WP = 0) to dry (RWC = 0%) state, the following ChlF parameters were measured repeatedly (in 10 min steps): (1) the effective quantum yield (ΦPSII) of photosystem II, (2) steady-state ChlF FS (for parameter definition and equations, see, e.g Roháček and Barták 1999; Roháček 2010). For this purpose, a PAM-2000 fluorometer (Walz, Germany) with a custom-programmed routine was used. Saturation pulses were applied on the samples at a light-acclimated state (exposure, 20 μmol m–2 s–1 of PAR) at 10 min intervals. The dehydration response curves of ΦPSII and FS were constructed (pooled data from three replicates for each species). Species-specific responses in the dehydration-induced change of ΦPSII and FS were evaluated; critical points for primary photosynthetic processes (RWC, WP) in which the most rapid changes occurred were measured.
Rapid light response curves of ETR
We measured light response curves of apparent electron transport rate (ETR) were measured on the moss samples that had been maintained fully hydrated for at least 24 h before the experiment. ETR curves were measured by a modulated fluorometer (PAM 2500; Walz, Germany) using a standard protocol that had been pre-programmed into the instrument during progressive drying at laboratory temperature. During desiccation, RWC was gravimetrically evaluated (see above). Readings of ETR were taken at 30 min intervals for three replicates of each species. This method comprised the measurements of rapid light curves (RLC; see White and Critchley 1999), i.e. measurements of ΦPSII in samples exposed to increasing intensities of actinic light (from 0 to 1050 µmol m–2 s–1 of PAR). We measured apparent ETR i.e. ETR exploiting the effective quantum yields of photosystem II (ΔF/FM' or ΦPSII) according to ETR = ΦPSII × PAR × 0.42. The coefficient 0.42 was used according to Schreiber et al. (1994). The time interval for each PAR intensity level was 20 s for full equilibration of photosynthetic reactions. Then, ETR data were plotted against PAR and the following parameters were evaluated: (1) α, the initial slope of RLC, which is related to the quantum efficiency of photosynthesis; (2) ETRmax, the maximum electron transport rate.
Spectral reflectance and absorbance measurements
Reflectance spectra of the moss species were measured by a PolyPen RP 400 ultraviolet–visible (UV–VIS) spectroreflectometer (Photon Systems Instruments, Drásov, Czech Republic) within the range of 380–800 nm. During gradual dehydration from a fully wet (RWC = 100%) to dry state (RWC = 0%), moss samples were measured repeatedly. The photochemical reflectance index (PRI) and normalised difference vegetation index (NDVI), as well as and several others (CARI, MCARI, GI, CI, CCRI, Pq—see Table 1 for definitions and equations) were calculated by the SpectraPen software.
Statistical analysis
Species-specific differences were evaluated for ChlF signals (FS), ChlF parameters (ΦPSII, NPQ and ETR dependent on RWC [independent variable]) for the rehydration and dehydration experiments. The species-specific differences in spectral reflectance were evaluated (using wavelength as an independent value) in wet and dry states, as well as by a paired t-test at the P = 0.005 level of significance. The same paired t test was used for the evaluation of species-specific differences in spectral reflectance indices during desiccation (using RWC as the independent variable).
Results
Pigment content
The moss species differed slightly in terms of Chl a and Chl b values, as well as total chlorophyll content (Chl a + b) (see Table 2). There was a statistically significant difference in chlorophyll b content; B. pseudotriquetrum had a higher concentration of Chl b than B. austro-glareosum. The total carotenoid content in B. pseudotriquetrum was higher than in B. austro-glareosum.
Rehydration
Rehydration response curves showed gradual restoration of primary photosynthetic processes in PS II (Figs. 1, 2) with time of hydration. For both species, 32 h was required to reach maximum values for FV/FM and ΦPSII. The rise of both parameters, however, was polyphasic, showing a lag phase (0–3 h for B. austro-glareosum and 0–5 h for B. pseudotriquetrum) with constant values. Then (2/5–30 h), FV/FM and ΦPSII increased and followed an S-curve in both species. Maximum values of FV/FM and ΦPSII were found to be somewhat lower in B. pseudotriquetrum compared with B. austro-glareosum. The rehydration response courses of NPQ were found to be species-specific. For B. austro-glareosum, NPQ curves were complex and polyphasic. Local minimum was found in the interval of 5–9 h of rehydration, as well as two maxima (after 45 min and 27 h). The NPQ minimum corresponded to the time at which FV/FM and ΦPSII exhibited the most rapid increase and the local maximum of steady-state ChlF (FS); B. pseudotriquetrum showed more or less a constant but slow rate of increase in NPQ values throughout the rehydration period. The FS values were found to be constant at the very beginning (0–9 h) but slightly decreased after 24 h of rehydration.
The time courses of chlorophyll fluorescence parameters recorded during a 32 h rehydration period in Brachythecium austro-glareosum (full symbols) and Bryum pseudotriquetrum (open symbols). Data points represent the means of five replicates ± standard error (bars). The symbol keys are as follows: FV/FM, potential yield of photochemical photosynthetic processes in PS II; ΦPSII, the effective yield of photochemical photosynthetic processes in PS II; NPQ, the non-photochemical quenching of chlorophyll fluorescence; FS, steady-state chlorophyll fluorescence; RFd, a relative decrease of chlorophyll fluorescence (vitality index); qF0, quenching of background chlorophyll fluorescence. The species-specific time courses of the parameters show a statistically significant difference (P = 0.005), except for RFd and qF0
Dehydration
Within the first phase of dehydration (RWC decrease from 100 to 40%) ΦPSII showed no change in terms of dehydration for both species. With pronounced desiccation (RWC decreased from 40 to 0%, i.e. from a water potential of 8.0 MPa; see Fig. 3), a rapid decline to the full inhibition at which ΦPSII = 0 was apparent. The decline was comparable in both species; however, B. pseudotriquetrum showed higher resistance to dehydration since the half of maximum value was found at a lower RWC compared with B. austro-glareosum (see Fig. 4). In both species, 40% of RWC equaled a value of – 7 MPa of water potential (see Fig. 3). The decline of ΦPSII was found in the WP range of –10 to –33 MPa in both species.
The relationship between the effective quantum yield of PS II (ΦPSII) and relative water content (RWC) in desiccating moss thalli from a fully wet (RWC = 100%) to dry (RWC = 0%) state. The ΦPSII courses show a statistical difference (P = 0.005). The RWCs in which half of the maximum ΦPSII value is reached is indicated by the arrow
During gradual dehydration, FS declined in a polyphasic manner and showed species-specific differences. The rapid dehydration-induced decline began at different RWCs. The starting point was found at a higher RWC for B. austro-glareosum (40%) than B. pseudotriquetrum (30% RWC). The decline in FS in low-to-moderate thallus dehydration (from RWC 100% to 40%) also differed between the two experimental species. This showed an S-like curve with FS values decreasing alongside dehydration (from right to left in Fig. 5); a slight increase in FS of approximately 14% was found during the decrease of RWC from 100 to 40% in B. austro-glareosum.
Rapid light curves during dehydration
The ETR responded differently to gradual desiccation in both species. An increase in ETR was found during the initial phase of dehydration (RWC decrease from 100 to 60%), followed by a decrease in ETR at an RWC below 60% in B. austro-glareosum. In B. pseudotriquetrum, a decrease in ETR was only found alongside an RWC decrease (Fig. 6). In the latter species, ETR in response to PAR did not change within the range of RWC decreasing from 100 to 40% and was followed by an ETR decrease beginning at 20% RWC. A substantial decrease was found for both species at an RWC below 10%. The experimental species differed regarding the α parameter (the effectivity of photosynthetic electron transport per PAR unit), which represented the initial slope of the curve in low light. Parameter α reached 0.25 and 0.14 electrons quanta–1 in fully-hydrated (RWC = 100%) B. austro-glareosum and B. pseudotriquetrum, respectively. In both species, α decreased with desiccation and reached minima of 0.08 and 0.04, respectively, at an RWC of approximately 10%.
The rapid light curves of electron transport rate (ETR) recorded for the thalli of Brachythecium austro-glareosum (upper panel) and Bryum pseudotriquetrum (lower panel) showing strong desiccation-induced limitation of the ETR at a relative water content (RWC) of approximately 20%. Species-specific differences in the ETR values recorded at a particular RWC show statistical significance (P = 0.005). Data points are the means of at least three samples and standard deviations were below 10% of the means
Spectral reflectance indices
Spectral reflectance curves and indices showed different sensitivity to dehydration (see Table 3 and Fig. 7). In the case of spectral curves, the most pronounced differences between wet and dry spectra were found in 530, 617 and 663 nm (B. austro-glareosum) and 530, 617 and 579 nm (B. pseudotriquetrum). The NDVI, GI and MCARI declined with a decrease in RWC. Despite similar trends in dehydration-response curves of NDVI, GI, MCARI, species-specific differences were found for these parameters (lower ranges in B. austro-glareosum compared with B. pseudotriquetrum throughout the entire RWC range).
The relationship between NDVI and RWC (see Fig. 8) appeared to be biphasic during dehydration. In B. pseudotriquetrum, NDVI slightly increased during the initial phase of dehydration (with a peak at 70%–80% of RWC); then, NDVI declined with an RWC decreased from 70 to 0% with a minimum of 0.45 found at extremely low RWCs. In general, the dehydration response curve of NDVI showed a curvilinear relationship with a pronounced decrease in NDVI value at RWCs below 40%. Additionally, B. austro-glareosum showed a similar dehydration-response curve for NDVI; however, the NDVI values were smaller in the best-fit curve and the extremely dehydrated thallus (NDVI, 0.4).
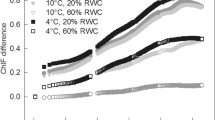
The relationship of non-photochemical quenching (qN) to spectral reflectance indices (photochemical reflectance index = linear relationship, normalised difference vegetation index = 3rd order polynomial) in the desiccating thalli of Brachythecium austro-glareosum (full symbols) and Bryum pseudotriquetrum (open symbols)
The PRI dehydration-response curve showed a different shape for B. austro-glareosum and B. pseudotriquetrum. In severely dehydrated thalli (RWC below 30%), an increase was apparent in the desiccating B. austro-glareosum while an opposite trend was observed for B. pseudotriquetrum. The PRI and NDVI were related to non-photochemical quenching (Fig. 8); however, the relationships were species-specific. For PRI, opposing trends of non-photochemical quenching (qN) were found in each species. The MCARI decreased alongside desiccation in both species. Absolute MCARI values were, however, generally lower in B. austro-glareosum compared with B. pseudotriquetrum. Generally, the GI declined with a decrease in RWC, except in the case of full hydration in B. pseudotriquetrum. Within the range of 80–100% RWC, GI increased with desiccation. In the RWC range of 0–80%, GI declined with desiccation.
The phaeophytisation index (Pq) increased with a decrease in RWC. However, it was found polyphasic in B. austro-glareosum but linear in B. pseudotriquetrum. Spectral reflectance indices CARI) CCRI, as well as CIred-edge decreased (B. austro-glareosum) and increased (B. pseudotriquetrum) in the dry states (see Table 3).
Absorption spectra of moss extracts
Absorption spectra are presented showing high peaks in the area of 190–220 nm and are more pronounced in B. pseudotriquetrum than B. austro-glareosum (Fig. 9). Other, much smaller absorbance peaks were evident at 270 and 300 nm on the spectral curve, followed by peaks related to carotenoids (450 nm) and chlorophyll (663 nm).
Discussion
Photosynthetic pigment content
Chlorophyll content has been widely used as an ecophysiological marker in vascular plants in Antarctica. In bryophytes, however, such studies are much scarcer. Our data on total Chl content are in good agreement with those of Russell (1985), who reported 0.143 and 1.357 mg g–1 DW (for Ditrichum strictum and Brachythecium rutabulum from Marion Island). Our data on total Chl content, however, were lower than those reported by Barcikowski and Loro (1999) from the King George Island, i.e. Sanionia georgico-uncinata (5.14 mg g–1 DW), Brachythecium austro-salebrosum (4.55 mg g–1 DW), Sanionia uncinata (4.47 mg g–1 DW) and Polytrichastrum alpinum (2.34 mg g–1 DW). It seems that such Chl values in mosses resulted from the natural fertilisation of habitats (ornithogenic soils) with organic matter (Tatur et al. 1997). Our samples from the James Ross Island were collected from sites with low organic matter availability, which suggests that they may have lower chlorophyll content. Other interacting factors affecting Chl contents in Antarctic mosses may be seasonal (Barcikowski and Loro 1999) and the negative effects of high UV-B (Robinson et al. 2005), which is apparent in Antarctica in September–November due to ozone-hole formation.
The total carotenoid content was higher in B. pseudotriquetrum than in B. austro-glareosum. However, the content was in a good agreement with the data reported by several authors for mosses from subpolar and Polar Regions (Lappalainen et al. 2008; Newsham 2002; Schroeter 2012). Carotenoids have photoprotective and antioxidative functions. They protect photosystem II from overenergisation by thermal dissipation of excess energy under a wide variety of environmental factors/stressors. Therefore, in Antarctica, the total carotenoid content may differ within the growing season, particularly from September to November, when increased levels of UV-B radiation are available as a result of stratospheric ozone depletion. It has been well-documented that ozone hole induced an increase in UV-B and led to an increase in UV-B absorbing compounds (Waterman et al. 2018) as well as total carotenoids in Antarctic mosses (Newsham 2002). Additionally, in situ experiments using the UV-B exclusion approach indicated that UV-B-exposed mosses had a higher total carotenoid content than mosses not exposed to UV-B (Singh and Singh 2014). Similarly, long-term experiments with UV-B enhancement led to an increased content of carotenoids in the sub-Arctic tundra (Arróniz-Crespo et al. 2011).
Rehydration
Rehydration time (30 h for maximum FV/FM) for the mosses included in this study was comparable to other moss species; however, shorter times were reported by ecophysiological studies for several moss species (1 h by Proctor et al. 2007a, 16 h by Csintalan et al. 1999, 20 h by Nabe et al. 2007 and 25 h by Nakaya and Saxena 2014). However, species-specific differences were observed in the re-wetting time that may be attributable to the length of the period of sample hydration before desiccation. Green et al. (2011) reported rapid recovery of photosynthetic processes in mosses from rock surfaces (fast and repetitive hydration/dehydration), while those that were wet for long periods before desiccation required a longer time to restore their photosynthetic processes. This could be supported by a follow-up experiment involving a desert moss conducted by Stark et al. (2013), who reported that the rate of drying was significantly affected following rehydration responses and prolonged recovery time of FV/FM (8 h for slowly desiccated and 72 h for rapidly desiccated). Since the two Antarctic mosses used in our study underwent rather rapid desiccation in the field (due to the dual action of wind speed and temperature), it is feasible to attribute the time of rehydration established in our study (32 h) to a site-related ecophysiological strategy. Based on this concept, the desiccation tolerance of the two moss species was related to the site-dependent rapidity of desiccation. A recent study by Pizzaro et al. (2019) reported requiring approximately 40 h to restore PS II efficiency in rehydrated Sanionia uncinata from King George Island, Antarctica.
Environmental factors, such as light and temperature may also play a role and interact during the rehydration period since both the photosynthesis and respiration that occurs during the rehydration time are light- and temperature-dependent. Li et al. (2014) documented a large difference in rehydration time for Bryum argenteum in light (3 h) and dark (24 h) conditions. In our data, FV/FM and ΦPSII were well- linked, indicating that for all thallus hydration and stages of cell function restoration, both potential and actual photosynthetic processes in PS II were well-coupled.
Non-photochemical quenching course alongside the time of rehydration for B. austro-glareosum showed two comparably high maxima followed by a decrease (Fig. 2). This phenomenon is comparable to data reported by Csintalan et al. (1999), who found an increase in NPQ followed by a decrease within the first 1 h. Our data showed this decrease lasted for at least 9 h. The following increase in NPQ, observed in the rehydration period (24–27 h), may be attributable to the protection of PS II during the adjustment of PSII full functioning (see FV/FM and ΦPSII courses between 24 and 27 h). However, the course of NPQ adjustment during rehydration was affected by a complex range of factors that may have interacted, causing a dynamically changing curve shape. For example, the rehydration time required for the final adjustment of NPQ varied between species and reflected peculiarities of the experimental set-up. It may be as fast as 1 h (Csintalan et al. 1999 for Rhytidiadelphus loreus) or may only be completed after 8 h (Mayaba et al. 2002 for Atrichum androgynum).
Dehydration response curves of ChlF parameters
In both species, the decline of FS at the RWCs below 45% could be attributable to a progressive loss of water from cells and a consequent decrease in the ChlF emission. The FS decline is comparable to the evidence for Rhizomnium punctatum (Giudici et al. 2018, 20% RWC). Similarly, Yamakawa et al. (2012) reported an FS decline with progressive dehydration in Rhytidium rugosum. However, some moss species do not show any FS decline during desiccation (RWC from 100 to 0%), e.g. Palustriella commutate and Rhytidiadelphus squarrosus (Giudici et al. 2018). The reason for the constant FS during moss desiccation remains unclear.
The dehydration response curve for ΦPSII showed that a substantial decrease in ΦPSII values in B. austro-glareosum (see the arrow in Fig. 4) began at a higher RWC (23%, − 9.9 MPa) than B. pseudotriquetrum (14%, − 17.0 MPa). The interspecific differences appear quite obvious. These differences were previously reported by Deltoro et al. (1998a) even for mosses from xeric environments. The authors found sensitive (Leucodon sciuroides, RWC of 30%) and resistant (Orthotrichum cupulatum, 5% RWC) species. The same authors reported much higher RWCs (10–40%) for mosses from hydric and mesic environments. Proctor et al. (2007a) reported RWC as high as 75% for Polytrichum formosum in a humid site (Exeter, UK). Our ΦPSII data indicate that B. austro-glareosum and B. pseudotriquetrum could be considered drought-resistant (based on Antarctic semi-desert conditions). The dehydration response curves of ΦPSII allowed us to classify the two experimental species in terms of desiccation tolerance. The majority of mosses are considered desiccation-tolerant; however, desiccation-sensitive species exists. Marschall et al. (2018) reported representatives of both groups: desiccation-tolerant Porella platyphylla) and desiccation-sensitive (Sphagnum angustifolium. In our experiment, the analysis of RWC0.5, i.e. the RWC at which the sample showed half of maximum ΦPSII, reflected the difference in desiccation tolerance between B. austro-glareosum and B. pseudotriquetrum. RWC0.5 reached 12.6% RWC in desiccation-tolerant (B. austro-glareosum) and 9.8% RWC in highly-tolerant (B. pseudotriquetrum) species. The concept of species-specific desiccation tolerance, however, is not generally accepted as valid since there are many interacting factors. These include the phenotypic (Proctor et al. 2007b) and ecotypic plasticity of the species, physiological ‘history’ of the sample, microclimate effects (temperature, light) and the rate of desiccation/dehydration, which may co-act and change the desiccation tolerance/sensitivity. The mechanism involved into desiccation tolerance can be divided into two groups, the first related to a chloroplastic apparatus and effective quenching of absorbed light energy (for details see below), while the second is related to the cellular response to dehydration. The latter one comprises sucrose accumulation, antioxidant synthesis (e.g. glutathione), stress proteins and the synthesis of dehydrins (for mosses reviewed by Proctor et al. 2007b).
Several protective mechanisms are activated in mosses during dehydration including energy dissipation performed by 3 types of quenchers (for a review see, e.g. Yamakawa et al. 2012). These quenchers are (1) protonation of a thylakoid protein, (2) thermal dissipation of energy from light-harvesting complexes (Heber et al. 2006, 2008) and (3) a third mechanism based on the reversible photo-accumulation of a chlorophyll radical in PS II RCs. Activation of these mechanisms provides an increase in non-photochemical quenching (qN, NPQ) in desiccating mosses. Heber (2012) showed that dissipation occurred faster than energy capture by functional reaction centres. When this mechanism is insufficient, the other mechanism described below permits energy dissipation from the RCs. It is allowed thanks to an efficient spillover, i.e. energy transfer from PS II to PS I (Slavov et al. 2013). This phenomenon is called desiccation-induced quenching and was described by Heber et al. (2006). as a property RCs of PSII in Rhytidiadelphus squarrosus. On protein level, both (1) PsbS and (2) light-harvesting complex stress-related proteins (LHCSRs) are expressed during stress and involved into non-photochemical quenching (Alboresi et al. 2011; Gerotto et al. 2012). A small protein (PsbS) is associated primarily with the energy quenching (qE) component of non-photochemical quenching (Nyiogi et al. 2005). Conversely, LHCSR protein is involved in thermal dissipation as reported by Stella et al. (2016) for Physcomitrella patens.
The above-specified molecular mechanisms were involved into PS II protection which was demonstrated as qN (NPQ) increase during desiccation. Our data support such an interpretation because qN tended to increase (exponentially or following an S-curve [data not shown]) at RWCs below 50 (B. austro-glareosum) and by 15%–30% (B. pseudotriquetrum).
In addition to the above-noted three quenching mechanisms, several antioxidative substrates and enzymes are involved in non-photochemical quenching during desiccation (ASC in Paciolla and Tommasi (2003), SOD, CAT, APX and POD in Seel et al. (1992b) and Pizarro et al. (2019)). In general, mosses possess 2 types of antioxidant defence mechanisms (Chobot et al. 2008). The first of these removes or reduces free radicals including enzymes and antioxidants SOD, CAT, POD, vitamin C, Car, GSH (reviewed by Zhang et al. 2017) and zeaxanthin (see Deltoro et al. 1998b; Nabe et al. 2007). The second one produces antioxidants, such as e.g. enzymes: GSR and APX (Oliver et al. 2005).
Photosynthetic electron transport curves during dehydration
In our data, ETR in response to light declined in severely desiccated mosses (RWC below 20%; see RLCs in Fig. 6). This was indicative of a dehydration-dependent decline in ΦPSII that was documented for mosses (see, e.g. Proctor et al. 2007a). However, several other factors may interact with and affect ETR and the shape of RLC in mosses. Among them, the light and temperature to which the samples were exposed and/or acclimated to before the experiment play an important role. Griffin-Nolan et al. (2018) showed that acclimation to low/high light caused differences in the ETR light-response curves in temperate moss species. Similarly, Schroeter et al. (2012) showed a substantial difference between ETR curves recorded for ´sun´ and ´shade´ ecotypes of B. argenteum. Peng et al. (2019) reported that short-term high light treatment led to the flattening of ETR curves (a lower ETRmax) due to photoinhibition in Physcomitrella patens deficient in LHC components. This was also supported by the data of Schroeter et al. (2012), who reported a 50% reduction in the ETRmax of shade ecotype B. argenteum treated by high light for 6 h. Interspecific differences found in maximum light utilization coefficient (α, see Results) could be interpreted as the better use of absorbed light energy in primary photosynthetic processes in B. austro-glareosum compared with B. pseudotriquetrum throughout a broad range of thallus dehydration. However, B. austro-glareosum showed a more rapid decline in ETR during dehydration (see the RLCs at 40% RWC in Fig. 6). For B. pseudotriquetrum, Kudoh et al. (2003) reported an ETRmax of 25, which is comparable to our data.
Spectral reflectance indices
Hydration-induced reflectance changes in the visible region were mainly due to high absorption by photosynthetic pigments. Malenovský et al. (2015) suggested that the reflectance in the wavelengths range of 530–600 nm could be used as a proxy of the content of xanthophyll cycle pigments that changes during desiccation as reported by Calatayud et al. (1997) and Deltoro et al. (1999) and which function as part of the protective mechanisms required by moss in response to oxidative stress in chloroplasts. The increase in the ratio of zeaxanthin to the total xanthophyll cycle pigments pool is typically reported in high light-exposed mosses (a photoprotective mechanism); however, it was also found in slow desiccating mosses (Fernández-Marín et al. 2013). A comparison of spectral reflectance curves in the range of 650–680 nm wavelengths revealed that both B. pseudotriquetrum and B. austro-glareosum had higher reflectance in a dry state, which is consistent with the data presented by Malenovský et al. (2015). Specifically, for B. pseudotriquetrum, lower reflectance values for a dry state compared with a wet state were found at a 520 nm wavelength, similar to Malenovský et al. (2015).
It has been well-established that in poikilohydric autotrophs, dehydration leads to significant changes in spectral reflectance, particularly in the visible (380–720 nm) range (Guyot 1990). Such changes bring about differences in PRI and NDVI, which are species-specific and based on structural changes and pigmentation during desiccation. Our data indicate a good comparison to the NDVI (0.835) and PRI –0.160) reported for hydrated Antarctic moss B. pseudotriquetrum by Lovelock and Robinson (2002). In partly dehydrated moss thalli, however, several interacting factors may play a role including the dehydration-dependent movements of leaves. During desiccation, the leaves of moss reduce photosynthetic activity and pass through shape changes and geometrical rearrangement, i.e. ‘shrinking’ and ‘curling’ (see Zotz and Kahler 2007). These changes affect spectral reflectance curves and spectral indices. Generally, the top of a wet moss cushion with expanded leaves has significantly higher near-infrared reflectance than in dry state (Malenovský et al. 2015). The shrunken shoots and curled leaves of dry moss allow photons to penetrate deeper inside the turf, where they are absorbed.
The dehydration-induced decline in NDVI and PRI has been reported for several species of the Sphagnum genus; however, substantial interspecific differences do exist (Harris 2008). Similarly, May et al. (2018) reported a desiccation-induced decline in NDVI for several moss species in a manipulated drying experiment. Field evidence (e.g. in Svalbard as denoted by Valøen 2019) supports a dehydration-induced decline in NDVI in Arctic mosses. Our data support a decline in NDVI in desiccating B. pseudotriquetrum and B. austro-glareosum. The physiological consequences of a dehydration-induced decline in NDVI are generally unknown for mosses. However, lower NDVI values and higher levels of red reflectance at a low RWC may act as mechanisms for minimising the absorption of incident light to prevent further water loss leading to cellular damage (Charron and Quatrano 2009; Cruz de Carvalho et al. 2014). In both species, non-photochemical quenching (qN) increased at NDVI values below 0.54, which indicated the involvement of a protective mechanism at low RWC (below 20%).
However, PRI values declined with desiccation only in B. pseudotriquetrum (similar to the data of Harris 2008). The reason for a biphasic change in PRI during desiccation (a decrease followed by an increase) in B. austro-glareosum remains unknown. Similar biphasic changes with an RWC decline was found in, e.g. a lichen (Physconia muscigena, Barták et al. 2018). The PRI decline caused both an increase (B. pseudotriquetrum) and a decrease (B. austro-glareosum) in qN. The reason for the two contrasting responses (see Fig. 8) was that PRI behaved differently at RWCs below 30%. In the RWC range of 0%–30%, PRI increased with desiccation in B. austro-glareosum and decreased in B. pseudotriquetrum. The latter response, including the non-photochemical quenching rise with desiccation, was reported for Sphagnum sp. (Van Gaalen et al. 2007). Similarly, Rastogi et al. (2019) found a decrease in PRI in desiccating Sphagnum, which was related to a decrease in the performance index (PI), a ChlF parameter derived from a rapid ChlF induction curve (OJIP). An earlier study by Orekhova (unpublished data, manuscript in preparation) showed an S-curve decline in the PI of Bryum pseudotriquetrum with a decrease in RWC. This indicated the significant potential of studies exploiting simultaneous ChlF and spectral reflectance measurements in the analysis of the relationship between the two groups of data.
Since low RWC (below 20%) led to high qN (above 0.8) in both experimental species, the involvement of xanthophyll cycle pigment in the protection of desiccating moss was considered. These changes, i.e. a desiccation-induced increase of zeaxanthin content (Deltoro et al. 1998b) and a de-epoxidation state of the xanthophyll cycle pigments (DEPS) are, however, associated with a decrease in PRI since the viola- to zeaxanthin conversion can be detected by changes in leaf absorbance at 505–515 nm or via reflectance (e.g. Gamon et al. 1990). Some lichens and mosses do not exhibit a decrease in PRI at low RWC, which raises questions about interspecific differences concerning the constitutive amount of xanthophyll cycle pigments pool and the shape of the dehydration response curves of PRI. While the majority of mosses and lichens show a decline in PRI with thallus desiccation at a low RWC, some exceptions exist (B. austro-glareosum in this study and Physconia muscigena in Barták et al. 2018).
Both MCARI and GI spectral indices showed a decline with an RWC decrease, which is comparable to evidence derived from desiccating lichens in the study of Barták et al. (2016). In current study, they exhibited a high sensitivity to dehydration. Therefore, the MCARI index could be used as an indicator of dehydration status in moss thalli. Since the GI courses were similar to NDVI, the GI can be used as a good proxy for dehydration state in mosses, similar to NDVI. For follow-up studies involving mosses, the analysis of several simultaneously measured spectral reflectance indices may be recommended since, other than the hydration status and general optical properties in a wet and dry state, such an approach will allow for distinguishing the difference between moss species and co-occurring components of vegetation types as shown by Erudel et al. (2017).
Spectral absorbance curves showed high peaks in the UV-range, indicating a high level of UV-B screens present in the two moss species (Fig. 9). Since both moss species were collected in Antarctica, the high content of UV-B screens could be attributed to the ozone hole and its effect on UV-B incidence on Antarctic terrestrial ecosystems. Recent climate change holds significant implications for the survival and physiological processes of Antarctic bryophytes (Robinson et al. 2018) due to stratospheric ozone depletion beginning in the 1970s, which produced a rapid increase in biologically damaging UV-B radiation. Additionally, B. pseudotriquetrum is reported to have high levels of UV absorbing compounds (Waterman et al. 2018). In Antarctic mosses, UV-B screening compounds were identified as cell wall-bound (Waterman et al. 2018) and soluble phenolics (Clark and Robinson 2008). These are found in lichens in large quantities (see, e.g. Klavina et al. 2015).
Author contribution statement
Contribution of particular co-authors: AO measurements of chlorophyll fluorescence and spectral reflectance data, manuscript writing (50%), MB experimental design and manuscript writing (40%), JH data processing, graphical outputs, manuscript writing (10%), JM sample handling, testing measuring protocols and measurements of chlorophyll fluorescence.
Abbreviations
- ChlF:
-
Chlorophyll fluorescence
- KK:
-
Kautsky kinetics of chlorophyll fluorescence
- FV/FM :
-
Potential yield of photochemical photosynthetic processes in PS II
- ΦPSII:
-
Effective yield of photochemical photosynthetic processes in PS II
- NPQ:
-
Non-photochemical quenching of chlorophyll fluorescence
- FS :
-
Steady-state fluorescence
- RLCs:
-
Rapid light curves
- ETR:
-
Electron transport rate
- RWC:
-
Relative water content
- WP:
-
Water potential
- NDVI:
-
Normalised difference vegetation index
- PRI:
-
Photochemical reflectance index
- PSII:
-
Photosystem II
- MCARI:
-
Modified chlorophyll absorption in reflectance index
- GI:
-
Greenness index
- CARI:
-
Carotenoid index
- CIred-edge :
-
Red-edge chlorophyll index
- CCRI:
-
Carotenoid/chlorophyll ratio index
- Pq:
-
Phaeophytisation index
References
Alboresi A, Gerotto C, Cazzaniga S, Bassi R, Morosinotto T (2011) A red-shifted antenna protein associated with photosystem II in Physcomitrella patens. J Biol Chem 286:28978–28987
Alpert P (2000) The discovery, scope, and puzzle of desiccation tolerance in plants. Plant Ecol 15:5–17
Arróniz-Crespo M, Gwynn-Jones D, Callaghan TV, Núñez-Olivera E, Martínez-Abaigar J, Horton P, Phoenix GK (2011) Impacts of long-term enhanced UV-B radiation on bryophytes in two sub-Arctic heathland sites of contrasting water availability. Ann Bot 108:557–565
Barcikowski A, Loro P (1999) Changes in chlorophyll content throughout the year in selected species of mosses on King George Island, South Shetland Islands, maritime Antarctic. Pol Polar Res 20(3):291–299
Barták M, Trnková K, Hansen ES, Hazdrová J, Skácelová K, Hájek J, Forbelská M (2015a) Effect of dehydration on spectral reflectance and photosynthetic efficiency in Umbilicaria arctica and U. hyperborea. Biol Plantarum 59(2):357–365
Barták M, Váczi P, Stachoň Z, Kubešová S (2015b) Vegetation mapping of moss-dominated areas of northern part of James Ross Island (Antarctica) and a suggestion of protective measures. Czech Polar Rep 5:75–87
Barták M, Hájek J, Amarillo AC, Hazdrová J, Carreras H (2016) Changes in spectral reflectance of selected Antarctic and South American lichens caused by dehydration and artificially-induced absence of secondary compounds. Czech Polar Rep 6:221–230
Barták M, Hájek J, Morkusová J, Skácelová K, Košuthová A (2018) Dehydration-induced changes in spectral reflectance indices and chlorophyll fluorescence of Antarctic lichens with different thallus color, and intrathalline photobiont. Acta Physiol Plant 40:177
Bartošková H, Nauš J, Výkruta M (1999) The arrangement of chloroplasts in cells influences the reabsorption of chlorophyll fluorescence emission. The effect of desiccation on the chlorophyll fluorescence spectra of Rhizomnium punctatum leaves. Photosynth Res 62:251–260
Baxter R, Emes MJ, Lee JA (1991) Short-term effects of bisulphite on pollution-tolerant and pollution-sensitive populations of Sphagnum cuspidatum Ehrh. (ex. Hoffm.). New Phytol 118(3):425–431
Bewley JD (1979) Physiological aspects of desiccation tolerance. Annu Rev Plant Physiol 30:195–238
Bewley JD, Krochko JE (1982) Desiccation tolerance. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology, vol 12B. Physiological Ecology II. Berlin, Springer-Verlag, pp 325–378
Bohuslavová O, Macek P, Redčenko O, Láska K, Nedbalová EJ (2018) Dispersal of lichens along a successional gradient after deglaciation of volcanic mesas on northern James Ross Island, Antarctic Peninsula. Polar Biol 41:2221–2232
Calatayud A, Deltoro VI, Barreno E, Del Valle-Tascon S (1997) Changes in in vivo chlorophyll fluorescence emission during desiccation and suggestion of zeaxanthin associated photoprotection. Physiol Plant 101:93–102
Charron AJ, Quatrano RS (2009) Between a rock and a dry place: the water-stressed moss. Mol Plant 2(3):478–486
Chobot V, Kubicová L, Nabbout S, Jahodár L, Hadacek F (2008) Evaluation of antioxidant activity of some common mosses. Verlag Der Zeitschrift Für Naturforschung, Tübingen Z Naturforsch C J Biosci 63(7–8):476–482
Clarke LJ, Robinson SA (2008) Cell wall-bound ultraviolet-screening compounds explain the high ultraviolet tolerance of the antarctic moss, Ceratodon Purpureus. New Phytol 179:776–783
Cruz de Carvalho RC, Bernardes da Silva A, Soares R, Almeida AM, Coelho AV, Marques da Silva J, Branquinho C (2014) Differential proteomics of dehydration and rehydration in bryophytes: evidence towards a common desiccation tolerance mechanism. Plant Cell Environ 37:1499–1515
Csintalan Z, Proctor MCF, Tuba Z (1999) Chlorophyll Fluorescence during drying and rehydration in the mosses Rhytidiadelphus loreus (Hedw.) Warnst., Anomodon viticulosus (Hedw.) Hook. and Tayl. and Grimmia pulvinata (Hedw.) Sm. Ann Bot 84:235–244
Daughtry CST, Walthall CL, Kim MS, Brown DE, Colstoun E, Mcmurtrey JE (2000) Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens Environ 74:229–239
Davey MC (1997) Effects of short-term dehydration and rehydration on photosynthesis and respiration by Antarctic bryophytes. Environ Exp Bot 37(2–3):187–198
Deltoro VI, Calatayud A, Gimeno WC, Barreno E (1998a) Water relations, chlorophyll fluorescence, and membrane permeability during desiccation in bryophytes from xeric, mesic, and hydric environments. Can J Bot 76:1923–1929
Deltoro VI, Calatayud A, Gimeno C, Abadia A, Barreno E (1998b) Changes in chlorophyll a fluorescence, photosynthetic CO2 assimilation and xanthophyll cycle interconversions during dehydration in desiccation-tolerant and intolerant liverworts. Planta 207:224–228
Deltoro VI, Morales ÁCF, Abadía A, Barreno E (1999) Changes in net photosynthesis, chlorophyll fluorescence and xanthophyll cycle interconversions during freeze-thaw cycles in the Mediterranean moss Leucodon sciuroides. Oecologia 120:499–505
Erudel T, Fabre S, Houet T, Mazier F, Briottet X (2017) Criteria comparison for classifying peatland vegetation types using in situ hyperspectral measurements. Remote Sens 9:748
Fernández-Marín B, Míguez F, Becerril JM, García-Plazaola JI (2011) Dehydration-mediated activation of the xanthophyll cycle in darkness: is it related to desiccation tolerance? Planta 234:579–588
Fernández-Marín B, Kranner I, Sebastián MS, Artetxe U, Laza JM, Vilas JL, Pritchard HW, Nadajaran J, Míguez F, Becerril JM, García-Plazaola JI (2013) Evidence for the absence of enzymatic reactions in the glassy state. A case study of xanthophyll cycle pigments in the desiccation-tolerant moss Syntrichia ruralis. J Exp Bot 64:3033–3043
Gamon JA, Field CB, Bilger W, Björkman O, Fredeen A, Peñuelas J (1990) Remote sensing of the xanthophyll cycle and chlorophyll fluorescence in sunflower leaves and canopies. Oecologia 85:1–7
Gao B, Zhang D, Li X, Yang H, Zhang Y, Wood AJ (2015) De novo transcriptome characterization and gene expression profiling of the desiccation tolerant moss Bryum argenteum following rehydration BMC. Genomics 16:416
Gerotto C, Alboresi A, Giacometti GM, Bassi R, Morosinotto T (2012) Coexistence of plant and algal energy dissipation mechanisms in the moss Physcomitrella patens. New Phytol 196:763–773
Gitelson AA, Vina A, Ciganda V, Rundquist DC, Arkebauer TJ (2005) Remote estimation of canopy chlorophyll content in crops. Geophy Res Lett 32(8)
Giudici GNM, Hájek J, Barták M, Kubešová S (2018) Comparative research of photosynthetic processes in selected poikilohydric organisms from Mediterranean and Central-European alpine habitats. Czech Polar Rep 8(2):286–298
Green TGA, Sancho LG, Pintado A (2011) Ecophysiology of desiccation/rehydration cycles in mosses and lichens. In: Lüttge U, Beck E, Bartels D (eds) Plant Desiccation Tolerance, Ecological Studies 215, Part 2. Springer-Verlag, Berlin, pp 89–120
Griffin-Nolan RJ, Zelehowsky A, Hamilton JG, Melcher PJ (2018) Green light drives photosynthesis in mosses. J Bryol 40(4):342–349
Guyot G (1990) Optical properties of vegetation canopies. In: Steven MD, Clark JA (eds) Applications of Remote Sensing in Agriculture. Butterworths, London, pp 19–43
Hamerlynck EP, Tuba Z, Csintalan Z, Nagy Z, Henebry G, Goodin D (2000) Diurnal variation in photochemical dynamics and surface reflectance of the desiccation-tolerant moss, Tortula ruralis. Plant Ecol 151:55–63
Harris A (2008) Spectral reflectance and photosynthetic properties of Sphagnum mosses exposed to progressive drought. Ecohydrol 1:35–42
Heber U (2008) Photoprotection of green plants: a mechanism of ultra-fast thermal energy dissipation in desiccated lichens. Planta 228(4):641–650
Heber U (2012) Conservation and dissipation of light energy in desiccation-tolerant photoautotrophs, two sides of the same coin. Photosynth Res 113:5–13
Heber U, Bilger W, Shuvalov VA (2006) Thermal energy dissipation in reaction centres and in the antenna of photosystem II protects desiccated poikilohydric mosses against photo-oxidation. J Exp Bot 57:2993–3006
Hu R, Xiao L, Bao F, Li X, He Y (2016) Dehydration-responsive features of Atrichum undulatum. J Plant Res 129:945–954
Ino Y (1990) Field measurement of net photosynthesis of mosses at Langhovde, East Antarctica. Ecol Res 5:195–205
Jassey VEJ, Signarbieux C (2019) Effects of climate warming on Sphagnum photosynthesis in peatlands depend on peat moisture and species-specific anatomical traits. Glob Change Biol 25:3859–3870
Jupa R, Hájek J, Hazdrová J, Barták M (2012) Interspecific differences in photosynthetic efficiency and spectral reflectance in two Umbilicaria species from Svalbard during controlled desiccation. Czech Polar Rep 2(1):31–41
Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI, Brestic M, Bussotti F, Calatayud A, Dąbrowski P, Elsheery NI, Ferroni L, Guidi L, Hogewoning SW, Jajoo A, Misra AN, Nebauer SG, Pancaldi S, Penella C, Poli D, Pollastrini M, Romanowska-Duda ZB, Rutkowska B, Serôdio J, Suresh K, Szulc W, Tambussi E, Yanniccari M, Zivcak M (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122:121–158
Klavina L, Springe G, Nikolajeva V, Martsinkevich I, Nakurte I, Dzabijeva D, Steinberga I (2015) Chemical composition analysis, antimicrobial activity and cytotoxicity screening of moss extract (moss phytochemistry). Molecules 20:17221–17243
Kudoh S, Kashino Y, Imura S (2003) Ecological studies of aquatic moss pillars in Antarctic lakes. Light response and chilling and heat sensitivity of photosynthesis. Polar Biosci 16:33–42
Lappalainen NM, Huttunen S, Suokanerva H (2008) Acclimation of a pleurocarpous moss Pleurozium schreberi (Britt.) Mitt. to enhanced ultraviolet radiation in situ. Glob Change Biol 14:321–333
Láska K, Barták M, Hájek J, Prošek P, Bohuslavová O (2011) Climatic and ecological characteristics of deglaciated area of James Ross Island, Antarctica, with a special respect to vegetation cover. Czech Polar Rep 1:49–62
Li J, Li X, Chen C (2014) Degradation and reorganization of thylakoid protein complexes of Bryum argenteum in response to dehydration and rehydration. Bryologist 117:110–118
Lichtenthaler HK, Welburn AR (1983) Determination of total carotenoids and chlorophylls a and b of extracts in different solvents. Biochem Soc T 11:591–592
Lovelock CE, Robinson SA (2002) Surface reflectance properties of Antarctic moss and their relationship to plant species, pigment composition and photosynthetic function. Plant Cell Environ 25(10):1239–1250
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Malenovský Z, Turnbull JD, Lucieer A, Robinson SA (2015) Antarctic moss stress assessment based on chlorophyll content and leaf density retrieved from imaging spectroscopy data. New Phytol 208:608–624
Marschall M, Borbély P, Pné-Kónya E, Süto S (2018) Background processes and the components of photoprotection and regeneration under rehydration in desiccation-tolerant and desiccation-sensitive bryophytes. In: Book for the Plant Biology Europe Conference in Copenhagen, p. 80
May LJ, Parker T, Unger S, Oberbauer SF (2018) Short term changes in moisture content drive strong changes in Normalized Difference Vegetation Index and gross primary productivity in four Arctic moss communities. Remote Sens Environ 212:114–120
Mayaba N, Minibayeva M, Beckett RP (2002) An oxidative burst of hydrogen peroxide during rehydration following desiccation in the moss Atrichum androgynum. New Phytol 155:275–283
Murray KJ, Tenhunen JD, Nowak RS (1993) Photoinhibition as a control on photosynthesis and production of Sphagnum mosses. Oecologia 96(2):200–207
Nabe H, Funabiki R, Kashino Y, Koike H, Satoh K (2007) Responses to desiccation stress in bryophytes and an important role of dithiothreitol – Insensitive non-photochemical quenching against photoinhibition in dehydrated states. Plant Cell Physiol 48(11):1548–1557
Nayaka S, Saxena P (2014) Physiological responses and ecological success of lichen Stereocaulon foliolosum and moss Racomitrium subsecundum growing in same habitat in Himalaya. J Fundam Appl Life Sci 4(3):167–179
Newsham KK, Hodgson DA, Murray AWA, Peat HJ, Lewis Smith RI (2002) Response of two Antarctic bryophytes to stratospheric ozone depletion. Glob Change Biol 8:972–983
Niyogi KK, Li X-P, Rosenberg V, Jung H-S (2005) Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot 56:375–382
Oliver MJ, Mishler BD, Quisenberry JE (1993) Comparative measures of desiccation-tolerance in the Tortula ruralis complex. I. Variation in damage control and repair. Am J Bot 80:127–136
Oliver MJ, Velten J, Wood AJ (2000) Bryophytes as experimental models for the study of environmental stress tolerance: Tortula ruralis and desiccation-tolerance in mosses. Plant Ecol 151:73–84
Oliver MJ, Velten J, Mischler BD (2005) Desiccation tolerance in bryophytes: a reflection of a primitive strategy for plant survival in dehydrating habitats. Integr Comp Biol 45:788–799
Oliver MJ, Hudgeons J, Dowd SE, Payton PR (2009) A combined subtractive suppression hybridization and expression profiling strategy to identify novel desiccation response transcripts from Tortula ruralis gametophytes. Physiol Plant 136:437–460
Paciolla M, Tommasi F (2003) The ascorbate system in two bryophytes: Brachythecium velutinum and Marchantia polymorpha. Biol Plantarum 47:387–393
Pannewitz S, Green TGA, Maysek K, Schlensog M, Seppelt R, Sancho LG, Türk R, Schroeter B (2005) Photosynthetic responses of three common mosses from continental Antarctica. Antarctic Sci 17:341–352
Peng X, Deng X, Tang X, Tan T, Zhang D, Liu B, Lin H (2019) Involvement of Lhcb6 and Lhcb5 in photosynthesis regulation in physcomitrella patens. response to abiotic stress. Int J Mol Sci 20:3665
Pizarro M, Contreras RA, Köhler H, Zúńiga GE (2019) Desiccation tolerance in the Antarctic moss Sanionia uncinata. Biol Res 52:46
Pressel S, Duckett JG (2010) Cytological insights into the desiccation biology of a model system: moss protonemata. New Phytol 185:944–963
Proctor MCF (1990) The physiological basis of bryophyte production. Bot J Linn Soc 104:61–77
Proctor MCF, Ligrone R, Duckett JG (2007a) Desiccation tolerance in the Moss Polytrichum formosum: Physiological and fine-structural changes during desiccation and recovery. Ann Bot 99:1243
Proctor MFC, Oliver MJ, Wood AJ, Alpert P, Stark LR, Cleavitt NL, Mishler BD (2007b) Desiccation-tolerance in bryophytes: a review. Bryologist 110:595–621
Putzke J, Pereira AB (2001) The Antarctic mosses with special reference to the Shetland Island. Lutheran University of Brazil, Editora da Ulbra, Canoas, p 196
Rastogi A, Strozecki M, Kalaji H, Łuców D, Lamentowicz M, Juszczak R (2019) Impact of warming and reduced precipitation on photosynthetic and remote sensing properties of peatland vegetation. Environ Exp Bot 160:71–80
Rastogi A, Antala M, Gąbka M, Rosadzinski S, Stróżecki M, Brestic M, Juszczak R (2020) Impact of warming and reduced precipitation on morphology and chlorophyll concentration in peat mosses (Sphagnum angustifolium and S. fallax). Scientific Rep 10:8592
Robinson SA, Wasley J, Popp M, Lovelock CE (2000) Desiccation tolerance of three moss species from continental Antarctica. Aust J Plant Physiol 27:379–388
Robinson SA, Turnbull JD, Lovelock CE (2005) Impact of changes in natural ultraviolet radiation on pigment composition, physiological and morphological characteristics of the Antarctic moss, Grimmia antarctici. Glob Change Biol 11:476–489
Robinson SA, King DH, Bramley-Alves J, Waterman MJ, Ashcroft MB, Wasley J, Turnbull JD, Miller RE, Ryan-Colton E, Benny T, Mullany K, Clarke LJ, Barry LA, Hua Q (2018) Rapid change in East Antarctic terrestrial vegetation in response to regional drying. Nat Clim Change 8:879–884
Roháček K (2010) Method for resolution and quantification of components of the non-photochemical quenching (qN). Photosynth Res 105:101–113
Roháček K, Barták M (1999) Technique of the modulated chlorophyll fluorescence: basic concepts, useful parameters, and some applications. Photosynthetica 37:339–363
Ronen R, Galun M (1984) Pigment extraction from lichens with dimethyl sulfoxide (DMSO) and estimation of Chlorophyll degradation. Environ Exp Bot 24:239–245
Rouse JW, Haas RH, Schell JA, Deering DW (1974) Monitoring vegetation systems in the Great Plains with ERTS. In: Freden SC, Mercanti EP, Becker M (eds) Third Earth Resources Technology Satellite-1 Symposium Technical Presentations, NASA SP-351, vol I. NASA, Washington, pp 309–317
Russell S (1985) Bryophyte production at Marion Island. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 200–203
Rütten D, Santarius KA (1992) Age-related differences in frost sensitivity of the photosynthetic apparatus of two Plagiomnium species. Planta 187(2):224–229
Schlensog M, Schroeter B (2001) A new method for the accurate in site monitoring of chlorophyll a fluorescence in lichens and bryophytes. Lichenologist 33(5):443–452
Schlensog M, Pannewitz S, Green TGA, Schroeter B (2004) Metabolic recovery of continental antarctic cryptogams after winter. Polar Biol 27:399–400
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. Ecol Stud 100:47–70
Schroeter B, Green TGA, Kulle D, Pannewitz S, Schlensog M, Sancho LG (2012) The moss Bryum argenteum var. muticum Brid. is well adapted to cope with high light in continental Antarctica. Antarct Sci 24:281–291
Seel WE, Baker NR, Lee JA (1992a) Analysis of the decrease in photosynthesis on desiccation of mosses from xeric and hydric environments. Phys Plant 86:451–458
Seel WE, Hendry GAF, Lee JA (1992b) The combined effects of desiccation and irradiance on mosses from xeric and hydric habitats. J Exp Bot 4:1023–1031
Singh J, Singh RP (2014) Adverse effects of UV-B radiation on plants growing at Schirmacher Oasis East Antarctica. Toxicol Int 21(1):101–106
Šinžar-Sekulić J, Sabovljević M, Stevanović B (2005) Comparison of desiccation tolerance among mosses from different habitats. Arch. Biol. Sci 57(3):189–192
Slavov CH, Reus M, Holzwarth AR (2013) Two different mechanisms cooperate in the desiccation-induced excited state quenching in Parmelia lichen. J Phys Chem B 117(38):11326–11336
Smith RCG, Adams J, Stephens DJ, Hick PT (1995) Forecasting wheat yield in a Mediterranean-type environment from the NOAA satellite. Aust J Agric Res 46:113–125
Stark LR, Greenwood JL, Brinda JC, Oliver MJ (2013) The desert moss Pterygoneurum lamellatum (Pottiaceae) exhibits an inducible ecological strategy of desiccation tolerance: effects of rate of drying on shoot damage and regeneration. Am J Bot 100(8):1522–1531
Stella GR (2016) Light stress and photoprotection in green algae, mosses and diatoms. Ph.D. thesis, University of Verona and University Pierre et Marie Curie, 144 p
Stoklasa-Wojtasz A, Rzepka A, Rit G (2012) Responses of mosses species on environment stress factors. In: Grzesiak MT, Rzepka A, Hura T, Grzesiak S (eds) Plant functioning under environmental stress The F Górski Institute of Plant Physiology. Polish Academy of Science, Cracow, pp 69–83
Tatur A, Myrcha A, Fabiszewski J, Niegodzisz J (1997) Formation of abandoned penguin colony ecosystems in maritime Antarctic. Polar Biol 17:405–417
Trnková K, Barták M (2017) Desiccation-induced changes in photochemical processes of photosynthesis and spectral reflectance in Nostoc commune (Cyanobacteria, Nostocales) colonies from polar regions. Phycol Res 65(1):44–50
Tuba Z, Csintalan Z, Proctor MCF (1996) Photosynthetic responses of a moss, Tortula ruralis ssp. ruralis, and the lichens Cladonia convoluta and C. furcata to water deficit and short periods of desiccation, and their ecophysiological significance: a baseline study at present-day concentration. New Phytol 133:353–361
Valøen K (2019) Stochastic rain events increase NDVI through moss water content: a High-Arctic field experiment. Master’s thesis. Norwegian University of Science and Technology, 46
Van Gaalen KE, Flanagan L, Peddle DR (2007) Photosynthesis, chlorophyll fluorescence and spectral reflectance in Sphagnum moss at varying contents. Oecologia 153(1):19–28
Wasley J, Robinson SA, Lovelock C, Popp M (2006) Some like it wet—Biological characteristics underpinning tolerance of extreme water stress events in Antarctic bryophytes. Funct Plant Biol 33(5):443–455
Waterman MJ, Bramley-Alves J, Miller RE, Keller PA, Robinson SA (2018) Photoprotection enhanced by red cell wall pigments in three East Antarctic mosses. Biol Res 51:49
White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59:63–72
Wood AJ (2007) The nature and distribution of vegetative desiccation-tolerance in hornworts, liverworts and mosses. Bryologist 110:163–177
Yamakawa H, Fukushima Y, Itoh S, Heber U (2012) Three different mechanisms of energy dissipation of a desiccation-tolerant moss serve one common purpose: to protect reaction centres against photo-oxidation. J Exp Bot 63:3765–3775
Zhang X (2016) Effects of drought stress and rehydration on chlorophyll fluorescence characteristics of Erythrodontium julaceum (Schwaegr.) par. In areas of puding karst rock desertification. Bangladesh J Bot 45(4):911–917
Zhang X, Zhao Y, Wang S (2017) Responses of antioxidant defense system of epilithic mosses to drought stress in karst rock desertified areas. Acta Geochim 36(2):205–212
Zhou X, Huang W, Kong W, Ye H, Dong Y, Casa R (2017) Assessment of leaf carotenoids content with a new carotenoid index: development and validation on experimental and model data. Int J Appl Earth Obs 57:24–35
Zhou X, Huang W, Zhang J, Kong W, Casa R, Huang Y (2019) A novel combined spectral index for estimating the ratio of carotenoid to chlorophyll content to monitor crop physiological and phenological status. Int J Appl Earth Obs 76:128–142
Zotz G, Kahler H (2007) A moss “canopy”—Small-scale differences in microclimate and physiological traits in Tortula ruralis. Morphol Distrib Funct Ecol Plants Flora 202:661–666
Acknowledgements
The authors thank the ECOPOLARIS and CzechPolar-I and II projects (CZ.02.1.01/0.0/0.0/16_013/0001708, LM2010009 and LM2015078) for providing facilities and the infrastructure used to conduct the research reported in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by M. Horbowicz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orekhova, A., Barták, M., Hájek, J. et al. Species-specific responses of spectral reflectance and the photosynthetic characteristics in two selected Antarctic mosses to thallus desiccation. Acta Physiol Plant 44, 6 (2022). https://doi.org/10.1007/s11738-021-03339-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-021-03339-6