Abstract
The aim of this study was to examine the effect of abscisic acid (ABA), sucrose, and auxin on grape fruit development and to assess the mechanism of these three factors on the grape fruit ripening process. Different concentrations of ABA, sucrose, and auxin were used to treat the grape fruit, and the ripening-related indices, such as physiological and molecular level parameters, were analyzed. The activity of BG protein activity was analyzed during the fruit development. Sucrose, ABA, and auxin influenced the grape fruit sugar accumulation in different ways, as well as the volatile compounds, anthocyanin content, and fruit firmness. ABA and sucrose induced, but auxin blocked, the ripening-related gene expression levels, such as softening genes PE, PG, PL, and CELL, anthocyanin genes DFR, CHI, F3H, GST, CHS, and UFGT, and aroma genes Ecar, QR, and EGS. ABA, sucrose, and glucose induced the fruit dry weight accumulation, and auxin mainly enhanced fruit dry weight through seed weight accumulation. In the early development of grape, starch was the main energy storage; in the later, it was glucose and fructose. Sucrose metabolism pathway-related gene expression levels were significant for glucose and fructose accumulation. BG protein activity was important in the regulation of grape ABA content levels. ABA plays a core role in the grape fruit development; sucrose functions in fruit development through two pathways: one was ABA dependent, the other ABA independent. Auxin blocked ABA accumulation to regulate the fruit development process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit development and ripening mechanism are complex processes. They involved lots of physiological changes, such as softening, coloring, and aroma appearance (Giovannoni 2001; Seymour et al. 2013). In these processes, plant hormone was important in regulating these physiological changes and induced the substrate translation (Su et al. 2015; Jia et al. 2011; Li et al. 2012). Sugar, in addition to being a carbon source, provided energy for plant organ growth, which could be metabolized in the fruit for lots of substance (Huang et al. 2016; Ai et al. 2016; Qin et al. 2016). In a manner similar to classical plant hormones, sugars acted as signaling molecules that controlled gene expression and developmental processes in plants. The emerging scenario in plants pointed to the existence of a complex signaling network that interconnected transduction pathways from sugars, other hormones, and nutrient signals (Solfanclli et al. 2006; Huang et al. 2016). These signal transduction pathways crossed to regulate the plant respiration, metabolism, growth, tissue development, and senescence (Solfanclli et al. 2006). In the plant tissues, fruit was the most important target for the researcher. So the sugar and hormone were very important for fruit quality during fruit development and ripening.
According to the patterns of respiration and ethylene production during maturation and senescence, fruits were classified as climacteric and non-climacteric (Giovannoni 2001). The mechanism of climatic fruit ripening had been understood that climatic fruits such as tomato showed an increase in ethylene production in association with increased rates of respiration during the ripening process (Giovannoni 2001; Giovannoni 2004; Alexander and Grierson 2002). In these fruits, the burst of ethylene production was essential as it coordinates the transcription and translation of many ripening-related genes and downstream proteins were responsible for normal fruit ripening. Abscisic acid (ABA) was also associated with ethylene production and fruit development (Zhang et al. 2009). However, the signal transduction in non-climacteric fruit has not been revealed. Such as in pepper (Lee et al. 2010) and grape (Villalobos-González et al. 2016), there were no respiratory burst, and ethylene responded little to the fruit maturation. A lot of reports used the physiological method to show that ABA (Zhang et al. 2009), methyl jasmonic acid (MeJA) (Jia et al. 2015), and brassinosteroids (BR) (Symons et al. 2006; Fu et al. 2008) accelerated the process of grape skin coloring. Auxin, as a classic plant hormone, could block ABA effect on the plant growth. For instance, the endogenous ABA levels had been shown to increase after veraison, but the treatment of the synthetic anxin-like compound on grape fruit delayed this increase and led to the delay in the onset of ripening (Corso et al. 2016), suggesting that the content of both endogenous hormones ABA and auxin coordinately played a role in the regulation of grape fruit ripening. However, little is known about their controlling fruit ripening mechanism or whether other plant growth regulators are involved in the process of fruit ripening. These results suggested that the non-climacteric fruit ripening maybe controlled by the integration of multiple signals, but there were no intensive researches on the molecular mechanism of grape fruit ripening.
In recent years, with the continuous development of test technology, a growing number of studies have shown that sugar could be used as a signal to participate in the regulation of every aspect of plant growth and development (Gibson 2005; Solfanclli et al. 2006; Baker et al. 2016; AI et al. 2016). Sugar could induce the expression of transcription factors. For example, the SUSIBA2 transcription factor of the WRKY superfamily could be induced by sucrose, and SUSIBA2 binds to the SURE and W box components in the iso1 gene promoter, controlling the starch accumulation in wheat endosperm (Sun et al. 2003). Sucrose and ABA could together regulate the expression of some genes. Cakir et al. (2003) found that in grapes, a gene named ASR (ABA-stress-ripening induced, ASR) associated with maturity was induced strongly by sucrose and ABA existing at the same time. This suggested that the sucrose linked with the ABA signal transduction system regulated the expression of functional genes in plant growth and development.
In this study, we presented the sucrose metabolism pathway in fruit development, and the sugar accumulation was affected by ABA and auxin in different ways. The ABA and sucrose metabolism-related gene expression levels were also different in the fruit development, and ABA and sucrose both promoted the grape fruit ripening. The NCED gene played an important role in the early fruit development of ABA accumulation, and the BG protein functioned better in the later fruit development. Auxin blocked the ABA accumulation, thus blocking the fruit coloring and softening. The purified VvBG protein of ABA biosynthesis released more freely active ABA in grape fruit to promote fruit ripening.
Results
Morphological and physiological changes during grape fruit development
Based on previous reports, and the seeds’ state, we defined seven developmental visual stages of grape fruit in 14 weeks post flowering (Wpf): at about 2, 4, 6, 8, 10, 12, and 14 Wpf, respectively (Fig. 1a). We observed that the size of the grape fruit had two swellings and rapid growth stages from 4 to 8 Wpf followed by 10 to 14 Wpf and one slow growth stage from 8 to 10 Wpf (Fig. 1b). However, the proportion of dry material increased continually from 4 to 12 Wpf, especially from 10 to 12 Wpf (Fig. 1c), whereas levels of soluble sugars (sucrose, glucose, and fructose), particularly glucose and fructose, showed rapid increase from 8 Wpf in the skin and 4 Wpf in the flesh and skin. But the sucrose content which increased a little from 6 to 14 Wpf was very low compared to glucose and fructose (Fig. 1d, e). Although sucrose accumulation of the sucrose transporter protein gene (VvSUTs) and sucrose phosphate synthase (SPS) increased during grape fruit development (Fig. S1A-C and Fig. 2a), the high levels of sucrose degradation enzyme activity of sucrose acid invertase (AI) and neutral invertase (NI) made the sucrose cleaved into other sugars quickly in grape (Fig. 2b, c). Notably, we observed under the electron microscope that there was a lot of starch granule present in the chloroplast in the fruit flesh and skin during the early fruit development, but it disappeared quickly with the fruit development (Fig. 3). Further research showed that the levels of starch declined continually at 2 Wpf in the flesh and skin, but in the skin, there was a peak of starch content on 8 Wpf (Fig. 4a), and in general the starch content was higher in the skin than that in the flesh. The starch metabolism-related enzyme activity of starch synthesis adenosine diphosphate glucose pyrophosphoyrlase (ADPase) (Fig. 4b) and starch degradation α-amylase (AMY) (Fig. 4c) and β-amylase (BMY) (Fig. 4d) also supports this view.
Morphological and physiological changes in the receptacle of grape fruit during developmental processes divided into the following seven stages: 2, 4, 6, 8, 10, 12, and 14 weeks post flowering. a Fruit color and seed state. b Changes in fruit size. c Changes in fruit dry weight and the dry weight percentage. d Changes in soluble sugar contents in fruit skin. e Changes in soluble sugar contents in fruit flesh (sucrose, glucose, and fructose). Values are means + SD of four biological replicates
Ultrastructrural changes in the skin and flesh of grape fruit during its development. ① Skin cell at 14 days after flowering, bar = 1 μm. ② Flesh cell at 14 days after flowering, bar = 1.2 μm. ③ Skin cell at 42 days after flowering, bar = 1 μm. ④ Flesh cell at 42 days after flowering, bar = 1.5 μm. ⑤ Skin cell at 70 days after flowering, bar = 0.7 μm. ⑥ Flesh cell at 70 days after flowering, bar = 0.8 μm. CW cell wall; PP polyphenolic; V vacuole; N nucleus; Ch chloroplast; M mitochondrion; OG osmiophilic globule; VE vesicle; PM plasma membrane; T tonoplast; ML middle lamella; ICS intercellular space; S starch granule
Changes in starch content and various kinds of sugar metabolism-associated enzyme activity in grape fruit. a Starch content in grape skin and flesh 1 to 12 weeks post flowering. b Changes in adenosine diphosphate-glucose pyrophosphoyrlase (ADPase). c α-Amylase (AMY). d β-Amylase (BMY) enzyme activity in grape skin and flesh. Values are means + SD of four biological replicates
The effect of sucrose, ABA, and auxin on grape fruit coloring and softening
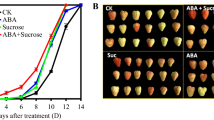
In order to investigate the function of sucrose, ABA, and auxin on the fruit development and ripening process, different concentrations of sucrose (8, 16, and 25 mM), glucose (222, 444, and 666 mM), and mannitol (666 mM) as a control were used to treat the fruit of 7 Wpf, as well as ABA (100 and 200 μM), ABA inhibitor nordihydroguaiaretic acid (NDGA) (100 mM), auxin (indole-3-acetic, IAA) (100 and 200 μM), and IAA transport inhibitor 1-N-naphthylphthalamic acid (NPA) (100 μM), and water as a control. The results showed that ABA, sucrose, and glucose markedly increased the anthocyanin content and stimulated cell wall metabolism enzyme activity (Table 1), and sucrose had much better effect than glucose in the regulation of fruit coloring and softening. NDGA and IAA inhibited the fruit ripening compared to water, but NPA had no more effect on the fruit ripening (Table 1). Notably, ABA or sucrose obviously promoted the grape berry ripening progress; conversely, IAA delayed this progress (Fig. 5). In order to further verify it, fruit dry weight was measured, and the results showed that ABA promoted the dry weight accumulation remarkably, compared to water or mannitol 20 or 30 days after treatment (Fig. 6a–c), and 30 days after treatment, glucose (222, 444, and 666 mM) and sucrose (16 mM) both promoted the dry weight accumulation and sucrose functioned much better (Fig. 6b). In the former stage, low concentration of glucose (222 mM) and sucrose (8 mM) and high sucrose concentration of 25 mM induced the soaking of fruit in the water to enhance the fresh weight of the fruit. ABA (100 and 200 μM) and a low concentration of IAA (100 μM) promoted the dry weight accumulation (Fig. 6c). IAA promoted the dry weight accumulation through enhancing the seed sugar accumulation (Table 2), but reduced the sugar content in other fruit tissues, suggesting that an appropriate IAA is necessary for the fruit ripening progress. High concentration of IAA (200 μM), ABA inhibitor NDGA, and IAA inhibitor NPA reduced the dry weight. And the IAA inhibitor NPA helped in soaking the fruit in the water compared to other tissues (Fig. 6a, b). Compared to the corresponding control, the IAA-, ABA-, and sucrose-treated fruit exhibited a large decrease and increase in sugar accumulation (Table 2). Different concentrations of ABA, sucrose, and glucose promoted the fruit tissue sugar accumulation in different ways (Table 2). Furthermore, ABA, sucrose, and IAA led to an increase and decrease, respectively, in the contents of alkanes, alcohol, ester, amine, and benzene compounds (such as 3-ethylhexane, octane,4-methyloctane, 1-hexanol, benzeneethanol, hexadecanoic acid, butyl ester, 1,2-benzenedicarboxylic acid, dibutyl ester, 4-octyl-N-(4-octylphenyl)-benzenamine, butylated hydroxytoluene, etc.), and conversely a decrease and increase in the contents of others such as alkanes, ketone, and ester compounds (such as 2,3,7-trimethyldecane, nonadecane, heptadecane, 2,6,10,15-tetramethyl, 5-methyl-5-propylnonane, tetratetracontane, tetracosane, 9-hexyl-heptadecane, 3-ethyl-5-(2-ethylbutyl)-octadecane, 2-methyl-2-hepten-4-one, 1,2-benzenedicarboxylic acid dibutyl ester, etc.) (Table 3). Based on the maturity of grape fruits containing more concentrations of multiple alkanes, alcohol, ester, amine, or benzene compounds and lower concentrations of some amount of alkanes, ketone, or ester compounds, overall, the changes of anthocyanin, aroma, and cell wall metabolism in the sucrose-, ABA-, or IAA-treated fruits collectively suggested that sucrose, ABA, and IAA promoted and inhibited grape fruit ripening progress, respectively.
Influence of exogenous ABA, IAA, sucrose, or ABA + sucrose on the grape berry ripening progress. Berries were treated with these exogenous concentrations of 100 μM ABA and IAA and 16 mM sucrose at 0, 8, and 16 post the first treatment. Percent colored berries were calculated for each observation time by counting the numbers of berries showing any degree of color development
Exogenous hormone and sugars on the grape fruit dry weight. a Twenty days after treatment. b Thirty days after treatment, 100 and 200 μm of ABA, 100 μm of ABA inhibitor NDGA, 100 and 200 μm of auxin (IAA), and 100 μm of auxin transport inhibitor NPA. c Thirty days after treatment, 222, 444, and 666 mM of glucose; 8, 16, and 25 mM of sucrose; and 666 mM of mannitol were used to treat the pre-veraison grape berry for three times, respectively. Asterisks indicate statistically significant differences at P < 0.05 as determined by Student’s test

Consistent with the observation of the changed patterns of the ripening-associated physiological parameters as described above, ABA, sucrose, and IAA remarkably modulated a series of gene expression levels implicated in fruit color and cell wall and aroma metabolism, including several anthocyanin synthesis-associated genes chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), UDP-glycose:flavonoid 3-o-glycosyltransgerase (UFGT), dihydroflavonol-4-reductase (DFR), and glutathione S-transferase (GST) (Fig. 7a); softening genes pectin esterase (PE), cellulose (Cell), polygalacturonase (PG), and pectate lyase (PL) (Fig. 7b); and aroma genes (E)-beta-caryophyllene synthase (ECar), eugenol synthase (EGS), and quinine oxidoreductase (QR) in a converse manner (Fig. 7c). Similar to sucrose, the sucrose analog turanose also induced the ripening-related gene expression levels (Fig. 8). In order to know if there are genes that are independent of ABA signal transduction that can be induced by sucrose, the ABA synthesis was blocked by NDGA and in the meantime induced by sucrose. The results showed that VvCHS, VvF3H, VvUFGT, VvPG, VvPL, VvSAAT, and VvQR were induced by sucrose + NDGA (Fig. 8). Collectively, these results implicated that ABA, sucrose, and IAA coordinately regulate the grape fruit development and ripening progress.
Exogenous sucrose, ABA, and IAA on fruit ripening-associated gene expression levels. Concentrations of 100 μm of ABA and IAA and 16 mM sucrose treat the pre-veraison grape berry. Seven days later, gene expression levels were analyzed. a Anthocyanin-related gene expression levels. b Softening-related gene expression levels. c Aroma synthesis-related gene expression levels. qRT-PCR was conducted using Vv18s as an internal control. Error bars represent the SE (n = 3). Asterisks indicate statistically significant differences at P < 0.05 as determined by Student’s test
Exogenous sucrose analog turanose, sucrose + NDGA on fruit ripening-associated gene expression levels. Concentrations of 16 mM turanose and 16 mM sucrose + 100 μm NDGA treat the pre-veraison grape berry. Seven days later, gene expression levels were analyzed. qRT-PCR was conducted using Vv18s as an internal control. Error bars represent the SE (n = 3). Asterisks indicate statistically significant differences at P < 0.05 as determined by Student’s test
The gene expression of sucrose, ABA, and IAA metabolism
In order to know the roles of main regulator factors in the fruit development, the ABA, sucrose, and IAA metabolism pathways in the fruit development were analyzed. The energy storage in grapes is in two forms: sugar and starch. Starch is mainly produced in the early stage of fruit development, and sugar is in the later stage. During the fruit development, there was a peak of ADPase, that is, starch synthesis enzyme, at 8 Wpf in the skin and not in the flesh, as well as the synthesis gene expression levels of VvAPS and VvAPL (Fig. S2A-B), but the starch contents of degradation enzymes VvAMY and VvBMY were opposite to the starch content in the skin and flesh—the same with their coding genes VvAMY and VvBMY (Fig. S2C-D). There are three sucrose transporter genes, namely, VvSUT1, VvSUT2, and VvSUT3, which had different expression levels during fruit development. The VvSUT1 and VvSUT2 gene expression increased from 2 to 14 Wpf; on the contrary, the VvSUT3 gene expression increased from 2 to 14 Wpf (Fig. S1A-C). The sucrose synthesis gene expression of sucrose phosphate synthase (SPS) increased from 4 to 8 Wpf (Fig. S1D), but then it reduced until ripening in the skin and flesh. For the sucrose degradation metabolism, different from the invertase gene expression patterns, the high levels of sucrose acid and neutral invertase activity increased from 2 to 14 Wpf in the flesh and skin, indicating that sucrose was cleaved quickly and hardly stored (Fig. S1D and 2B–C). ABA metabolism is a critical step during fruit development. There are three NCED genes of ABA synthesis found in grapes: VvNCED1, VvNCED2, and VvNCED3. The VvNCED2 gene expression levels were consistent with the fruit ABA content changes, but the other two VvNCED1 and VvNCED3 genes’ expression decreased and increased during fruit development, respectively; however, the ABA degradation-related gene CYP707A transcript levels were also consistent with ABA content to balance the ABA content during fruit development (Fig. 9a, b). The ABA receptor PYR which controls the intensity of ABA increased during fruit development and had a reduction in the ripening fruit, whereas the expression levels of negative regulator VvPP2C and VvSnRK2.6 genes existing in the ABA signal transduction pathway all declined during fruit development (Fig. 9c). Consistent to the auxin content in the fruit, the gene expression of the auxin transporter VvPin gene expression level also declined with grape fruit development and had an expression peak at 10 Wpf (Figs. 9a, c). Three genes were found coding the BG protein which are VvBG1, VvBG2, and VvBG3. The VvBG1 gene expression levels increased from 2 to 14 Wpf; however, VvBG2 or VvBG3 gene expression levels declined in the fruit ripening (Fig. 10).
The relationship of sucrose, ABA, and IAA
In order to analyze the relationship of sucrose, ABA, and IAA in grape, their synthesis-associated genes were selected. The results showed that sucrose induced the ABA synthesis VvNCED2 gene expression and reduced the ABA degradation VvCYP707A gene expression, but had no more effect on the IAA gene expression (Fig. 11a). On the contrary, auxin induced the ABA degradation VvCYP707A gene expression and reduced ABA synthesis VvNCED1, VvNCED2, VvNCED3, VvBG1, VvBG2, and VvBG3 gene expression (Fig. 11b). At the same time, auxin can induce sucrose transporter SUT gene expression and sucrose degradation acid invertase gene expression (Fig. 11b). ABA also reduced the IAA accumulation gene auxin transporter gene VvPIN, synthesis gene indole synthase (INS), indole-3-pyruvate monooxygenase YUCCA (YUC), and tryptophan aminotransferase of Arabidopsis1 (TAA1) expression levels and induced the VvSUT gene expression (Fig. 11c).
Relationship of ABA, IAA, and sucrose in grape berry. Concentrations of 100 μM of ABA and IAA and 16 mM sucrose treat the pre-veraison grape berry. Seven days later, gene expression levels were analyzed. a–c qRT-PCR was conducted using Vv18s as an internal control. Error bars represent the SE (n = 3). Asterisks indicate statistically significant differences at P < 0.05 as determined by Student’s test
The function of BG protein of ABA synthesis
In addition to free ABA, there is another form of ABA-GE for the fruit to store ABA. ABA-GE was hydrolyzed by β-glucosidase (BG) protein into the free ABA facing different stress or physiological changes. In order to verify the function of the BG protein in fruit development, the VvBG1 gene was selected and an expression system of pET30a-VvBG1 was constructed and the target protein purified. A total of 10 mg protein was purified to be used for further research (Fig. S3). The seven-stage samples were grinded into liquid and incubated with the purified BG protein; the results showed that the BG protein released more ABA and in the later stages more ABA forming ABA-GE to meet emergencies (Fig. 12). In the meantime, the BG enzyme activities also increased during fruit development (Fig. 10). Collectively, these results suggested that the BG protein is related to plant ABA accumulation.
Discussion
The sucrose metabolism during fruit development
The growth pattern of developing grape berries (Vifis vinifera L.) can be described as a double sigmoidal curve with an initial rapid increase in size followed by a lag period during which berry volume does not increase (Fig. 1a, c) (Coombe 1992). The lag period is followed by a second phase of growth during which ripening occurs. Unlike the berry volume, the fruit dry weight increased quickly from a small fruit to a ripening fruit and did not have a lag period, and the dry weight percentage also increased continuously during fruit development (Fig. 1b). These findings suggest that the fruit volume changes are responsible for the fruit water intake capacity. In the early fruit development, the fruit turgor is lower and needs more water to reduce the turgor to make the fruit soft (Robinson et al. 1988). In veraison of fruit, the conversation of material was intense; it needs a little water. After this period, the material accumulated quickly and the fruit became soft and had color followed by having an increased fruit volume; the dry weight accumulation producing the turgor needed more water to reduce the turgor to make the fruit soft. So during the ripening process, the increase in volume is accompanied by an increase in berry softness, accumulation of hexoses in the berries, and decrease in the level of malic and tartaric acids, and in red grape varieties the skin becomes colored due to the accumulation of anthocyanins (Fig. 1d, e) (Coombe 1992).
During grape berry ripening, sucrose transported from the leaves is accumulated in the berry vacuoles as glucose and fructose. High levels of SUT gene expression levels and SPS enzyme activity benefited to the sucrose transported from the sink (Fig. 2a and Fig. S1). Although a lot of sucrose was poured into the grape fruit, there was little sucrose accumulation in the grape berries. Hardy (1967) introduced the sucrose that was marked by radioactive carbon C14 into the detached grape fruit, discovering that sucrose was cleaved quickly, and the fructose content was higher than glucose in the radioactive component within 2 h, followed by the increasing glucose content. During fruit development, the high level of sucrose-transported gene VvSUTs and sucrose phosphate synthase activity made lots of sucrose accumulation (Fig. 2a and Fig. S1), but we found that there were high levels of acid invertase and neutral invertase that led to sucrose being cleaved into glucose and fructose in grapes (Fig. 2b, c). Therefore, the sucrose invertase is responsible for the sucrose accumulation. The strawberry fruit accumulated high levels of sucrose during fruit development, due to its low levels of acid invertase (Basson et al. 2010); however, the tomato (Lycopersicon esculentum Mill.) has high levels of invertase activity and stores little sucrose (Wang et al. 2016). The accumulation of sugar in the form of glucose and fructose within the vacuole is one of the main features of the ripening process in grape berries. It suggested that sucrose was metabolized quickly in the grape.
Aside from sugar, there is another energy storage, which is starch (Nardozza et al. 2013). Our microscope study of grape fruit showed that starch appeared mainly in small fruits and disappeared during the later stages of fruit development (Fig. 3). The fruit is very hard in the early stage of grape development, and the starch works as a cell content playing a role in structural support to maintain the cell turgor pressure (Robinson et al. 1988). But the starch accumulation form was varied in grape flesh and skin; at 8 Wpf, there was a starch peak in the skin but low levels of starch content in the flesh (Fig. 4a), due to the different starch metabolic enzyme activities. In the early fruit development stage, the grape fruit sugar content was very low, the photosynthesis material in grape transported from the leaves was stored with the starch form, and high levels of starch synthesis enzyme ADPase or low levels of starch degradation enzymatic activity AMY and BMY led to more starch accumulation in the fruit. With the fruit development, the fruit needs more energy to maintain its quick growth, material conversion, and quality formation; the starch began to convert by high levels of starch degradation enzymes AMY and BMY into other energy forms, glucose and fructose (Fig. 4b–d and Fig. S1). Different to flesh, the grape skin had a starch peak at 8 Wpf. Grape skin coloring represents the fruit ripening, sugar is the substance for anthocyanin production (Neta-Sharir et al. 2000), starch converted into sugar quickly, at 8 Wpf, the starch in the skin and flesh began to decline quickly, the starch synthase ADPase began to decline, and the starch degradation enzymes AMY and BMY immediately increased quickly which led to the starch being cleaved into glucose and glucose. Meanwhile, the starch was degraded into soluble sugar completely and metabolized, which made the cell tension decline (Kaplan and Guy 2005), leading to the fruit softening (Fig. 4b–d and Fig. S1). The sugar and starch metabolism in the fruit are very important in fruit coloring and softening. In the skin, there was higher starch content than that in the flesh, so the starch in the skin can be converted indirectly into sucrose. But in the flesh, there was little starch with fruit ripening (Fig. 4a). So sucrose in the flesh was mainly from leaf transportation, and the expression levels of sucrose transporter SUT genes were higher in the flesh than those in the skin (Fig. 2a and Fig. S2A-C). But the expression levels of sucrose phosphate synthase or their coding genes were lower with the fruit ripening (Fig. 2b and Fig. S2D), which showed that glucose and fructose used for sucrose synthesis became less with the fruit ripening process. And the sucrose degradation rate is faster with the fruit ripening, which leads to the higher content of glucose and fructose with the fruit ripening.
Influence of hormone and sucrose on the fruit ripening
In grape, the changing content of hormone ABA was consistent with the fruit development (Fig. 9a), but there was a high content of ABA in the early developmental stages of grape fruit swelling and cell division and expansion, with a high content of IAA, GA, and CTK to meet the fruit growth (Fig. 9a) (Coombe 1992) and balance the hormone content (Fig. 9a).
In this study, we observed that ABA, sucrose, and auxin have integrated regulation during grape fruit ripening (Fig. 5). In these three regulators, ABA plays a core role in the fruit ripening. ABA promoted the sucrose accumulation, which led to the fruit dry weight enhancement (Fig. 6), and promoted cell wall, anthocyanin, and aroma metabolism (Tables 1, 2, and 3). Notably, the sugar acts as material to synthesize the anthocyanin and fruit aroma to induce fruit ripening. What is more, ABA speeded the aroma production (Table 3) and in the meantime blocked the auxin accumulation to accelerate the fruit ripening (Fig. 11b). In the process of fruit development, the grape skin anthocyanin represents the fruit ripening stage. We used ABA to treat the grape fruit at 7 Wpf. After 20 days, they obviously promoted the fruit anthocyanin accumulation compared to the water-treated fruits (control) (Table 1). Meanwhile, the gene expression levels of anthocyanin, softening, or aroma associated were also induced by the ABA treatment (Fig. 7a–c). The sucrose accumulation gene SUT and the auxin accumulation gene expression levels were induced and blocked by ABA treatment, respectively (Fig. 11b). On the contrary, compared to the control fruit, using ABA inhibitor NDGA to treat with the fruits delayed the anthocyanin accumulation (Table 1) and stopped the fruit dry weight accumulation (Fig. 6a, b). These suggested that ABA is involved in the fruit ripening progress.
Pre-veraison auxin treatments delayed grape berry ripening, but auxin treatments maintained the berry in the pre-veraison state as judged usually by a delay in the physical and biochemical changes normally associated with ripening (Corso et al. 2016), but there is limited information about their effects on berry development and fruit volatile compounds or sugar accumulation (Yuan and Carbaugh 2007; Trainotti et al. 2007; Anderson et al. 2012). In our study, the auxin treatments delayed the grape fruit ripening progress (Fig. 5) and also reduced the transcript levels of ABA synthesis genes (Fig. 11a) and induced the transcription of some genes normally expressed during the pre-ripening period, such as anthocyanin-, softening-, and aroma-related genes (Fig. 7a–c), and in some cases, the corresponding activities of the encoded products, such as anthocyanin content, sugar content, and volatile compounds (Table 1, 2, and 3) and the low concentration of IAA, promoted the seed development and sugar accumulation, which led to the increase in grape dry weight (Fig. 6). The IAA inhibitor did not obviously induce the fruit ripening (Tables 1 and 2), but reduced the fruit dry weight accumulation (Fig. 6a, b), suggesting that in the later stage of grape fruit development, the IAA is also necessary for fruit ripening.
Sucrose, as a signal, is also involved in the grape fruit dry weight and skin anthocyanin accumulation. Although not all the concentrations of sucrose were effective for the grape fruit ripening process, we found that a suitable level of sucrose was 16 mM (Tables 1 and 2). This concentration obviously promoted the grape fruit dry weight and skin anthocyanin accumulation (Fig. 6a, b and Table 1). Similar to hormone ABA, sucrose also influenced the ripening-related gene expression levels (Fig. 7a–c). Unlike sucrose, all of the different concentrations of glucose promoted the grape fruit dry weight and skin anthocyanin accumulation, and the high concentration of 666 mM was the reliable dose (Fig. 6c and Table 1). In order to further verify the sucrose function during the fruit development, we used sucrose analog turanose which did not degrade into other materials, but could mimic the function of sucrose (Fernie et al. 2001). The results showed that turanose was the same with sucrose to promote the grape fruit ripening-related gene expression levels (Fig. 8), suggesting that the function of sucrose in the fruit ripening was of its own, but not degraded into glucose and fructose. Interestingly, although ABA and sucrose influenced each other, sucrose played a role that was independent of ABA. When the ABA signal transduction was blocked by ABA inhibitor NDGA, some ripening-related genes were also induced by sucrose (Fig. 8), suggesting that sucrose played roles in grape fruit in two ways: one was ABA-dependent, and another was ABA-independent. Collectively, exogenous ABA, sucrose, and glucose treatment positively influenced the fruit ripening process, and auxin negatively affected the fruit development.
ABA signal transduction during grape fruit ripening
There are a number of evidences related to fruit begging for fruit maturity (Koyama et al. 2010; Tadiello et al. 2016). However, the molecular mechanism of ABA in regulation of grape fruit development, especially grape fruit maturity, is questioned. The beginning of the maturity of grapes relies on the rapid rise of ABA (Fig. 9a). Furthermore, high levels of sugars led to the accumulation of ABA (Fig. 11c) (Price et al. 2004). Based on this, we cloned an ABA receptor PYR whose expression levels were low in the early fruit development and increased rapidly in the former phase of the grape fruit. The expression changes of receptor PYR were consistent with ABA content, in response to ABA, to pass the signal to PP2C to relieve the positive factor SnRK to start the downstream gene expression (Fig. 9c). Therefore, not only does hormonal effect depend on the hormone content in the affected part (or the number of target cells inside and outside), but also the number and activity of receptors are closely related to signal transduction components of protein (Xing et al. 2016). The expression level of PP2C declined during fruit development, but the positive regulator of SnRK family member SnRK2.6 did not increase rapidly during the fruit development (Fig. 9c). On the contrary, its expression levels declined in the ripening fruit (Fig. 9c). A previous report showed that SnRK2.6 in strawberry fruit is an ortholog of open stomata 1 (OST1) which negatively regulated the fruit development and ripening progress, but other members such as SnRK2.1 gene expression levels were consistent with the fruit development (Han et al. 2015). This suggests that the grape fruit ripening is controlled by a complex ABA signaling transduction pathway.
As for the ABA synthesis genes, presently, the NCED gene is considered to be the key gene in the regulation of ABA accumulation (Nambara and Marion-poll 2005). In the present study, we found three NCED genes in the fruit, and they showed different expression patterns (Fig. 9b). In addition to the active ABA, ABA has another inactive form, ABA-GE, stored to respond to stresses. The BG enzyme can release the inactive form ABA glucose ester (ABA-GE) to active ABA (Lee et al. 2006). In grape, we found three BG genes in the fruit, and their expressions were also different (Fig. 10). For all the BG genes, the BG1 gene expression level was relatively high and consistent to the ABA content changes, suggesting that the ABA synthesis in the fruit is complex and regulated by two forms: synthesis and storage. Based on the NCED and BG expression level patterns, we found that in the early stage of fruit development, the ABA accumulation was due to the NCED gene expression, and in the later stage of fruit development, the high content of ABA was mainly from the BG protein activity (Fig. 10). At the same time, the ABA content was also affected by the ABA degradation CYP707A gene expression, and in turn, its expression level was also consistent with the ABA content to balance the ABA content (Fig. 9b). We used the BG protein to incubate with the different stages of grape fruit grinding liquid to determine the ABA content. With the fruit development, there were more and more ABA released (Fig. 12), suggesting that there are more active ABA to be stored with the ABA-GE form to respond to fruit ripening. Therefore, in the late period, the BG protein that regulates the ABA levels to promote fruit ripening has important biological significance. All in all, the ABA signal transduction pathway is very important in revealing the ABA function during the grape fruit development.
Conclusions
IAA, ABA, and sucrose delay and promote the grape fruit anthocyanin accumulation, fruit firmness and cell wall metabolism, sugar content increase, and aroma spread through regulation of their metabolism-associated gene expression levels. ABA plays a core role to increase sugar and decrease IAA content in inducing the fruit ripening process. IAA inhibits ABA and sugar accumulation in fruit to maintain fruit un-ripening. Sucrose induces and reduces ABA and IAA contents to involve fruit anthocyanin and aroma appearance, and its low content is due to high sucrose invertase. ABA metabolism-related enzyme BG is important in the regulation of the ABA content in the fruit ripening process. Taken together, ABA is a core signal that is positively and negatively influenced by sucrose and IAA during the fruit ripening process.
Materials and methods
Plant material
Grapevine (Vitis vinifera) fruits of cultivar “Fujiminori” 8 years old were collected from a commercial vineyard during the 2014 season. Approximately 300 berries from at least 50 bunches were tagged, treated, and collected at 10-day intervals throughout berry development from 2 weeks after flowering until harvest (Cakir et al. 2003). All samples were pictured and frozen in liquid nitrogen and stored at −80 °C until analyzed.
RNA extraction and qRT-PCR analysis
Total grapevine RNA was isolated from the various plant tissues (Cakir et al. 2003). Genomic DNA was eliminated using an RNase-free DNase I kit (Takara, Japan) according to the manufacturer’s instructions. The quality and quantity of total RNA samples were assessed by agarose gel electrophoresis and by its A260/A230 and A260/A280 ratios. The complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the PrimeScript™ RT Reagent Kit (Takara, Japan) according to the manufacturer’s instructions. Primers used for qRT-PCR, designed using Primer 5 software (http://www.premierbiosoft.com/), are listed in Table S1. 18sRNA was used as an internal control gene. All primer pairs were tested by PCR. The presence of a single product of the correct size for each gene was confirmed by agarose gel electrophoresis and double-strand sequencing (Invitrogen, www.invitrogen.com). The amplified fragment of each gene was sub-cloned into the pMD18-T vector (Takara) and used to generate standard curves by serial dilution. qRT-PCR reactions (20 μl) contained 10 μl SYBR Premix Ex Taq (perfect real-time buffer containing dNTPs, MgCl2, and DNA polymerase; Takara, Japan), 0.4 μl of 10 μM forward specific primer, 0.4 μl of 10 μM reverse specific primer (Sangon, Shanghai, P.R. China), and 2 μl cDNA template. The mixture was placed in a Bio-Rad iQ5 Sequence Detector (Bio-Rad, Hercules, CA, USA), and DNA amplification was conducted using the following thermocycling programs: 1 cycle of 95 °C for 2 min, and 40 cycles of template denaturation at 94 °C for 20 s, primer annealing at 56 °C for 20 s, and primer extension at 72 °C for 30 s, and 71 cycles increasing from 60 to 95 °C at 0.5 °C per cycle for 30 s. The sequence detector was programmed to measure fluorescence only during the annealing step. At this temperature, no incorporated uniprimer was in the hairpin conformation contributing to fluorescence measurements.
Determination of anthocyanin, ABA, auxin, soluble sugar content, and volatile compound
Anthocyanin concentration was measured by extracting grape receptacle and skin surface of equal weight (five replications) with 1% HCl methanol and determining the absorbance at 530 and 657 nm. The formula A = A530 − 0.25 A657 was used to compensate for the contribution of chlorophyll and its degradation products to the absorption at 530 nm. The anthocyanin concentration was a relative value, and we set A = 0.01 equal to 1 unit (Jia et al. 2011). ABA, auxin, and soluble sugar contents were determined as described by Jia et al. (2011). Volatile compound production by grape berry was characterized by headspace solid-phase micro-extraction and gas chromatography mass spectrometry as described (Dong et al. 2013). The relative content of each volatile compound was calculated using the peak areas and expressed as a percentage of the total volatiles.
6xHis-tagged protein expression and purification
To express VvBG1 in bacteria, the pET-30a vector with a 6xHis tag at the N-terminal end was used to construct the pET-30a-VvBG1 vector (Qiagen). To clone VvBG1 cDNA in the same reading frame as the 6xHis affinity tag, its opening reading frame was modified (boldface letters) by PCR using the primers (forward: 5′-GGATCCATGGCGTTCGGAAGATTCATC-3′ and reverse: 5′-CTCGAGGGGAGAATTCAAGAAGTTC-3′). In parallel, BamH I and XhoI restriction sites (underlined sequence in the primer above) suitable for cloning were introduced at the 5′ end, respectively. For the construct, the PCR product was checked by sequencing before and after ligation. The E. coli BL21 (DE3) host strain was transformed with the pET-30a-VvBG1 construct. A Luria-Bertani culture medium (25 g/mL kanamycin) was inoculated (1:500) with overnight culture and grown at 28 °C with vigorous shaking until an OD600 of 0.6 was reached. After induction with 2 mM isopropylthiogalactoside (IPTG), the culture was incubated for an additional 4 to 5 h at 37 °C. Cells were harvested at different times to determine the best expression level and then frozen in liquid nitrogen. Total proteins were extracted under non-denaturing conditions, and VvBG1 purification by nickel-nitrilotriacetic acid agarose (Ni-NTA) affinity chromatography and elution were achieved under native conditions according to the recommended QIA expressionist protocol (Qiagen). After dialysis against the column fixation buffer, the VvBG1 protein was purified a second time by Ni-NTA affinity chromatography and concentrated by centrifugation in a centricon tube (3000 D; Pharmacia).
Determination of the dry weight percentage
Firstly, the fresh grapes were collected and weighed; secondly, the grape fruits were cut and dried under 80 °C for 2 days and then weighed. The ratio of dry/fresh weigh of grape represents the accumulation of dry material percentage.
Determination of the total β-glucosidase activity
An assay of the total β-glucosidase activity of strawberry pulp was performed spectrophotometrically by measuring the release of reducing sugars by the p-NPG method (Wegrzyn and MacRae 1992), in which the synthetic substrate p-nitrophenyl-β-d-glucopyranoside (p-NPG) was used to detect β-glucosidase activity. Fifty milliliters of crude β-glucosidase solution was made from 5 g strawberry pulp in a citrate, respectively. A volume of 0.6 ml of the β-glucosidase solution and 0.4 ml p-NPG were added to 2 ml buffer in a test tube, and the mixture was kept in a water bath at 56 °C for 60 min. The reaction was interrupted by the addition of 4 ml 1 M Na2CO3. The p-nitrophenol released in the reaction was determined by a spectrophotometer at 400 nm. A p-nitrophenol calibration curve had previously been prepared. One unit of enzyme activity (U) was defined as the amount of β-glucosidase which would release 1 μmol p-nitrophenol/min under experimental conditions.
Cell wall metabolism enzyme activity measure
Ten grams of frozen pulp was ground with liquid nitrogen and mixed with 20 ml of cold 5% (v/v) polyethylene glycol (PEG) supplemented with 1 ng ml−1 of leupeptin. After centrifugation (16,000×g, 30 min), the supernatant was discarded, and the sediment was washed with 10 ml of 5% (v/v) of PEG (plus 1 ng ml−1 leupeptin) before further centrifugation (16,000×g, 10 min). The supernatant was discarded, and the residue was re-suspended by vibration for 10 min in 25 ml of 20 mM EDTA and 1.25 M NaCl, pH 6.5. After centrifugation (16,000×g, 30 min), the supernatant was made up to 1 l with a solution of 100 mM NaCl that included 5 ng of leupeptin and this was kept at pH 6.5. After 12 h of dialysis followed by centrifugation (16,000×g, 20 min), the supernatant (extract) was used to assess enzyme activity. Assays for polygalacturonase (EC3.2.1.15), pectin methylesterase (EC3.1.1.11), and cellulose were conducted according to previously published methods (Andrews and Li 1995; Engelen et al. 1995).
Microscope of grape fruit
The fruit was cut with a double-sided blade into 1–2-mm2 small pieces and fixed with 3% glutaraldehyde (pH 7.2 phosphoric acid buffer preparation) for 3 h at room temperature and in the meantime had a suction until slices sink in glutaraldehyde. After washing by phosphate buffer (pH 7.2), fruit pieces were fixed with 1% hungry acid (pH 7.2 phosphoric acid buffer preparation), fixed for 1.5 h at room temperature, and rinsed again with phosphoric acid buffer, dehydrated in an ethanol series, and embedded in Spurr’s resin. Sections were cut at 90 nm, collected on formvar-coated copper grids, and observed under JEM-1005 transmission electron microscopy (Hardie et al. 1996).
Exogenous sucrose, glucose, ABA, IAA, NDGA, and NPA application of detached grape fruit
The grape fruits 60 days after flowering were used. A bunch of 30 fruits were collected, and 120 fruits of similar size were selected. These fruits were soaked into the following solutions: sucrose (8, 16, and 25 mM, n = 8, respectively), glucose (222, 444, and 666 mM, n = 8, respectively), ABA (100 and 200 μM, n = 8, respectively), auxin (indole-3-acetic, IAA) (100 and 200 μM, n = 8, respectively), NPA (100 μM, n = 8), NDGA (100 μM, n = 8), and water (control, n = 8). They were vacuumed three times, each time for 10 min, and then with a sealed plastic bag on the earth; after 7 days, the samples were frozen with liquid nitrogen frozen after peeling, and seed were removed and stored at −80 °C until use.
Abbreviations
- ABA:
-
Abscisic acid
- IAA:
-
Indole-3-acetic acid
- NDGA:
-
Nordihydroguaiaretic acid
- NPA:
-
1-N-Naphthylphthalamic acid
Reference
Ai TN, Naing AH, Arun M, Lim SH, Kim CK (2016) Sucrose-induced anthocyanin accumulation in vegetative tissue of Petunia plants requires anthocyanin regulatory transcription factors. Plant Sci 252:144–150
Alexander L, Grierson D (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53:2039–2055
Anderson JV, Doğramacı M, Horvath DP, Foley ME, Chao WS, Suttle JC, Thimmapuram J, Hernandez AG, Ali S, Mikel MA (2012) Auxin and ABA act as central regulators of developmental networks associated with paradormancy in Canada thistle (Cirsium arvense). Functional Integrative Genomics 12(3):515–531
Andrews PK, Li S (1995) Cell wall hydrolytic enzyme activity during development of nonclimacteric sweet cherry (Pruns avium L.) fruit. J. Hortic Sci 70:561–567
Baker RF, Leach KA, Boyer NR, Swyers MJ, Benitez-Alfonso Y, Skopelitis T, Luo A, Sylvester A, Jackson D, Braun DM (2016) Sucrose transporter ZmSut1 expression and localization uncover new insights into sucrose phloem loading. Plant Physiology pp.00884.
Basson CE, Groenewald JH, Kossmann J et al (2010) Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: invertase is the main sucrose hydrolysing enzyme. Food Chem 121(4):1156–1162
Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15:2165–2180
Coombe BG (1992) Research on development and ripening of the grape berry. Am J Enol Vitic 43:101–110
Corso M, Vannozzi A, Ziliotto F, Zouine M, Maza E, Nicolato T, Vitulo N, Meggio F, Valle G, Bouzayen M, Müller M, Munné-Bosch S, Lucchin M, Bonghi C (2016) Grapevine rootstocks differentially affect the rate of ripening and modulate auxin-related genes in cabernet sauvignon berries. Front Plant Sci 7(382)
Dong J, Zhang YT, Tang XW, Jin WM, Han ZH (2013) Differences in volatile ester composition between Fragaria×ananassa and F. vesca and implications for strawberry aroma patterns. Sci Horti (Amsterdam) 150:47–53
Engelen FAV, Molthoff JW, Conner AJ, Nap JP, Pereira A, Stiekema WJ (1995) pBINPLUS: an improved plant transformation vector based on pBIN19. Transgen Res 4:288–290
Fernie AR, Roessner U, Geigenberger P (2001) The sucrose analog palatinose leads to a stimulation of sucrose degradation and starch synthesis when supplied to discs of growing potato tubers. Plant Physiol 125:1967–1977
Fu FQ, Mao WH, Shi K, Zhou YH, Asami T et al (2008) A role of brassinosteroids in early fruit development in cucumber. J Exp Bot 59:2299–2308
Gibson SI (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8:93–102
Giovannoni JJ (2001) Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 52:725–749
Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell 16:170–180
Han Y, Dang RH, Li JX, Jiang J, Zhang N, Jia MR, Wei LZ, Li ZQ, Li BB, Jia WS (2015) SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE2.6, an ortholog of OPEN STOMATA1, is a negative regulator of strawberry fruit development and ripening. Plant Physiol 167(3):915–930
Hardie WJ, O'brien TP, Jaudzems VG (1996) Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust J Grape Wine Res 2(2):97–142
Hardy J (1967) Sucrose breakdown and synthesis in the ripening grape berry. Aust J Biol Sci 20:465–470
Huang H, Xie S, Xiao Q, Wei B, Zheng L, Wang Y, Cao Y, Zhang X, Long T, Li Y, Hu Y, Yu G, Liu H, Liu Y, Huang Z, Zhang J, Huang Y (2016) Sucrose and ABA regulate starch biosynthesis in maize through a novel transcription factor, ZmEREB156. Sci Rep 6:27590
Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157:188–199
Jia HF, Zhang C, Pervaiz T, Zhao PC, Liu ZJ, Wang BJ, Wang C, Zhang L, Fang JG, Qian JP (2015) Jasmonic acid involves in grape fruit ripening and resistant against botrytis cinerea. Functional Integrative Genomics 16(1):1–16
Kaplan F, Guy CL (2005) RNA interference of Arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J 44(5):730–743
Koyama K, Sadamatsu K, Goto-Yamamoto N (2010) Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Functional Integrative Genomics 10(3):367–381
Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126:1109–1120
Lee S, Chung EJ, Joung YH, Choi D (2010) Non-climacteric fruit ripening in pepper: increased transcription of EIL-like genes normally regulated by ethylene. Functional Integrative Genomics 10:135–146
Li Q, Li P, Sun L, Wang Y, Ji K, Sun Y, Dai S, Chen P, Duan C, Leng P (2012) Expression analysis of β-glucosidase genes that regulate abscisic acid homeostasis during watermelon(Citrullus lanatus) development and under stress conditions. Journal Plant Physiology 169(1):78–85
Nambara E, Marion-poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Nardozza S, Boldingh HL, Osorio S et al (2013) Metabolic analysis of kiwifruit (Actinidia deliciosa) berries from extreme genotypes reveals hallmarks for fruit starch metabolism. J Exp Bot 64(16):5049–5063
Neta-Sharir I, Shoseyov O, Weiss D (2000) Sugars enhance the expression of gibberellin-induced genes in developing petunia flowers. Physiol Plant 109(2):196–202
Price J, Laxmi A, St Martin SK, Jang JC (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16:2128–2150
Qin G, Zhu Z, Wang W, Cai J, Chen Y, Li L, Tian S (2016) A tomato vacuolar invertase inhibitor mediates sucrose metabolism and influences fruit ripening. Plant Physiology pp.01269.
Robinson NL, Hewitt JD, Bennett AB (1988) Sink metabolism in tomato fruit I. Developmental changes in carbohydrate metabolizing enzymes Plant Physiology 87(3):727–730
Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit development and ripening. Annu Rev Plant Biol 64:219–241
Solfanclli C, Poggi A, Loreti E (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646
Su L, Diretto G, Purgatto E, Danoun S, Zouine M, Li Z, Roustan JP, Bouzayen M, Giuliano G, Chervin C (2015) Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol 15:114
Sun C, Palmqvist S, Olsson H (2003) A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell 15:2076–2092
Symons G, Davies C, Shavrukov Y, Dry I, Reid J et al (2006) Grapes on steroids. Brassinosteroids are involved in grape berry ripening Plant Physiol 140:150–158
Tadiello A, Ziosi V, Negri AS, Noferini M, Fiori G, Busatto N, Espen L, Costa G12, Trainotti L (2016) On the role of ethylene, auxin and a GOLVEN-like peptide hormone in the regulation of peach ripening. BMC Plant Biol 16:44
Trainotti L, Tadiello A, Casadoro G (2007) The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot 58:3299–3308
Villalobos-González L, Peña-Neira A, Ibáñez F, Pastenes C (2016) Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: gene expression andmetabolite content. Plant Physiol Biochem 105:213–223
Wang N, Duhita N, Ariizumi T, Ezura H (2016) Involvement of vacuolar processing enzyme slvpe5 in post-transcriptional process of invertase in sucrose accumulation in tomato. Plant Physiology Biochemistry 108:71–78
Wegrzyn TF, MacRae EA (1992) Pectinesterase, polygalacturonase, and β-galactosidase during softening of ethylene-treated kiwifruit. Hortscience 27:900–902
Xing L, Zhao Y, Gao J, Xiang C, Zhu JK (2016) The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Sci Rep 6:27177
Yuan RC, Carbaugh DH (2007) Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden supreme’ and ‘Golden delicious’ apples. Hortscience 42:101–105
Zhang M, Leng P, Zhang GL, Li XX (2009) Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol 166:1241–1252
Acknowledgements
We would like to express our gratitude to Jiangsu Academy of Agricultural Sciences for providing the grape material. This work was supported by the Jiangsu Natural Science Fund (BK20140707), Key Laboratory of the Ministry of Agriculture (ZW2014009), China National Natural Science Fund (31401847), China National Natural State Key Laboratory of the Ministry of Agriculture (NYB-201508-1) Science Fund (31361140358), and State Key Laboratory of the Ministry of Agriculture (NYB-201508-1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jia, H., Xie, Z., Wang, C. et al. Abscisic acid, sucrose, and auxin coordinately regulate berry ripening process of the Fujiminori grape. Funct Integr Genomics 17, 441–457 (2017). https://doi.org/10.1007/s10142-017-0546-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-017-0546-z