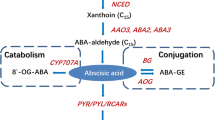
Abstract
Phytohormones regulate numerous aspects of plant growth and development. Green-mature banana fruit were treated with deionized water (control), abscisic acid (ABA), indole-3-acetic acid (IAA) and ABA + IAA, respectively, to investigate the role of ABA and IAA in fruit ripening. Results showed that ABA accelerated fruit ripening, but IAA delayed the process. However, treatment of ABA + IAA showed little difference in fruit color and firmness. The acceleration of ABA and delay of IAA on banana ripening process seems to be neutralized by ABA + IAA. Digital gene expression revealed that ABA + IAA treated fruit maintained the similar color phenotype with the control by regulating the expression of chlorophyll degradation-related gene PaO (GSMUA_Achr6G25590_001), and carotenoid biosynthesis-related genes DXR (GSMUA_Achr3G20790_001) and PSY (GSMUA_Achr2G12480_001, GSMUA_Achr4G17270_001, GSMUA_Achr4G17290_001). Moreover, ABA + IAA treated fruit maintained the similar softening phenotype with the control by adjusting the expression of pectin degradation-related genes PME (GSMUA_Achr3G05740_001) and PL (GSMUA_Achr6G28160_001, GSMUA_Achr7G04580_001). ABA + IAA treatment nearly abolished the action of individual ABA or IAA through equilibrating the expression of specific genes involved in chlorophyll degradation, carotenoid biosynthesis and pectin degradation pathways in the postharvest ripening of banana. The interaction between ABA and IAA might exercise as an antagonistic mechanism of neutralizing the specific gene expression either induced by ABA or reduced by IAA in the postharvest ripening of banana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Fruit ripening results from developmentally and physiologically marked changes of expressed and/or suppressed genes, which finally cause transformations of color, flavor, texture, aroma, and nutritional value of the flesh that make fruit visually attractive and edible (Bapat et al. 2010; Klee et al. 2011). Banana fruit respond to ethylene by inducing rapid changes of color, flavor, sweetness and nutritional composition (Du et al. 2016). Despite the predominant regulatory role of ethylene in climacteric fruit ripening, ABA and auxin also function as significant hormones in controlling fruit ripening (Galpaz et al. 2008; Su et al. 2015). Numerous evidences revealed that exogenous ABA could promote fruit maturation and ABA content reached to the maximum level before ripening. ABA accelerated banana ripening associated with color change and softening (Jiang et al. 2000). Many researches have suggested that ABA act as an upstream regulatory factor for the initiation of fruit ripening by triggering ethylene production (Zhang et al. 2009; Leng et al. 2009). At the same time, ABA may induce the expression of many ethylene-independent genes (Zhang et al. 2009). Indole-3-acetic acid (IAA), an auxin compound, has been reported in many studies in regulating fruit ripening. Exogenous IAA reduced fruit softening through suppressing activities of various cell wall hydrolases in banana (Seemi et al. 2004). Application of exogenous IAA in the pre-ripening stage delayed ripening in both climacteric fruits and non-climacteric fruits (Su et al. 2015; Bottcher et al. 2012; Symons et al. 2012).
However, current studies largely focused on the function of a single plant hormone on fruit ripening. Researches about cross-talk of different phytohormones involved in fruit ripening were very limited. Many studies have showed that ethylene and auxin interacted with each other to regulate many physiological processes in plants (Liu et al. 2005; Muday et al. 2012). Result that gene expression in the auxin-related pathway regulated by ethylene and gene expression in the ethylene-related pathway regulated by auxin demonstrated an ethylene–auxin interaction in tomato and peach (Jones et al. 2002; Trainotti et al. 2007; Li et al. 2017). The induction of ethylene-related ACS and ethylene-response-factor2 (ctg2116) genes by NAA was more evident than that by ethylene. Aux/IAA-related genes were either up- (as ctg671 and ctg42) or down-regulated (as ctg57 and ctg84) by both NAA and ethylene (Trainotti et al. 2007). Our previous study indicated that a combination of exogenous ABA and IAA did not affect the ripening of strawberry fruit, suggesting receptor-like kinases and ubiquitin ligases played a role in the cross-talk between ABA and IAA (Chen et al. 2015). Nevertheless, the underlying mechanisms of the interaction between ABA and IAA in fruit are not yet deciphered.
In this study, we employed Illumina RNA sequencing to analyze banana after application of exogenous ABA, IAA or ABA + IAA. Digital gene expression (DGE) method was actualized to elucidate the molecular regulatory mechanisms of the interaction between ABA and IAA in the postharvest banana ripening process.
Materials and methods
Plant material and treatments
Banana fruit (Musa acuminate L. AAA group, cv. Brazilian) were harvested from Hainan Province of China at 80–85% maturity (100–110 days after flower shoot formation). For each experiment, 128 banana fingers from 8 hands (16 fingers each hand) were equally divided into four groups to avoid difference in ripening behaviors among different hands (Choudhury et al. 2008). All selected fingers were surface sanitized by immersing in 1% sodium hypochlorite solution for 1 min, then dipping in 0.05% Sporgon for 3 min to prevent fungal disease, and followed by rinsing three times in ddH2O and brief surface drying at room temperature. Fingers of four groups were vacuum-infiltrated with 1.0 μmol/L ABA, 1.0 μmol/L IAA, mixture of 1.0 μmol/L ABA and 1.0 μmol/L IAA, and H2O (control), respectively, in a vacuum container (SHZ-D III, Mingyuan Instrument Co., Ltd., China). The vacuum-infiltration condition (80 kPa, 20 min) and concentration of ABA and IAA were based on Vendrell (1985) and our preliminary experiments. After infiltration, fingers were washed three times by ddH2O and surface-dried at room temperature. All the finger samples were kept in polyethylene plastic bags (0.01 mm thickness) at 20 °C and 90% relative humidity (RH) in the dark for 7 days. After color and firmness determination, the fruit peel was frozen in liquid nitrogen and stored at – 80 °C for further analyses. Three biological replicates were performed in this study.
Determination of color and firmness
The Chroma meter CR-400 (Konica Minolta Sensing Inc., Osaka, Japan) was used to measure peel color at four locations around equatorial region on each fruit finger. L*, a*, b* were recorded and results were expressed as H* (hue angle). The firmness of fruit peels was measured by the TA-XT2i Texture Analyzer (Stable Micro Systems Ltd., Surrey, UK) with a 5-mm-diameter flat probe. The penetration depth was 10 mm at a rate of 1 mm/s. Firmness was recorded as N/cm2.
Total chlorophyll and carotenoids
Chlorophyll and carotenoids were detected according to Zhu et al. (2014). The peel sample was ground to powder in liquid nitrogen, and mixed with hexane/acetone (60:40, v/v) over night at 4 °C in the dark. The mixture was centrifuged at 10,000g for 30 min at 4 °C. The cell debris was repeatedly extracted with fresh solvent until colorless and the supernatants were measured using a UV–VIS spectrophotometer (UV-1750, Shimadzu Corp., Japan), meanwhile hexane was used as the blank control. The pigment contents (total chlorophyll and carotenoid) were calculated in the following equations: chlorophyll (mg/ml) = 8.02 OD663 + 20.2 OD647, carotenoids (mg/ml) = OD450/0.25.
RNA extraction
Total RNA was extracted from the peels using the cetyltrimethylammonium bromide (CTAB) method (Jaakola et al. 2001). The peel sample was ground to powder under liquid nitrogen. A total of 1 g of the powder was added into 4 ml of preheated 2% CTAB extraction buffer with 2% (v/v) β-mercaptoethanol, and centrifuged at 10,000g for 10 min at 4 °C after incubating at 65 °C for 10 min. The supernatant was extracted with an equal volume of chloroform and the mixture was then centrifuged at room temperature. After centrifugation, the supernatant was mixed with 0.2 volume of 12 mol/l LiCl and stored overnight at 4 °C. The supernatant was centrifuged at 10,000g for 20 min at 4 °C, and the RNA was dissolved in preheated SSTE buffer. The liquid was added with chloroform and then centrifuged at room temperature. RNA was precipitated by mixing the solution with two volumes of pre-chilled absolute ethanol at – 80 °C for 30 min. After centrifugation, the RNA pellet was washed with pre-chilled 75% ethanol. At last, the RNA pellets were dissolved in RNase-free water. NanoDrop 2000 was used to check the quality of the RNA samples.
cDNA library construction and Illumina sequencing
The total RNA was constructed by four individual cDNA libraries, which represented four treatments with six fruit fingers in each group. This study provided two biological replicates for each treatment. A total amount of 3 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA) according to the recommendations by the manufacturer. AMPure XP system and Agilent Bioanalyzer 2100 system were used to analyze the PCR products.
After cluster generation of the index-coded samples using TruSeq PE Cluster Kit v3-cBot-HS (Illumina), the qualified libraries were sequenced on Illumina Hiseq 2000 platform in two lanes and 100 bp single-end reads were generated. After removing the low-quality reads, the clean reads were aligned to the Musa acuminate genome (ftp://ftp.ensemblgenomes.org/pub/release-23/plants/fasta/musa_acuminata/dna/).
Bioinformation analysis
The expression levels of all genes were estimated by RSEM (Li and Dewey 2011). Differential expression analysis of two samples was performed using the DEGseq (differential expressed genes) R package (1.18.0) (Wang et al. 2010). P value was adjusted using Q value (Storey and Tibshirani 2003). Fragments per kilobase of transcript per million mapped reads (FPKM) values of one or more were considered as differentially expressed genes.
For further analysis, gene ontology (GO) enrichment analysis was performed by the GOseq R package. GO terms with corrected P value less than 0.05 were considered significantly. GO-annotated unigenes belonged to the biological processes, molecular functions, and cellular components. Kyoto encyclopedia of genes and genomes (KEGG) database provided resource to understand high-level functions and utilities of the biological systems. The statistical enrichment of DEGs in KEGG pathways was tested by the software KOBAS.
Results
Influence of ABA and IAA on banana ripening
The color (hue angle) and firmness in banana peels gradually decreased during the whole storage period. Yellowing and softening processes were slowed down by IAA, but accelerated by ABA, while there was little difference between ABA + IAA and control (Fig. 1a–c). Total chlorophyll content in the peel decreased rapidly while carotenoids increased during storage. Comparing with the control, ABA-treated fruit showed to be much lower in chlorophyll content as well as higher in carotenoid content, while IAA-treated fruit had higher chlorophyll and lower carotenoid content. However, ABA + IAA treated fruit showed similar chlorophyll content and carotenoid content with control (Fig. 1d, e). ABA accelerated the ripening process and IAA delayed the process. However, the ripening phenotype of ABA + IAA groups was similar with control.
Differentially expressed genes in response to ABA and IAA
Volcano plot showed overall distribution of differentially expressed genes in four groups. The numbers of genes showing statistically significant changes were plotted in Figure S1. Compared to control, there were 185 differentially expressed genes in ABA groups, including 67 up-regulated genes and 118 down-regulated genes (Fig. S1A). As high as 904 genes were differentially expressed in IAA group, containing 482 up-regulated genes and 422 down-regulated genes (Fig. S1B). In addition, there were 292 differential genes expressed in ABA + IAA treatment, involving 186 up-regulated genes and 106 down-regulated genes (Fig. S1C).
Mixture of ABA and IAA showed a different transcript pattern from the individual ABA or IAA (Fig. S2A). Venn diagrams displayed that there was an overlap of 44 differentially expressed genes between ABA and IAA, 56 genes between ABA and ABA + IAA, and 144 genes between IAA and ABA + IAA. There was an overlap of 16 genes among ABA, IAA and ABA + IAA groups (Fig. S2B). As shown in Figure S2, there were 108 differentially expressed genes only found in ABA + IAA group, which were mostly involved in glucose metabolism pathway, plant hormone pathway, amino acid metabolism pathway.
In addition, top 30 most enriched GO terms (q < 0.05) of each group were selected from all the terms. Figure S3A showed that 131 differentially expressed genes in ABA group were found in 14 biological processes and 16 molecular functions (Fig. S3A). There were 614 differentially expressed genes in IAA group found in 15 biological terms and 15 molecular function terms (Fig. S3B). For ABA + IAA group, 204 differentially expressed genes were found in 19 biological processes, 8 molecular functions and especially 3 cellular components, including integral to membrane, intrinsic to membrane and extracellular to membrane (Fig. S3C). All the enriched GO and gene information were displayed in Table S1.
Furthermore, all DEGs were subjected to KEGG pathway enrichment analysis in order to identify specific pathways affected by exogenous ABA and IAA. Analysis suggested that the DEGs of three groups contained genes associated with many different KEGG pathways (Table S2). There were three up-regulated and four down-regulated KEGG pathways (q < 0.05) following the application of exogenous ABA. Seven up-regulated and two down-regulated KEGG pathways were considered to be the most enriched pathways when exogenous IAA was applied. In addition, three up-regulated and seven down-regulated KEGG pathways were highly enriched by ABA + IAA.
Differentially expressed genes involved in the chlorophyll degradation pathway
Banana color change from green to yellow was accompanied with chlorophyll degradation that was regulated by a series of enzymes. As shown in Fig. 2a, chlorophyll degradation was catalyzed mainly by chlorophyllase (CLH), metal chelating substance (MCS), pheophytinase (PPH), pheophorbide an oxygenase (PaO) and red chlorophyll catabolite reductase (RCCR). RNA-seq analysis showed six PaO genes, one CLH gene and one RCCR gene were expressed in this study (Fig. 2b, Table S3).
Analysis of the putatively identified genes involved in chlorophyll degradation pathway. a Schematic diagram of chlorophyll degradation. b Expression heatmap of 8 genes encoding enzymes in chlorophyll degradation pathway in control, ABA, IAA and ABA + IAA banana fruit. c Fragments per kilobase of exon per million fragments mapped (FPKM) levels determined by RNA-seq of PaO in control, ABA, IAA and ABA + IAA banana fruit. Dotted lines represent multistep reactions. CLH, chlorophyllase; MCS, metal chelating substance; PPH, pheophytinase; PaO, pheophorbide a oxygenase; RCCR, red chlorophyll catabolite reductase
PaO has been considered the key enzyme during chlorophyll degradation, which catalyzes the opening of the porphyrin ring of pheophorbide a, forming the primary red chlorophyll catabolite (RCC) (Ma et al. 2012). Transcript of PaO (GSMUA_Achr6G25590_001) was highly up-regulated by ABA, down-regulated by IAA, but little influenced by ABA + IAA (Fig. 2c). The expression pattern of PaO (GSMUA_Achr6G25590_001) was highly synchronized with chlorophyll content.
Differentially expressed genes involved in the carotenoid biosynthesis pathway
Apart from chlorophyll degradation, color change from green to yellow was also related with the accumulation of carotenoids during ripening (Jaiswal et al. 2016). Carotenoids biosynthesis including carotene and lutein was obtained by a series of catalytic reactions (Fig. 3a). Heat map showed the putatively identified genes involved in carotenoid biosynthesis pathway (Fig. 3b, Table S3).
Analysis of the putatively identified genes involved in carotenoid biosynthesis pathway. a Schematic diagram of carotenoid biosynthesis. b Heatmap showed the expression of 20 genes encoding enzymes in this pathway in control, ABA, IAA and ABA + IAA banana fruit. FPKM levels were determined by RNA-seq of DXPS (c), DXR (d) and PSY (e–g) in control, ABA, IAA and ABA + IAA banana fruit. Dotted lines represent multistep reactions. DXPS, 1-deoxy-d-xylulose-5-phosphate synthase; DXR, 1-deoxy-d-xylulose-5-phosphate reductoisomerase; PSY, phytoene synthase; PDS, phytoene desaturase; Z-ISO, zeta-carotene isomerase; ZDS, zeta-carotene desaturase; CRTISO, carotenoid isomerase; LCYB, lycopene beta cyclase
In carotenoid biosynthesis pathway, one 1-deoxy-d-xylulose-5-phosphate synthase (DXPS) gene, one 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR) gene, and three phytoene synthase (PSY) genes were detected. Transcripts of DXPS (GSMUA_Achr5G24730_001) in ABA-treated fruit and IAA-treated fruit were detected, while no expression of DXPS was detected in control fruit and ABA + IAA treated fruit (Fig. 3c).
ABA treatment promoted the transcript of DXR (GSMUA_Achr3G20790_001), but IAA treatment inhibited its expression in fruit. However, the influence of ABA + IAA on DXR (GSMUA_Achr3G20790_001) expression was not significant comparing with control (Fig. 3d).
Three PSY genes (GSMUA_Achr2G12480_001, GSMUA_Achr4G17270_001, GSMUA_Achr4G17290_001_001) all showed the similar expression patterns in different treatments (Fig. 3e–g). ABA slightly promoted the expressions of PSYs, but IAA obviously inhibited their expressions. However, ABA + IAA did not appear to be influencing the transcripts of PSYs.
Differentially expressed genes involved in the pectin degradation pathway
Fruit softening is related with degradation of cell wall polysaccharides through activities of hydrolases (Han et al. 2016). Pectin, as a kind of polysaccharides, is the main component of cell wall in fruit (Fig. 4a). Heat map showed the putatively identified genes involved in pectin degradation pathway (Fig. 4b, Table S3).
Analysis of the putatively identified genes involved in pectin degradation pathway. a Schematic diagram of pectin degradation. b Heatmap showed the expression of 43 genes encoding enzymes in this pathway in control, ABA, IAA and ABA + IAA banana fruit. FPKM levels were determined by RNA-seq of PME (c), PL (d, e) in control, ABA, IAA and ABA + IAA banana fruit. PME, pectin methylesterase; PL, pectate lyase; PG, polygalacturonase
Our data revealed that ABA promoted the expression of pectin methylesterase (PME) gene (GSMUA_Achr3G05740_001), which played a role in production of pectate from pectin due to the hydrolysis of methylester groups, but IAA inhibited the expression of PME (GSMUA_Achr3G05740_001) (Fig. 4c). No significant difference on PME (GSMUA_Achr3G05740_001) transcript level was found between ABA + IAA treated fruit and the control fruit (Fig. 4c). Digalacturonate was produced via pectate lyase (PL) from pectate (Fig. 4a). The expression of PL gene (GSMUA_Achr6G28160_001, GSMUA_Achr7G04580_001) with application of ABA was higher than that of control, and the expression of PL gene with application of IAA was lower than that of control (Fig. 4d, e). However, the PL (GSMUA_Achr6G28160_001, GSMUA_Achr7G04580_001) mRNA level showed a little change in response to ABA + IAA treatment (Fig. 4d, e).
Discussion
Phytohormones play key roles in regulation of fruit ripening. However, the information on the interplays between ABA and IAA on banana ripening is still limited (Klee and Giovannoni 2011; Seymour et al. 2013; Du et al. 2016). Present study showed that ABA promoted banana fruit ripening, while IAA delayed the process. However, mixture of ABA and IAA showed little influence on the ripening process.
Banana ripening was associated with a series of metabolic processes, which led to yellowing and softening. Degradation of chlorophyll and accumulation of carotenoids were thought to be the main reason for yellowing of banana fruit. PaO pathway has been described to be the major chlorophyll catabolic pathway for various plant species (Ma et al. 2012; Guyer et al. 2014). PaO, a key control enzyme in the overall regulation of chlorophyll degradation, showed different expression patterns when fruit responded to different stresses (Chung et al. 2006; Mittelberger et al. 2017). Present data showed that ABA promoted the mRNA level of PaO (GSMUA_Achr6G25590_001) but IAA suppressed its expression. However, the mRNA level of PaO (GSMUA_Achr6G25590_001) in ABA + IAA treated fruit was maintained at similar levels with the control fruit. This result also confirmed that PaO (GSMUA_Achr6G25590_001) played an important role in the fruit coloration of banana fruit.
Chlorophyll breakdown was usually accompanied by carotenoid accumulation during fruit ripening. Carotenoids were synthesized by a series of reactions catalysed by many enzymes, including DXPS, DXR, PSY, PDS. In this study, the transcript level of DXR (GSMUA_Achr3G20790_001) was highly induced by ABA, but reduced by IAA. However, its expression following ABA + IAA application tended to be close to the control. PSY played a role in production of phytoene from two geranylgeranyldiphosphate (GGPS) molecules (Ruiz-Sola et al. 2014). Present data showed that three PSY (GSMUA_Achr2G12480_001, GSMUA_Achr4G17270_001, GSMUA_Achr4G17290_001) genes transcripts were slightly accumulated by ABA, but significantly inhibited by IAA. However, the amounts of PSYs expression in ABA + IAA treatment were close to the control. Treatment of ABA + IAA might maintain the color in banana peel by regulating the expressions of DXR and PSY to neutralize the influence of individual ABA or IAA.
Pectins are a family of galacturonic acid-rich polysaccharide and galacturonic acid (Albersheim et al. 1996; Christiaens et al. 2016). PME and PL have been considered as the important enzymes in degradation of pectin (Cação et al. 2012; Cao 2012). Pectin could be de-esterified by a major hydrolase, PME (Wen et al. 2013). PL catalyzes the cleavage of glycosidic α-(1,4) linkages via a β-elimination reaction mechanism, which lead to the formation of a double bond between C-4 and C-5 of the newly formed nonreducing end. In this study, IAA significantly inhibited the expression of PME and PL. There was no significant differences between ABA + IAA treated fruit and the control fruit on PME (GSMUA_Achr3G05740_001) and PL (GSMUA_Achr6G28160_001, GSMUA_Achr7G04580_001) transcript levels. In addition, pectin contains many polysaccharide-formed galactoses, such as galactans and arabinans. Genes encoding enzymes involved in degradation of galactans and arabinans showed no significant differences. These results indicated that ABA + IAA treatment exhibited little influence on softening phenotype by maintaining the transcript levels of PME (GSMUA_Achr3G05740_001) and PL (GSMUA_Achr6G28160_001, GSMUA_Achr7G04580_001).
The response of fruits to the mixture of ABA and IAA can be either cooperative or antagonistic between the two hormones. The results showed that PaO, DXR, PSY mRNA levels in ABA + IAA treated fruit were close to the control, which was consistent with the coloration process. In addition, PME and PL mRNA levels in ABA + IAA treated fruit were close to the control, which was also consistent with the softening phenotype. Therefore, ABA + IAA treatment might inhibit the action of the individual ABA or IAA through the antagonistic mechanism of neutralizing the specific gene expression either induced by ABA or reduced by IAA in the postharvest ripening of banana.
Author contribution statement
MLC and LWJ conceived and designed the experiments. LWJ, CJX, HXY and RXC performed the experiments. LWJ analyzed the data and wrote the manuscript. YTJ and LZS also have contributed to the data interpretation and writing.
References
Albersheim P, Darvill AG, O’Neill MA, Schols HA, Voragen AGJ (1996) An hypothesis: the same six polysaccharides are components of the primary cell walls of all higher plants. Pectins and pectinases. Elsevier Sciences B.V., Amsterdam, pp 47–53
Bapat VA, Trivedi PK, Ghosh A, Sane VA, Ganapathi TR, Nath P (2010) Ripening of fleshy fruit: molecular insight and the role of ethylene. Biotechnol Adv 28:94–107
Bottcher C, Boss PK, Davies C (2012) Delaying riesling grape berry ripening with a synthetic auxin affects malic acid metabolism and sugar accumulation, and alters wine sensory characters. Funct Plant Biol 39(9):745–753
Cação SM, Leite TF, Budzinski IG, dos Santos TB, Scholz MB, Carpentieri-Pipolo V, Domingues DS, Vieira LG, Pereira LF (2012) Gene expression and enzymatic activity of pectin methylesterase during fruit development and ripening in Coffea arabica L. Genet Mol Res 11(3):3186–3197
Cao J (2012) The pectin lyases in Arabidopsis thaliana: evolution, selection and expression profiles. PLoS ONE 7(10):e46944
Chen J, Tan RK, Guo XJ, Fu ZL, Wang Z, Zhang ZY, Tan XL (2015) Transcriptome analysis comparison of lipid biosynthesis in the leaves and developing seeds of Brassica napus. PLoS ONE 10(5):e0126250
Choudhury SR, Roy S, Sengupta DN (2008) Characterization of transcriptional profiles of MA-ACS1 and MA-ACO1 genes in response to ethylene, auxin, wounding, cold and different photoperiods during ripening in banana fruit. J Plant Physiol 165(18):1865–1878
Christiaens S, Van Buggenhout S, Houben K, Jamsazzadeh Kermani Z, Moelants KR, Ngouémazong ED, Van Loey A, Hendrickx ME (2016) Process-structure-function relations of pectin in food. Crit Rev Food Sci Nutr 56(6):1021–1042
Chung DW, Pruzinská A, Hörtensteiner S, Ort DR (2006) The role of pheophorbide a oxygenase expression and activity in the canola green seed problem. Plant Physiol 142(1):88–97
Du L, Song J, Forney C, Palmer LC, Fillmore S, Zhang Z (2016) Proteome changes in banana fruit peel tissue in response to ethylene and high-temperature treatments. Hortic Res 3:16012. https://doi.org/10.1038/hortres
Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J (2008) Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J 53(5):717–730
Guyer L, Hofstetter SS, Christ B, Lira BS, Rossi M, Hörtensteiner S (2014) Different mechanisms are responsible for chlorophyll dephytylation during fruit ripening and leaf senescence in tomato. Plant Physiol 166(1):44–56
Han Y, Ban Q, Hou Y, Meng K, Suo J, Rao J (2016) Isolation and characterization of two persimmon xyloglucan endotransglycosylase/hydrolase (XTH) genes that have divergent functions in cell wall modification and fruit postharvest softening. Front Plant Sci 7:624. https://doi.org/10.3389/fpls.2016.00624 (eCollection)
Jaakola L, Pirttilä AM, Halonen M, Hohtola A (2001) Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol 19(2):201–203
Jaiswal P, Jha SN, Kaur PP, Bhardwaj R, Singh AK, Wadhawan V (2016) Prediction of textural attributes using color values of banana (Musa sapientum) during ripening. J Food Sci Technol 51(6):1179–1184
Jiang Y, Joyce DC, Macnish AJ (2000) Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. J Plant Growth Regul 19(1):106–111
Jones B, Frasse P, Olmos E, Zegzouti H, Li ZG, Latche A, Pech JC, Bouzayen M (2002) Down-regulation of DR12, an IAA-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J 32:603–613
Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59
Leng P, Zhang GL, Li XX, Wang LH, Zheng ZM (2009) Cloning of 9-cis-epoxycarotenoid dioxygenase (NCED) gene encoding a key enzyme during abscisic acid (ABA) biosynthesis and ABA-regulated ethylene production in detached young persimmon calyx. Sci Bull 54(16):2830–2838
Li B, Dewey C (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform 12:323
Li JY, Tao XY, Bu JW, Ying TJ, Mao LC, Luo ZS (2017) Global transcriptome profiling analysis of ethylene-auxin interaction during tomato fruit ripening. Postharvest Biol Technol 130:28–38
Liu KD, Kang BC, Jiang H, Moore SL, Li HX, Watkins CB et al (2005) A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol Biol 58(4):447–464
Lohani Seemi, Trivedi Prabodh K (2004) Pravendra nath.: changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol Technol 31:119–126
Ma N, Ma X, Li A, Cao XC, Kong LG (2012) Cloning and expression analysis of wheat pheophorbide a oxygenase gene; TaPaO. Plant Mol Biol Report 30(5):1237–1245
Mittelberger C, Yalcinkaya H, Pichler C, Gasser J, Scherzer G, Erhart T, Schumacher S, Holzner B, Janik K, Robatscher P, Müller T, Kräutler B, Oberhuber M (2017) Pathogen-induced leaf chlorosis: products of chlorophyll breakdown found in degreened leaves of Phytoplasma-infected apple (malus× domestica borkh.) and apricot (Prunus armeniaca L.) trees relate to the pheophorbide a oxygenase/phyllobilin pathway. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.6b05501 (Epub ahead of print)
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17(4):181–195
Ruiz-Sola MÁ, Arbona V, Gómez-Cadenas A, Rodríguez-Concepción M, Rodríguez-Villalón A (2014) A root specific induction of carotenoid biosynthesis contributes to ABA production upon salt stress in arabidopsis. PLoS ONE 9(3):e90765
Seymour GB, Ostergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit development and ripening. Annu Rev Plant Biol 64:219–241
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100:9440–9445
Su L, Diretto G, Purgatto E, Danoun S, Zouine M, Li Z et al (2015) Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol 15(1):114
Symons GM, Chua YJ, Ross JJ, Quittenden LJ, Davies NW, Reid JB (2012) Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot 63:4741–4750
Trainotti L, Tadiello A, Casadoro G (2007) The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot 58:3299–3308
Vendrell M (1985) Effect of abscisic acid and ethephon on several parameters of ripening in banana fruit tissue. Plant Sci 40:19–24
Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26:136–138
Wen B, Ström A, Tasker A, West G, Tucker GA (2013) Effect of silencing the two major tomato fruit pectin methylesterase isoforms on cell wall pectin metabolism. Plant Biol (Stuttg) 15(6):1025–1032
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60(6):1579–1588
Zhu MK, Chen GP, Zhou S, Tu Y, Wang Y, Dong TT et al (2014) A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol 55(1):119–135
Acknowledgements
This research is supported by the National Natural Science Foundation of China (31772365) and the National Basic Research Program (973 program) of China (2013CB127101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J.-H. Liu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2018_2621_MOESM1_ESM.tif
Figure S1. Volcano plot showing the number of unigenes significantly differentially expressed in response to exogenous ABA (A), IAA (B) and ABA+IAA (C). Red dots represents up regulated genes, green dots indicates down regulated genes. Supplementary material 1 (TIFF 473 kb)
11738_2018_2621_MOESM2_ESM.tif
Figure S2. H cluster (A) and Venn diagram (B) showing the distribution of differentially-expressed genes that are unique or common among treatments of ABA, IAA and ABA+IAA. Supplementary material 2 (TIFF 179 kb)
11738_2018_2621_MOESM4_ESM.xlsx
Table S1. All the enriched GO and genes information of banana peel in response to exogenous ABA and IAA. Supplementary material 4 (XLSX 30 kb)
11738_2018_2621_MOESM5_ESM.xlsx
Table S2. All the enriched KEGG and genes information of banana peel in response to exogenous ABA and IAA. Supplementary material 5 (XLSX 53 kb)
11738_2018_2621_MOESM6_ESM.xls
Table S3. Significant expressed genes involved in pigment metabolism and pectin degradation pathways. Supplementary material 6 (XLS 55 kb)
Rights and permissions
About this article
Cite this article
Lu, W., Mao, L., Chen, J. et al. Interaction of abscisic acid and auxin on gene expression involved in banana ripening. Acta Physiol Plant 40, 46 (2018). https://doi.org/10.1007/s11738-018-2621-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2621-z