Abstract
Flowering at a suitable time is critical for ensuring reproductive success in the plant life cycle. The transition from vegetative growth to reproduction development is finely tuned by environmental and endogenous signals. To date, control of flowering involves five genetically defined pathways. However, the role of type-A response regulator genes in regulation of this process remains largely unclear. In the present study, we cloned and characterized a type-A response regulator gene (RhRR1) in rose. The expression of RhRR1 significantly increased in axillary bud during the transition from the vegetative growth to the start of floral differentiation, and in rose flowers in response to exogenous cytokinin or 1-methylcyclopropene (1-MCP) treatments, while that expression was markedly repressed by ethylene treatment. RhRR1 has the highest degree of sequence homology to AtARR8 and AtARR9, and is localized in the nucleus. Ectopic expression RhRR1 in Arabidopsis promoted early flowering, accompanied with the less rosette leaf number at bolting, and shorter bolting time after transferring the plants into pots. In addition, the expression of flowering regulatory genes in RhRR1 transgenic Arabidopsis, including FLOWERING LOCUS D, GA REQUIRING 1, LUMINIDEPENDENS, LEAFY, and TWIN SISTER OF FT clearly increased. These results allow us to infer that RhRR1 plays a key role in the control of flowering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The timing of flowering is critical for reproductive success in the plant life cycle. Premature flowering restricts vegetative growth and as a result that plants accumulate insufficient resources (Johansson and Staiger 2015). On the other hand, delayed flowering ultimately delays seed development resulting in progeny that may be affected by the harmful environmental conditions exhibited during the autumn and winter seasons (Johansson and Staiger 2015). Accordingly, the transition from vegetative growth to reproduction development is finely tuned by environmental and endogenous signals (Iwata et al. 2012). To date, five pathways controlling flowering have been defined, including: vernalization, photoperiod, gibberellin pathway, autonomous, and plant age pathways (Srikanth and Schmid 2011). In addition, key genes involved in regulation of flowering time have been identified. FLOWERING LOCUS D (FD) in photoperiod pathway positively regulates flowering through activation of downstream targets, such as APETALA1 (AP1) (Wigge et al. 2005). GA REQUIRING 1 (GA1) catalyzes the first step in gibberellic acid biosynthesis, and is an activator of flowering in gibberellin pathway (Achard et al. 2007). LUMINIDEPENDENS (LD) belonging to autonomous pathway functions in promotion of flowering (Koornneef et al. 1991). VERNALIZATION 2 (VRN2) in vernalization pathway plays a critical role in regulation of flowering via affecting of the methylated state of FLOWERING LOCUS C (FLC) (Gendall et al. 2001). In addition, SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) in age-regulated pathway is also involved in flowering regulation (Wang et al. 2009).
Rosa hybrida (rose) is one of the most valuable ornamental flowers worldwide, with their wide use as cut flowers and landscaping plants (Debener and Linde 2009). Roses are also utilized in several commercial products, such as rose oil, perfume, and food (Kawamura et al. 2011). In China, more than 4 billion stems of cut roses were produced every year (Luo et al. 2013). As a woody perennial plant, roses have the ability to continuously flower under favorable environment conditions; however, understanding of the molecular mechanism that controls this important ornamental characteristic is not well characterized (Iwata et al. 2012). One regulator of this characteristic in rose is TERMINAL FLOWER 1 (TFL1) which plays a role in maintaining vegetative growth and modifying flowering seasonality in roses (Iwata et al. 2012). To identify other genes involved in this mechanism, 13 genes were screened in rose that may function in the transition between the vegetative and floral bud stages, and were implicated in either the gibberellic acid (GA) signaling pathway, control of photoperiod, or floral development (Foucher et al. 2008). However, the role of genes involved in the cytokinin signaling pathway in the regulation of flowering is unknown.
In the past decade, genes which control the critical steps in cytokinin biosynthesis and signaling have been categorized in Arabidopsis thaliana (Arabidopsis) (Uraoa et al. 2000). Histidine kinases (HKs), histidine phosphotransfer proteins (HPs), and response regulators (RRs) constitute the cytokinin signal transduction pathway (Hutchison and Kieber 2002). In Arabidopsis, cytokinin is sensed by the cytokinin-binding CHASE domain of HKs (AHKs), whereby HPs (AHPs) mediate His-to-Asp phosphotransfer from the cytoplasm to the nucleus (Brandstatter and Kieber 1998; Müller and Sheen 2007; Rashotte 2003). Type-B Arabidopsis RRs (ARRs) transcription factors (ARR1, 2, 10–14, and 18–21) then directly modulate expression of type-A ARRs (ARR3-9, 15–17), which are known as the primary cytokinin target genes (Hutchison and Kieber 2002), while the type-A ARRs function to negatively regulate type-B ARRs expression, as a negative feedback loop to control cytokinin response (Hutchison and Kieber 2002). Research has shown that type-A RR genes are involved in regulation of plant growth and development, including the control of the circadian period in Arabidopsis, alteration of rice morphology and cytokinin metabolism, and regulation of cytokinin-modulated rhizoid organogenesis (Hirose et al. 2007; Gao et al. 2013; Salome et al. 2006).
Here, we identified a rose homologue of the type-A RR gene, RhRR1, which plays a critical role in the regulation of flowering in rose by conducting both physiological and molecular analyses, including: (1) measuring the expression pattern of RhRR1 in axillary buds during the transition from vegetative growth to the start of floral differentiation and in rose flowers by exogenous phytohormone treatments, (2) phylogenetic tree construction and protein sequence alignment of RhRR1 with analogues, (3) localization and biological function analysis of RhRR1, and (4) dissection of regulatory mechanism of RhRR1. These findings provide further insight into the role of RhRR1 in regulation of flowering.
Materials and methods
Plant materials and growth conditions
To investigate the expression patterns of RhRR1, cut rose (Rosa hybrida ‘Samantha’) flowers were harvested from a local commercial green house in Beijing. The flowers were transported to the laboratory within 1 h after being harvested. The floral bud initiation stages (stage − 4 to − 1), cut flower opening stages (stage 1–4), and flower senescence stages (stage 5–6) in rose were defined as described previously (Horridge and Cockshull 1974; Ma et al. 2005; Moe and Kristoffersen 1968; Wu et al. 2017). Petal samples were collected from the same middle whorl of the flowers (stages 1–6), and the floral bud samples (stage − 4 to − 1) were collected at different stages of bud development.
For exogenous phytohormone treatments, cut rose flowers at floral stage 2 were used. Flowers were placed in vases with 0.1% dimethyl sulfoxide, and extra supplement with 100 µM abscisic acid (ABA), 100 µM 6-Benzylaminopurine (6-BA), 100 µM jasmonic acid (JA), or 100 µM 1-naphthylacetic acid (NAA) for 24 h. Mock samples were only placed in 0.1% dimethyl sulfoxide. For the ethylene and 1-MCP treatments, rose flowers were treated with 10 µl L−1 ethylene, or 2 µl L−1 1-MCP in an airtight chamber for 24 h, and flowers exposed only to air were used as controls. To prevent CO2 accumulation, 1 mol L−1 NaOH was placed in the chamber (Lü et al. 2014).
Arabidopsis seeds were sterilized, plated on Murashige and Skoog medium, and stratified in the dark for 2 d at 4 °C. The plates were transferred to a growth chamber with temperature maintained at 22 ± 2 °C with 60% relative humidity and a 16 h light/8 h dark photoperiod. After 7 days, the seedlings were grown in pots containing a 1:1 mixture of vermiculite and peat moss (Clough and Bent 1998).
RNA extraction and cloning of RhRR1
Total RNA was extracted from rose petals and floral bud samples as described previously (Wu et al. 2017). Total RNA was extracted from Arabidopsis leaves using an EasyPure Plant RNA kit (Huayueyang Biotechnology Co., Ltd., China). Cloning the full length of RhRR1 was performed by the SMART™ RACE cDNA Amplification Kit (Clontech, USA). Briefly, Two primers, RhRR1-3′-F1 and RhRR1-3′-F2, were designed based on the sequence of RU47281 as two forward primers and were used together with the ‘Universal Primer A Mix’ provided by the Kit to amplify potential RhRR1 3′-sequences. In addition, RhRR1-5′-F1 and RhRR1-5′-F2 were designed as reverse primers, and were used for individual reactions with the ‘Universal primer A Mix’ to amplify the RhRR1 5′-sequence. Finally, the complete sequence of RhRR1 was assembled by the 5′ and 3′ sequences. All of the amplified DNA fragments were inserted into the pMGE-T Easy vector (Promega, USA), and then which were transformed into Escherichia coli DH5α cells before sequencing. All primers mentioned above are listed in Table S1.
Plasmid construction and plant transformation
For the construction of 35S::RhRR1-GFP expression vector, the ORF of RhRR1 was inserted into 35S::GFP vector. A. tumefaciens leaf infiltration and the floral-dip methods were used for tobacco leaves and Arabidopsis transformation, respectively (Batoko et al. 2000; Clough and Bent 1998).
Microscopy
To investigate the subcellular localization of RhRR1, transiently transformed tobacco leaves with 35S::RhRR1-GFP were mounted on glass slides, and were viewed under a confocal laser scanning microscope (CLSM). 35S::GFP vector was used as a control.
Sequence analysis
Protein sequence alignment of RhRR1 with analogues was done through Clustal X and DNAMAN. And phylogenetic tree analysis was performed through MEGA with the calculation pattern of neighbor-joining algorithm with 1000 bootstrap replicates. The amino acid sequences of receiver domain of RhRR1 were used for phylogenetic tree.
Immunoblot assays
Arabidopsis protein extraction and immunoblot analysis assays were executed as described previously (Meng et al. 2014). Briefly, ground Arabidopsis leaves mixed with 2 × SDS sample buffer-containing 5% β-mercaptoethanol were boiled at 100 °C for 5 min. The above supernatant was collected after centrifuge at 13,000g for 5 min. To detect RhRR1-GFP protein, western blot analysis was executed by probing with Rabbit anti-GFP antibody (diluted 1:2000) and anti-Rabbit HRP (horse radish peroxidase), respectively. Finally, GFP was detected using ECL-detecting reagent (WBKLS0500).
Data analysis
Data were analyzed by one-way ANOVA and SPSS version 16.0 (SPSS Inc., USA). Effects were indicated to be significant if the p value was less than 0.05. Three biological and technical replicates were performed.
Results
Cloning and characterization of RhRR1
To investigate the function of type-A response regulator genes in rose, four putative type-A response regulator transcripts, including RU28916, RU12149, RU47281, and RU02862, were identified from an ethylene-treated rose petal transcriptome database (Wu et al. 2017). Transcripts of these genes in petals during various floral development stages (stages 1–6) were evaluated by RT-PCR. As shown in Fig S1, among four genes, the expression pattern of RU47281 exhibited a significant decrease following floral stage 4. Therefore, we selected RU47281 for further analysis.
To clone the full-length cDNA of RU47281 from rose, the RACE amplification method was applied. RU47281 is 1292 bp in length with a 738 bp predicted open reading frame (ORF), encoding a deduced protein of 246 amino acids (27.39 kDa, pI 4.88). The RU47281 sequence was submitted to GenBank under the accession number KY014463. Sequence alignment showed that RU47281 contained a conserved receiver domain with three invariant residues ‘DDK’ and a variable region of 11–21 amino acids. Subsequently, the gene corresponding to RU47281 was named Rosa hybrida RR1 (RhRR1). RhRR1 has a divergent C-terminus, less than 100 amino acids long, and lacks the GARP domain and a Glu- and Pro-rich region (Fig. 1a). To assess the similarity between RhRR1 and Arabidopsis type-A RR proteins, we constructed a phylogenetic tree by the neighbor-joining (NJ) method. RhRR1 had a high degree of sequence homology to AtARR8 and AtARR9 (Fig. 1b).
A phylogenetic tree and protein sequence alignment of RhRR1 with analogues. a Protein sequence alignment of RhRR1 with analogues; the red highlighted region indicates the conserved receiver domain. The three invariant residues ‘DDK’ among all type-A RRs are marked with asterisk. Amino acid sequences are from Fragaria vesca FvRR8 (GenBank accession number XP_004290880.1), Prunus mume PmRR9 (XP_008219624.1), Malus domestica MdRR9 (XP_008382445.1), Pyrus x bretschneideri PbRR9 (XP_009351080.1), and Arabiposis thaliana AtARR8 (1009112986) and AtARR9 (1009119738). b A phylogenetic tree of RhRR1 with all type-A response regulator proteins of Arabidopsis was constructed by MEGA5 software through the neighbor-joining method (1000 bootstrap replications). The amino acid sequences of receiver domain of RhRR1 were used for phylogenetic tree
Localization of RhRR1
To investigate the characteristics of RhRR1, the subcellular localization of RhRR1 was tested in Nicotiana benthamiana through a transient expression assay. Leaf pavement cells of transiently transformed tobacco showed that RhRR1-GFP was localized in nucleus (Fig. 2). This suggests that RhRR1 acts as a nuclear protein that may function as a transcriptional regulator.
Expression pattern of RhRR1 gene
To investigate the expression pattern of RhRR1 gene, transcript levels of RhRR1 were evaluated in rose from floral bud initiation to floral senescence, and in rose flowers after treatment with exogenous phytohormones using qRT-PCR. RhRR1 expression markedly increased in the axillary bud during transition from vegetative growth stage (stage − 4) to the start of floral differentiation (stage − 3), and then gradually decreased (Fig. 3a). In addition, RhRR1 expression was clearly induced by cytokinin or 1-MCP treatments, while ethylene treatment significantly decreased its expression. Abscisic acid (ABA), 1-naphthylacetic acid (NAA), and jasmonic acid (JA) treatments did not alter RhRR1 expression (Fig. 3b).
Expression patterns of RhRR1. a Quantitative RT-PCR analysis of RhRR1 expression in rose from floral initiation to flower senescence. Floral initiation stages (stage − 4 to − 1), flower opening stages (stages 1–4), and flower senescence stages (stages 5–6). b Quantitative RT-PCR analysis of RhRR1 expression in rose flowers in response to exogenous phytohormones. RhActin5 was used as an internal control. Error bars represent the standard deviation of three biological replicates. Letters indicate significant differences according to Duncan’s multiple range test (p < 0.05), and asterisks indicate significant differences according to Student’s t test (*p < 0.05, **p < 0.01)
A characteristic of floral development that has been previously shown is that buds at the top of the stem flower much earlier than those further down (Johnson 1924). Therefore, we also evaluated the time course of RhRR1 expression in five axillary buds of the same stem (named 1–5 from top to bottom) after removal of the terminal flower by qRT-PCR. The expression level of RhRR1 increased from the fourth axillary bud to the third axillary bud, but then decreased significantly (Fig. S2). This RhRR1 expression trend was similar to that of RhRR1 in the axillary bud during transition from the vegetative growth stage (stage − 4) to the start of floral differentiation (stage − 3) (Fig. 3a). Based on the results of expression pattern of RhRR1, we speculated that RhRR1 may play a key role in regulation of flowering.
Phenotype of transgenic Arabidopsis overexpression RhRR1
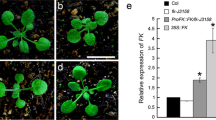
To evaluate the role of RhRR1 in development, we generated an Arabidopsis transgenic line overexpressing RhRR1-GFP. T2 homozygous plants were used for analysis. Protein levels of RhRR1 in both wild-type and overexpression lines were assessed using western blot probed with anti-GFP antibody (Fig. 4b). Line #1, #4, and #6 were used for further characterization. Overexpression of RhRR1 in Arabidopsis did not show any changes in morphogenesis, including formation of roots, stem, leaves, and flowers of adult plants, but showed early or premature flowering time (Fig. 4a). Accordingly, the rosette leaf number at bolting was clearly less in RhRR1-ox plants than that in the wild type, with only 7.50 ± 0.41 leaves in line 6# compared with 11.80 ± 0.87 leaves in the wild type (Fig. 4c). In addition, the bolting timing after transferring the plants into pots was also significantly different between wild-type and transgenic lines, with only 7.10 ± 0.36 days in line 6# compared with 10.78 ± 0.61 days in the wild type (Fig. 4d).
Ectopic expression of RhRR1 in Arabidopsis. a Phenotypes of 20-day-old seedlings of wild-type and transgenic plants. b Immunoblot analysis of RhRR1-GFP in Arabidopsis leaves. 10 µg of total protein were loaded in each lane. A Coomassie Brilliant Blue-stained blot (CBB) is shown to confirm equivalent sample loading. c Rosette leaf number at bolting in wild-type and 35S:: RhRR1-GFP transgenic plants. d The bolting timing after transferring the plants into pots. Error bars represent the standard deviation of three biological replicates. Letters indicate significant differences according to Duncan’s multiple range test (p < 0.05)
Transcripts of key genes involved in flowering regulation
To uncover the mechanism of RhRR1 in the regulation of flowering, the expressions of six key genes belonging to five flowering pathways in Arabidopsis were evaluated by qRT-PCR. Transcripts of FD in the photoperiod pathway, GA1 in the GA pathway, LD in the autonomous pathway, and the floral integrators LEAFY (LFY) markedly increased in the overexpression lines than that in the wild type (Fig. 5a–d), while no markedly changes of a flowering integrator gene FT expression were observed (Fig. 5e). Intriguingly, the TSF transcripts, the FT paralogue, also significantly increased in the overexpression lines (Fig. 5f). Although the expression of RhRR1 was induced by cytokinin treatment in rose flowers in Fig. 3b, it is unclear whether the early flowering phenotype in RhRR1 overexpression lines was related to cytokinin regulation. To confirm it, the expressions of two cytokinin signaling pathway genes ARR4 and ARR5 were evaluated in transgenic Arabidopsis. However, no significant difference of the transcripts of ARR4 and ARR5 was measured between wild-type and RhRR1 overexpression lines (Fig. 3s).
The expression levels of flowering regulatory genes in Arabidopsis. Quantitative RT-PCR analysis of expression of FD (a), GA1 (b), LD (c), FT (d), LFY (e), and TSF (f) in 10-day-old Arabidopsis leaves. TUB2 was used as an internal control. Error bars represent the standard deviations of three biological replicates. Asterisks indicated significant differences according to Duncan’s multiple range test (p < 0.05)
Discussion
Plants adjust the time of floral initiation for successful reproduction in their environment (Iwata et al. 2012; Johansson and Staiger 2015; Srikanth and Schmid 2011). Several genes involved in regulation of flowering have been identified and characterized in detail, but cytokinin response regulator gene function in regulation of flowering has not yet illuminated (Srikanth and Schmid 2011). Our results imply that RhRR1 is a member of the type-A response regulator family, and is involved in the regulation of flowering in rose. This is supported by the expression pattern of RhRR1, which was significantly increased in axillary bud during the vegetative growth stage (stage − 4) transition to the start of floral differentiation (Fig. 3). In addition, the early flowering phenotype exhibited by overexpression of RhRR1 in Arabidopsis, as shown in Fig. 4, which was accompanied by a decreased rosette leaf number at bolting and shorter bolting time also demonstrated the role of RhRR1 in rose floral development. Although we targeted RhRR1 to analyze its function in flowering, other type-A RRs in rose may also play similar role, especially those showing trends similar to RhRR1. Therefore, future experiments are required to determine whether other type-A RRs in rose have functions in controlling flowering.
Although cytokinins (CTKs) act as anti-senescence factors in flowers, cytokinin also promotes flowering in plants, including rose (Wang et al. 2002; Wu et al. 2017; Zeng et al. 2013). The expressions of type-A response regulator genes, including ARR3, ARR5, ARR7, and ARR8, are known to increase in response to cytokinin treatment; however, their function in controlling of flowering is not clear (Lee et al. 2007; Li et al. 2013; Osakabe et al. 2002; Salome et al. 2006). In Rosa hybrida, the transcripts of RhRR1 were also induced by exogenous cytokinin treatment (Fig. 3b). In addition, the expression of RhRR1 significantly decreased by ethylene treatment and induced by ethylene inhibitor 1-MCP treatment (Fig. 3b). This expression pattern of RhRR1 is similar to the expression patterns exhibited by type-A RRs in Arabidopsis, such as ARR5, ARR7, and ARR15, in response to ethylene treatment (Shi et al. 2012). Ethylene is an important plant hormone, involved in the regulation of various developmental processes including seed germination, flowering, and organ senescence (Pei et al. 2013). It was also reported that ethylene is related to flowering in pineapple (Ananas comosus) (Burg and Burg 1966). Based on the expression patterns of RhRR1, we speculated that RhRR1 may function as a key node to integrate ethylene and cytokinin signals in regulation of plant flowering.
Altered expression of five flowering time activator genes including FD, GA1, LD, LFY, and TSF was observed in Arabidopsis leaves with overexpression RhRR1, suggesting that RhRR1 may play a critical role in modulating floral initiation via affecting the photoperiod pathway, GA pathway, and autonomous pathway (Fig. 5). Phylogenetic analysis revealed that the determined amino acid sequence of RhRR1 showed high similarity to those of AtARR8 and AtARR9 in Arabidopsis, containing a conserved receiver domain with three invariant residues ‘DDK’ (Fig. 1a). In addition, a subcellular localization assay showed that RhRR1 is localized in the nucleus and may act as a transcriptional regulator (Fig. 2a). The localization may underlie the regulation of flowering by RhRR1 via regulation of genes involved in several flowering pathways.
Interestingly, the expression of FLOWERING LOCUS T (FT), a flowering time activator, was not significantly difference between RhRR1 overexpression lines and wild type (Fig. 5e). However, the expression level of the paralogue of FT, TWIN SISTER OF FT (TSF), was significantly increased in RhRR1 overexpression lines (Fig. 5f). This may be because the promotion of flowering by cytokinin in Arabidopsis does not require FT, but rather activates its paralogue, TSF, as well as FD, which encodes a partner of TSF (D’Aloia et al. 2011). Our findings provide a new insight that a type-A response regulator gene in rose, RhRR1, plays crucial role in regulation of flowering through controlling of expression of FD, GA1, LD, LFY, and TSF.
Author contribution statement
LYQ and LHL designed the experiment. FM and JYC performed the experiments and interpreted the results. WL and JYS wrote the paper.
References
Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, Harberd NP (2007) DELLAs contribute to plant photomorphogenesis. Plant Physiol 143:1163–1172. https://doi.org/10.1104/pp.106.092254
Batoko H, Zheng HQ, Hawes C, Moore I (2000) A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12:2201–2217. https://doi.org/10.1105/tpc.12.11.2201
Brandstatter I, Kieber JJ (1998) Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell 10:1009–1019. https://doi.org/10.1105/tpc.10.6.1009
Burg SP, Burg EA (1966) Auxin-induced ethylene formation: its relation to flowering in the pineapple. Science 152:1269. https://doi.org/10.1126/science.152.3726.1269
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
D’Aloia M, Bonhomme D, Bouche F, Tamseddak K, Ormenese S, Torti S, Coupland G, Perilleux C (2011) Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. Plant J 65:972–979. https://doi.org/10.1111/j.1365-313X.2011.04482.x
Debener T, Linde M (2009) Exploring complex ornamental genomes: the rose as a model plant. Crit Rev Plant Sci 28:267–280. https://doi.org/10.1080/07352680903035481
Foucher F, Chevalier M, Corre C, Soufflet-Freslon V, Legeai F, Hibrand-Saint Oyant L (2008) New resources for studying the rose flowering process. Genome 51:827–837. https://doi.org/10.1139/G08-067
Gao B, Fan L, Li X, Yang H, Liu F, Wang L, Xi L, Ma N, Zhao L (2013) RcRR1, a Rosa canina type-A response regulator gene, is involved in cytokinin-modulated rhizoid organogenesis. PLoS One 8:e72914. https://doi.org/10.1371/journal.pone.0072914
Gendall AR, Levy YY, Wilson A, Dean C (2001) The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107:525–535. https://doi.org/10.1016/S0092-8674(01)00573-6
Hirose N, Makita N, Kojima M, Kamada-Nobusada T, Sakakibara H (2007) Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol 48:523–539. https://doi.org/10.1093/pcp/pcm022
Horridge JS, Cockshull KE (1974) Flower initiation and development in the glasshouse rose. Sci Hortic 2:273–284. https://doi.org/10.1016/0304-4238(74)90036-3
Hutchison CE, Kieber JJ (2002) Cytokinin signaling in Arabidopsis. Plant Cell 14(suppl 1):s47–s59. https://doi.org/10.1105/tpc.010444
Iwata H, Gaston A, Remay A, Thouroude T, Jeauffre J, Kawamura K, Oyant LH, Araki T, Denoyes B, Foucher F (2012) The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J 69:116–125. https://doi.org/10.1111/j.1365-313X.2011.04776.x
Johansson M, Staiger D (2015) Time to flower: interplay between photoperiod and the circadian clock. J Exp Bot 66:719–730. https://doi.org/10.1093/jxb/eru441
Johnson DS (1924) The influence of insolation on the distribution and on the developmental sequence of the flowers of the giant cactus of Arizona. Ecology 5:70–82. https://doi.org/10.2307/1929166
Kawamura K, Hibrand-Saint Oyant L, Crespel L, Thouroude T, Lalanne D, Foucher F (2011) Quantitative trait loci for flowering time and inflorescence architecture in rose. Theor Appl Genet 122:661–675. https://doi.org/10.1007/s00122-010-1476-5
Koornneef M, Hanhart CJ, Van der Veen JH (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet 229:57–66. https://doi.org/10.1007/BF00264213
Lee DJ, Park JY, Ku SJ, Ha YM, Kim S, Kim MD, Oh MH, Kim J (2007) Genome-wide expression profiling of ARABIDOPSIS RESPONSE REGULATOR 7 (ARR7) overexpression in cytokinin response. Mol Gen Genet 277:115–137. https://doi.org/10.1007/s00438-006-0177-x
Li Y, Kurepa J, Smalle J (2013) AXR1 promotes the Arabidopsis cytokinin response by facilitating ARR5 proteolysis. Plant J 74:13–24. https://doi.org/10.1111/tpj.12098
Lü P, Zhang C, Liu J, Liu X, Jiang G, Jiang X, Khan MA, Wang L, Hong B, Gao J (2014) RhHB1 mediates the antagonism of gibberellins to ABA and ethylene during rose (Rosa hybrida) petal senescence. Plant J 78:578–590. https://doi.org/10.1111/tpj.12494
Luo J, Ma N, Pei H, Chen J, Li J, Gao J (2013) A DELLA gene, RhGAI1, is a direct target of EIN3 and mediates ethylene-regulated rose petal cell expansion via repressing the expression of RhCesA2. J Exp Bot 64:5075–5084. https://doi.org/10.1093/jxb/ert296
Ma N, Cai L, Lu W, Tan H, Gao J (2005) Exogenous ethylene influences flower opening of cut roses (Rosa hybrida) by regulating the genes encoding ethylene biosynthesis enzymes. Sci China Ser C Life Sci 48:434. https://doi.org/10.1360/062004-37
Meng Y, Ma N, Zhang Q, You Q, Li N, Ali Khan M, Liu X, Wu L, Su Z, Gao J (2014) Precise spatio-temporal modulation of ACC synthase by MPK6 cascade mediates the response of rose flowers to rehydration. Plant J 79:941–950. https://doi.org/10.1111/tpj.12594
Moe R, Kristoffersen T (1968) The effect of temperature and light on growth and flowering of Rosa ‘Baccara’ in greenhouses. Acta Hortic 14:157–166. https://doi.org/10.17660/actahortic.1969.14.16
Müller B, Sheen J (2007) Advances in cytokinin signaling. Science 318:68–69. https://doi.org/10.1126/science.1145461
Osakabe Y, Miyata S, Urao T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2002) Overexpression of Arabidopsis response regulators, ARR4/ATRR1/IBC7 and ARR8/ATRR3, alters cytokinin responses differentially in the shoot and in callus formation. Biochem Bioph Res Co 293:806–815. https://doi.org/10.1016/S0006-291X(02)00286-3
Pei H, Ma N, Tian J, Luo J, Chen J, Li J, Zheng Y, Chen X, Fei Z, Gao J (2013) An NAC transcription factor controls ethylene-regulated cell expansion in flower petals. Plant Physiol 163:775–791. https://doi.org/10.1104/pp.113.223388
Rashotte AM (2003) Expression profiling of cytokinin action in Arabidopsis. Plant Physiol 132:1998–2011. https://doi.org/10.1104/pp.103.021436
Salome PA, To JP, Kieber JJ, McClung CR (2006) Arabidopsis response regulators ARR3 and ARR4 play cytokinin-independent roles in the control of circadian period. Plant Cell 18:55–69. https://doi.org/10.1105/tpc.105.037994
Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S (2012) Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 24:2578–2595. https://doi.org/10.1105/tpc.112.098640
Srikanth A, Schmid M (2011) Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci 68:2013–2037. https://doi.org/10.1007/s00018-011-0673-y
Uraoa T, Yamaguchi-Shinozakia K, Shinozakib K (2000) Two-component systems in plant signal transduction. Trends Plant Sci 5:67–74. https://doi.org/10.1016/S1360-1385(99)01542-3
Wang GY, Yuan MF, Hong Y (2002) In vitro flower induction in roses. Vitro Cell Dev Biol Plant 38:513–518. https://doi.org/10.1079/IVP2002340
Wang JW, Czech B, Weigel D (2009) miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138:738–749. https://doi.org/10.1016/j.cell.2009.06.014
Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309:1052–1056. https://doi.org/10.1126/science.1115983
Wu L, Ma N, Jia Y, Zhang Y, Feng M, Jiang CZ, Ma C, Gao J (2017) An ethylene-induced regulatory module delays flower senescence by regulating cytokinin content. Plant Physiol 173:853–862. https://doi.org/10.1104/pp.16.01064
Zeng S, Liang S, Zhang YY, Wu KL, Teixeira da Silva JA, Duan J (2013) In vitro flowering red miniature rose. Biol Plant 57:401–409. https://doi.org/10.1007/s10535-013-0306-4
Acknowledgements
This work was supported by National Natural Science Foundation of China (31701972), Natural Science Foundation of Chongqing Municipal Science and Technology Commission (cstc2017jcyjAX0233), the Science and Technology Research Program of Chongqing Education Commission of China (KJ1711276), the Foundation for High-Level Talents of Chongqing university of Arts and Sciences (R2016TZ04), and Chongqing Horticulture Key Discipline (CQCDXK220170828-4). In addition, we sincerely appreciated Deka Mohamed, PhD candidate from University of Toronto Scarborough, for proofreading our manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by P. Wojtaszek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, L., Feng, M., Jia, Y. et al. Involvement of cytokinin response regulator RhRR1 in the control of flowering. Acta Physiol Plant 41, 121 (2019). https://doi.org/10.1007/s11738-019-2903-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2903-0