Abstract
Axillary bud outgrowth is regulated by both environmental cues and internal plant hormone signaling. Central to this regulation is the balance between auxins, cytokinins, and strigolactones. Auxins are transported basipetally and inhibit the axillary bud outgrowth indirectly by either restricting auxin export from the axillary buds to the stem (canalization model) or inducing strigolactone biosynthesis and limiting cytokinin levels (second messenger model). Both models have supporting evidence and are not mutually exclusive. In this study, we used a modified split-plate bioassay to apply different plant growth regulators to isolated stem segments of chrysanthemum and measure their effect on axillary bud growth. Results showed axillary bud outgrowth in the bioassay within 5 days after nodal stem excision. Treatments with apical auxin (IAA) inhibited bud outgrowth which was counteracted by treatments with basal cytokinins (TDZ, zeatin, 2-ip). Treatments with basal strigolactone (GR24) could inhibit axillary bud growth without an apical auxin treatment. GR24 inhibition of axillary buds could be counteracted with auxin transport inhibitors (TIBA and NPA). Treatments with sucrose in the medium resulted in stronger axillary bud growth, which could be inhibited with apical auxin treatment but not with basal strigolactone treatment. These observations provide support for both the canalization model and the second messenger model with, on the one hand, the influence of auxin transport on strigolactone inhibition of axillary buds and, on the other hand, the inhibition of axillary bud growth by strigolactone without an apical auxin source. The inability of GR24 to inhibit bud growth in a sucrose treatment raises an interesting question about the role of strigolactone and sucrose in axillary bud outgrowth and calls for further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The above ground architecture of plants is post-embryonically determined by the outgrowth of axillary buds which develop from axillary meristems formed in the axils of leaves. Interaction of the plant hormones auxin, cytokinins, and strigolactones is prominent in the physiological control of bud outgrowth regulation (Domagalska and Leyser 2011). The main mechanism of bud outgrowth regulation is attributed to the action of auxin and cytokinin, the balance of which controls apical dominance (Sachs and Thimann 1967; Cline 1991). Auxin is produced in the young expanding leaves of the shoot apex (Ljung et al. 2001) and is transported towards the roots in the stem (Friml et al. 2003). This basipetal transport is facilitated by PIN and ABCB auxin efflux proteins with basipetal transport mainly established by the PIN1 proteins in the basal membranes of the xylem parenchyma cells (Petrásek and Friml 2009). The further action of auxin to inhibit axillary bud outgrowth happens indirectly (Ongaro and Leyser 2008). Evidence exists for two non-mutually exclusive models: the second messenger model and the canalization model (Domagalska and Leyser 2011). The second messenger model puts another signal or hormone downstream from auxin to directly influence bud outgrowth. Both cytokinin and strigolactone fit in this model as cytokinin biosynthesis was shown to be downregulated by auxin (Tanaka et al. 2006), while strigolactone biosynthesis was upregulated by auxin (Foo et al. 2005; Mashiguchi et al. 2009). Strigolactone was further reported to be able to inhibit axillary bud outgrowth by direct application to the bud (Gomez-Roldan et al. 2008). The canalization model attributes the inhibitive action of auxin to a source–sink relationship between the shoot apex, axillary buds, and stem. The apex and axillary buds compete for the availability to export auxin to the stem. Outcompeted axillary buds are deprived of auxin export to the stem and do not grow out. Strigolactone’s proposed function is to restrict the auxin transport from bud to stem by interfering with the mobilization of the PIN1 transport proteins in the basal membranes of the xylem parenchyma cells (Ongaro and Leyser 2008). Supporting evidence is that application of exogenous strigolactone could increase axillary bud competition by suppressing PIN1 auxin transport (Crawford et al. 2010; Shinohara et al. 2013).
Recently, the role of sucrose in the control of apical dominance has also been reevaluated and sucrose was proposed, over auxin, as the initial regulator of apical dominance and bud outgrowth after loss of apical dominance (Mason et al. 2014; Barbier et al. 2015). The main argument for this case is the observation that bud outgrowth occurred in decapitated pea plants, prior to changes in auxin content in the stem (Morris et al. 2005). It is further argued that sugar also functions as a signaling molecule to trigger bud outgrowth. This is evidenced by non-metabolizable sucrose analogues that are able to trigger bud outgrowth (Rabot et al. 2012). Furthermore, sucrose has been reported to upregulate auxin (Mishra et al. 2009) and cytokinin biosynthesis (Kushwah and Laxmi 2014).
To study the effects of plant hormones or other external treatments on axillary bud outgrowth, a split-plate assay can be used. The concept of the assay is to put an excised nodal stem segment, with one or more axillary buds, in a petri dish between two pieces of agar. In this way, axillary bud growth can be measured with different treatments of plant hormones, or other chemicals, that can be added either to the top or bottom piece of agar. This method was first described by Chatfield et al. (2000) in a study on the shoot branching of Arabidopsis (Arabidopsis thaliana), demonstrating that apical auxin treatment could inhibit axillary bud growth and that basal cytokinin treatments could release auxin inhibition.
The same method has been used in several other studies with different species, including red clover (Van Minnebruggen et al. 2013), pea (Young et al. 2014; Brewer et al. 2015), and chrysanthemum (Liang et al. 2010; Chen et al. 2013). Liang et al. (2010) showed inhibition of only basal axillary buds in two-node stem segments treated with 5 µM of the synthetic strigolactone GR24. Furthermore, they observed inhibition of bud growth in one-node segments treated with GR24 only in combination with an apical auxin treatment. Also in chrysanthemum, Chen et al. (2013) reported that axillary bud inhibition by apical auxin NAA was weakened by basal cytokinin (BAP).
In this study, we used a modified split-plate assay to analyze bud outgrowth in chrysanthemum and to test the effect of different plant growth regulators on the bud outgrowth. The effect of the auxin indole acetic acid (IAA) and cytokinin (zeatin, thidiazuron (TDZ), 2-isopentenyladenine (2-iP) treatments was verified to show auxin cytokinin antagonism in axillary bud outgrowth. Strigolactone (GR24) treatments were tested separately and in combination with auxin transport inhibitors [1-N-naphthylphthalamic acid (NPA) and 2,3,5-triiodobenzoic acid (TIBA)] to investigate the role of basipetal auxin transport in the inhibitory action of strigolactone. Furthermore, we combined sucrose treatments with auxin and strigolactone to address the role of sucrose in apical dominance. In our modified assay, we tested apical and basal treatments on defoliated nodal stem segments by adding plant growth regulators to a top or bottom agar plate without nutrients or sucrose. This way the different treatments could be easily combined, and effects on bud growth could be measured in a period of just 5 days. Furthermore, possible influences of plant hormones or assimilates from leaves on axillary bud growth could be avoided.
Materials and methods
Plant material
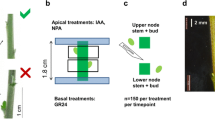
Chrysanthemum morifolium Ramat. cut flower genotype C17 (provided by Dekker Chrysanten BV., The Netherlands) was used in this study. Cuttings were rooted in a standard greenhouse at 20 °C under long day light (16 h SON-T 100 µmol/m2s) conditions for 3 weeks. Nodal stem segments of 1.8 cm, bearing two nodes, were cut from the young shoots. To obtain comparable segments in the same physiological status, we selected nodal positions 5–6 and 7–8 (starting from the nodal position with the first fully unfolded leaf under the shoot apex) (Fig. 1a). These positions contained axillary buds of roughly the same size that were inhibited by apical dominance. Leaves and stipules were removed from the segments.
Split-plate assay
Split-plate experiments were set up according to Chatfield et al. (2000) with minor modifications. Stem segments were sandwiched between two petri dishes with 15 ml of 0.7% agar medium, so that about 2 mm of the stem segment went into the agar at each end (Fig. 1b, c). Treatments were given to the apical or basal end of the stem segments by adding plant growth regulators from 1-mg/ml stock solutions to the solidifying agar in the top or bottom petri dish. IAA (0.5, 2, 5, 20 µM), NPA (5, 10 µM), and TIBA (5 µM) treatments were given apically and treatments with TDZ (1 µM), zeatin (20 µM), 2-iP (20 µM), and GR24 (5, 15, 25, 50 µM) were given basally. Sucrose treatments (1.5%) were given in both top and bottom petri dish. Table 1 gives an overview of the different treatment combinations that were used in the experiments.
Growth conditions and measurements
Plates containing stem segments were placed in a growth chamber (20 ± 1 °C, 19 h day length) with fluorescent light (Philips TL-D Super 80 58 W/840 cool white) with an intensity of 90 µmol * m−2 s−1 at the height of the nodal stem segments inside the petri dish. Axillary bud lengths were measured on stereomicroscopy (Leica M165FC) pictures of the stem segments with ImageJ software (Fig. 1d). For all experiments, five nodal stem segments were used per treatment. Each nodal stem segment contained two axillary buds. Bud outgrowth rate (BOR) was calculated as the difference in bud length between two subsequent days divided by the interval of days (2) between these measurements.
Statistical analysis
For all the experiments, a two-way ANOVA (SPSS 23, IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) was used to test interaction effects between the treatments and the position of the axillary bud on the stem segment (lower bud versus upper bud). There was no significant interaction between treatment and axillary bud position in any of the reported experiments, except for the treatment with 5-µM basal GR24 (Fig. 4c). In all experiments, the data are, therefore, represented as mean axillary bud lengths ± standard error (SE) of 10 axillary buds per treatment (five nodal stem segments with each two axillary buds). In Fig. 4, the axillary bud length is shown separately for the lower and upper axillary buds for each treatment.
Results
Axillary bud outgrowth inhibition with IAA
The initial bud length in all treatments was around 1 mm at day 1. In the 0 µM IAA control treatment, axillary bud outgrowth occurred over the 12-day period (Fig. 2a) with a visible change from bud to unfolded leaf at days 4 and 5 (Fig. 2c). Treatments with 0.5, 2, and 5 µM IAA, over the period of 12 days, showed an inhibition of axillary bud growth compared to the 0 µM IAA control treatment (Fig. 2a) with significant differences between control and IAA treatments from day 3 to day 12. This inhibition was stronger with increasing concentrations of IAA, culminating with 5 µM IAA. In the 5 µM IAA treatment, bud growth was strongly inhibited in the first 5 days of recording. After day 5, limited bud growth occurred with leaf expansion and visible outgrowth from day 8 onwards. The bud outgrowth rate was consistently higher in the control treatment during the first 5 days compared to the other treatments, where BOR decreased with each increase in IAA concentration (Fig. 2b). The control treatment also showed a peak of BOR at day 4. This indicated that in this experimental setup, the highest bud growth response occurred in the first 5 days. Based on these results, we used 5 µM IAA as the concentration to inhibit axillary bud outgrowth and measured axillary bud growth in five consecutive days in the experiments that followed.
Axillary bud length in the split-plate assay with IAA treatments on chrysanthemum nodal stem segments Apical treatments (marked with A in the legend) with 0-µM IAA, 0.5 µM, 2-µM IAA, and 5-µM IAA were assessed by measuring axillary bud outgrowth every 24 h for 12 consecutive days. a Bud length on the y-axis measured for 12 days. b Bud outgrowth rate between two subsequent days on the y-axis starting from day 2. c Images of one single axillary bud measured over 12 days for the 0-µM IAA treatment and the 5-µM IAA treatment
Cytokinin and NPA mitigate IAA axillary bud outgrowth inhibition
The treatment with 5-µM IAA inhibited axillary bud outgrowth (Fig. 3a). Treatments with a combination of 5-µM IAA and cytokinins 1 µM TDZ, 20 µM zeatin (Fig. 3a) or 20 µM 2-iP (Fig. 3b) showed reduced bud outgrowth inhibition compared to 5 µM IAA. A similar reaction was also observed for treatment with 5 µM of the auxin transport inhibitor NPA combined with 5 µM IAA (Fig. 3c).
Axillary bud length in the split-plate assay with cytokinin and NPA treatments on chrysanthemum nodal stem segments. Bud length is shown on the y-axis measured over 5 days on day 1, day 3, and day 5 for the different treatments. a 5-µM apical IAA combined with 2-µM basal zeatin or 1-µM basal TDZ. b 5-µM apical IAA combined with 20-µM basal 2-iP. c 5-µM apical IAA combined with 5-µM apical NPA. Apical and basal treatments are marked in the legend with A or B, respectively
Axillary bud outgrowth inhibition with strigolactone GR24
For the experiment with GR24, a significant difference between the lower and upper bud at day 4 and day 5 was observed. Therefore, the axillary bud lengths were split up in the lower node (LN) and the upper node (UN) (Fig. 4). Treatment with 5-µM GR24 showed an inhibiting effect only in the lowest axillary bud of the two buds on the stem segment. In the control treatment (Fig. 4a), both the LN and UN axillary buds grew out in equal lengths. The treatment with 5-µM IAA (Fig. 4b) and the treatment with 5-µM IAA and 5-µM GR24 (Fig. 4d) showed both LN and UN buds to be equally inhibited. The treatment with 5-µM GR24 (Fig. 4c) showed outgrowth of the UN bud and inhibition of the LN bud.
Axillary bud length in the split-plate assay with GR24 and IAA treatments on chrysanthemum nodal stem segments Bud length is shown on the y-axis measured over 5 days with separate upper node bud length (UN) and lower node bud length (LN). a Control treatment. b Apical treatment with 5-µM IAA. c Basal treatment with 5-µM GR24. d Apical treatment with 5-µM IAA and basal treatment with 5-µM GR24. Significant difference (t test) between LN and UN is marked in the figure with *
Basal treatments with a concentration gradient of GR24 were tested to achieve inhibition of both axillary buds on the stem segment (Fig. 5a). The treatment with 50-µM GR24 showed the strongest inhibition of bud outgrowth with no significant differences between lower and upper nodes. Hence, this concentration was subsequently used in further tests with GR24.
Axillary bud length in the split-plate assay with GR24, NPA, and TIBA treatments on chrysanthemum nodal stem segments. Bud length is shown on the y-axis measured over 5 days on day 1, day 3, and day 5 for the different treatments. a 5,- 15-, 25-, and 50-µM basal GR24. b 50-µM basal GR24 combined with 10-µM apical NPA. c 50-µM basal GR24 combined with 5-µM apical TIBA
TIBA and NPA mitigate GR24 axillary bud outgrowth inhibition
Basal applications with 50-µM GR24 were combined in two experiments with apical treatments with the auxin transport inhibitors NPA (Fig. 5b) and TIBA (Fig. 5c). An application of 10-µM apical NPA combined with 50-µM basal GR24 increased the bud length growth compared to the 50-µM GR24 treatment but bud length growth was lower than in the control treatment (Fig. 5b). The application of 5-µM apical TIBA together with 50-µM basal GR24 showed an increased bud length growth, similar to the control treatment, compared to the restricted growth in the 50-µM GR24 treatment (Fig. 5c). Treatments with either NPA or TIBA were included as a reference for the effect of these auxin transport inhibitors on bud outgrowth (S. 1). Treatment with 10-µM NPA or 20-µM TIBA showed a slight decrease in bud outgrowth compared to control treatment.
Increased axillary bud outgrowth with sucrose is inhibited by IAA but not by GR24 treatment
Treatment with 1.5% sucrose showed increased axillary bud growth compared to the control without sucrose. When 5-µM IAA was applied in the 1.5% sucrose treatment, there was an inhibition of bud outgrowth compared to the 1.5% sucrose control but the bud outgrowth was not inhibited beyond the no sucrose control (Fig. 6a). Treatment of 1.5% sucrose with 20-µM IAA showed inhibition of bud growth compared to the other treatments except the no sucrose 5-µM IAA treatment, which showed the strongest inhibition of bud outgrowth. The treatment with 1.5% sucrose and 50-µM GR24 showed no inhibition of axillary bud outgrowth (Fig. 6b).
Axillary bud length in the split-plate assay with sucrose, IAA and GR24 treatments on chrysanthemum nodal stem segments. Bud length is shown on the y-axis measured over 5 days on day 1, day 3, and day 5 for the different treatments. All treatments with sucrose had 1.5% sucrose in the basal and apical medium. a 1.5% sucrose with 5-µM apical IAA. b 1.5% sucrose in combination with 20-µM apical IAA or 50-µM basal GR24
Discussion
Bud outgrowth assay
In the experiment where apical auxin was applied for 12 days (Fig. 2), bud outgrowth occurred in the control treatment during the 12-day period that was measured (Fig. 2a). The rate of this bud outgrowth decreased after reaching a peak at day 4 (Fig. 2b). The axillary bud length itself reached a plateau at day 10. This indicates that after reaching a growth peak within the first 5 days, axillary bud growth decreased and halted. The arrested growth after the initial 5 days might be explained by the fact that no sucrose or other nutrients were added to the medium and that also the leaves were removed. In previous reports, it was shown that defoliation could have effects on bud outgrowth. In sorghum, defoliation led to an inhibition of axillary bud outgrowth (Kebrom et al. 2010). Similarly, defoliated pea plants showed inhibited axillary bud growth after decapitation (Mason et al. 2014). These observations have been suggested to be linked with sugar requirements of the growing buds (Rameau et al. 2015). Differences in leaf area could therefore influence the capacity of axillary buds to grow out (Kebrom and Mullet 2015). Accordingly, in our experiments, defoliated stem segments were used to eliminate the influence of auxin or sucrose from the leaves. The defoliation did not restrict the capability of the axillary buds to grow out, evidenced by the observed bud outgrowth occurring in a 5-day period in the control treatments.
Auxin and cytokinin bud outgrowth physiology
The apical treatment with auxin showed a correlation between the inhibition of axillary bud outgrowth and the concentration of apical IAA (Fig. 2). During the first 5 days, there was complete inhibition of axillary bud outgrowth with 5-µM IAA. After day 5, the axillary buds started expanding and showed bud outgrowth from day 8 to day 12. This demonstrated that the auxin treatment caused a delayed axillary bud outgrowth with initial inhibition. This has also been observed in Arabidopsis stem segments treated with NAA, and it has been argued that the eventual outgrowth could be due to synthesis of cytokinins in the cut stem (Chatfield et al. 2000). Another possibility could be that a decapitation signal, besides auxin, could induce the bud outgrowth (Morris et al. 2005).
The results with auxins and cytokinins showed that the inhibitory effect of IAA could be alleviated with basal application of three cytokinins, zeatin, TDZ and 2iP (Fig. 3). These observations are in line with previous reports in Arabidopsis (Chatfield et al. 2000) and chrysanthemum (Chen et al. 2013) where basal treatment with the cytokinin BA could reduce the inhibition of apical treatment with the auxin NAA. Apical treatment with the auxin transport inhibitor NPA showed a similar alleviation of axillary bud inhibition by apical IAA. NPA blocks ABCB and PIN auxin transport proteins that enable the polar auxin transport (Petrásek et al. 2006; Blakeslee et al. 2007). These results were also consistent with alleviation of NAA inhibition of axillary bud outgrowth by NPA in Arabidopsis (Chatfield et al. 2000).
Strigolactone bud outgrowth physiology
In the first experiment with GR24, 5 µM was applied basally and both the length of the upper and lower node was recorded (Fig. 4). A clear difference in outgrowth was observed: the upper node axillary bud grew out while the lower node axillary bud was inhibited. These results showed that 5-µM GR24 was not sufficient to inhibit the axillary bud outgrowth in both nodes of the stem segment. This confirms the results of a previous report of a treatment with 5 µM on chrysanthemum (Liang et al. 2010). A possible explanation could be that the physiological action of strigolactone is dependent on an apical auxin source, as proposed in the canalization model. The action of GR24 would dampen polar auxin transport by constraining accumulation of PIN1 auxin transport proteins. This would limit the amount of auxin that can be transported from axillary bud to stem and is required for the outgrowth of the bud. Furthermore, a competition would occur between the two adjacent axillary buds for the ability to transport auxin into the stem (Crawford et al. 2010). In our treatment with 5-µM GR24, the UN bud grew out (Fig. 4c). This would then, together with the basal GR24, limit the availability of auxin export for the LN bud, causing it to be fully inhibited. Similar observations that show strigolactone inhibition only in the presence of an apical auxin source have been made in Arabidopsis and pea (Bennett and Leyser 2006; Ongaro and Leyser 2008; Crawford et al. 2010).
When GR24 was applied at high concentrations (50 µM), axillary bud outgrowth was inhibited without the addition of an apical auxin source (Fig. 5a). This corresponds with the results in other experiments with pea and Arabidopsis (Brewer et al. 2009, 2015; Dun et al. 2013) where basal GR24 treatments could also inhibit axillary buds in the absence of an apical auxin source. This new observation in chrysanthemum is in line with the second messenger model where the action of strigolactone is proposed to be downstream and independent of auxin transport capacity. In both the canalization model and the second messenger model, the action of strigolactone is downstream of auxin; the difference being that in the second messenger model, the strigolactones can act independently of auxin, while in the canalization model, the action of strigolactone is auxin dependent. Thus, one could reason that if the upstream auxin signal is weakened by polar auxin transport inhibitors, the axillary bud inhibition by strigolactone would be weakened in the case of the canalization mechanism. Therefore, basal application of GR24 was combined with apical application of the auxin transport inhibitors NPA and TIBA. TIBA was proposed to compete with natural IAA for transport and binding sites without showing auxin activity (Katekar and Geissler 1977). Apical application with NPA or TIBA, in combination with basal GR24, showed reduced inhibition of axillary bud growth when compared to the basal GR24 treatment alone (Fig. 5b, c). These results seem to contradict the observation that GR24 could inhibit bud outgrowth independent of an apical auxin source since, when auxin transport is restricted, the inhibitive effect of GR24 is alleviated and could be seen as support for the auxin canalization model. From another perspective, the increased bud outgrowth that was observed in the treatment with GR24 in combination with NPA could be explained by previous observations that strigolactones inhibit or promote axillary bud outgrowth depending on the auxin transport status (Shinohara et al. 2013). There it was shown that in stems that are already deprived of auxin transport, which is the case in NPA-treated plants, strigolactone can actually promote axillary bud outgrowth. This also fits into the canalization model (Waldie et al. 2014).
The difference between intact plants and the isolated stem segments that are used in this study could also have effects on the action of auxin to inhibit axillary bud growth. It was shown in decapitated strigolactone deficient pea plants that apical auxin application could not inhibit axillary bud growth (Beveridge et al. 2000). Consistently, intact Arabidopsis mutant plants with increased auxin biosynthesis (yuc1D) showed decreased branching, whereas double mutants with increased auxin biosynthesis (yuc1D) and strigolactone deficiency (max3) showed an increased branching similar to max3 plants (Brewer et al. 2015). This demonstrated that strigolactone is required for the inhibition of axillary buds by auxin, fitting to the secondary messenger model. In contrast, in isolated stem segments of strigolactone deficient pea plants, apical auxin treatment could inhibit bud outgrowth (Young et al. 2014). The fact that auxin could not inhibit bud growth in the intact plants has been ascribed to the cytokinins provided by the roots that would counteract auxin and promote bud outgrowth (Foo et al. 2007; Young et al. 2014). In the isolated stems, the reported strigolactone independent bud growth inhibition could then be caused by auxin suppressing cytokinin biosynthesis (Young et al. 2014).
Sucrose bud outgrowth physiology
In the treatments with sucrose, stem segments on 1.5% sucrose medium showed stronger axillary bud growth compared to stem segments on sucrose free medium. Stem pieces treated with 5-µM IAA in sucrose medium showed inhibition of axillary bud outgrowth compared to stems on 1.5% sucrose but a similar extent of bud growth as compared to the sucrose free control (Fig. 6a). It should be noted that the 5-µM IAA treatment in sucrose medium inhibited bud growth until day 3, while at day 5, the bud growth of the IAA-treated stems surpassed that of the control treatment. This indicates that the apical IAA induced an initial inhibition of bud outgrowth but, compared to IAA treatments in sucrose free medium, bud outgrowth occurred with a shorter delay. This shows that apical auxin maintained an inhibitory effect on axillary bud outgrowth but that sucrose decreased the delay period of outgrowth. These observations are still in line with a central role of auxin in the regulation of axillary bud outgrowth but also show the importance of the interaction with sucrose requirement.
Unlike IAA application, GR24 treatment in combination with sucrose did not result in axillary bud outgrowth inhibition (Fig. 6b). This observation contrasts with the bud outgrowth inhibition that was seen in treatment with GR24 in the sucrose free medium. This indicates that the effect of GR24 alone could not inhibit axillary bud growth when sucrose was supplied to the plant. Interestingly, in the sucrose medium, treatment with apical auxin could still restrict axillary bud growth compared to the control. These observations do not comply with the second messenger model. According to this model the action of strigolactone is downstream of auxin so at least some bud growth inhibition would be expected. A possible explanation could be that other factors would play a more influential role on bud outgrowth in decapitated plants than strigolactones. Strigolactone could also be upstream from sucrose, making GR24 treatment ineffective in bud inhibition. The inhibition that was seen with IAA treatment could then have been due to strigolactone independent factors, like repressed cytokinin biosynthesis (Young et al. 2014) or reduced sink strength of the stem for auxin transport from the axillary buds according to the canalization model.
In addition to the canalization and second messenger model, these observations could be explained by considering separate bud outgrowth mechanisms for intact plants and for decapitated plants (Mason et al. 2014). In intact plants, the hormonal balance between auxins, strigolactones, and cytokinins would maintain the apical dominance and control over outgrowth of axillary buds. In decapitated plants a more rapid response would occur (Morris et al. 2005), making sucrose available for axillary buds that could subsequently grow out. After bud outgrowth these axillary shoots would become a new source of auxin, reestablishing the hormonal balance. Stem segments that were supplied with sucrose in the assay presented here would in this way represent decapitated plants with a rapid bud outgrowth response, independent from hormonal control, explaining the ineffectiveness of GR24 to inhibit bud growth. This, however, leaves out an explanation for the bud growth inhibition that was still seen in treatments with apical IAA and sucrose that fits best with the auxin canalization model.
Conclusions
In this work, a modified split-plate assay for chrysanthemum nodal stem segments was used and axillary bud outgrowth with applications of IAA, cytokinins (TDZ, zeatin, 2-iP) strigolactone (GR24), and sucrose were examined. Apical IAA treatments inhibited axillary bud outgrowth within a 5-day period and severely delayed bud growth after 8 days. Basal cytokinin treatments, as well as apical NPA treatment, could reduce bud growth inhibition by apical IAA. Treatment with 5-µM GR24 showed inhibition of the lower axillary bud and outgrowth of the upper axillary bud, consistent with the auxin canalization model. On the other hand, treatment with 50-µM GR24 could inhibit both LN and UN axillary bud growth without an apical auxin source, which is in line with the second messenger model. However, limiting the polar auxin transport with NPA and TIBA reduced the inhibition by 50-µM GR24, a result that also fits the auxin canalization model. With application of sucrose, apical IAA application could inhibit bud outgrowth, unlike basal GR24 treatment. These observations have raised some important questions about the inhibition mechanisms of auxin and strigolactone. The observed lack of bud outgrowth inhibition in stem segments treated with GR24 and sucrose warrants further investigation. For this purpose, the split-plate bioassay offers an easy and practical method which can be combined with hormone measurements, gene expression analysis and in situ hybridization to reveal the underlying molecular mechanisms.
Author contribution statement
RD designed and performed the experiments and wrote the manuscript. JVH, EDK, ED, JDR, and DVDS provided conceptual guidance in outlining the paper and revised the manuscript. JVH and ED assisted in the setup of the figures and statistics.
References
Barbier F, Peron T, Lecerf M et al (2015) Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66:2569–2582. doi:10.1093/jxb/erv047
Bennett T, Leyser O (2006) Something on the side: axillary meristems and plant development. Plant Mol Biol 60:843–854. doi:10.1007/s11103-005-2763-4
Beveridge CA, Symons GM, Turnbull CG (2000) Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol 123:689–698
Blakeslee JJ, Bandyopadhyay A, Lee OR et al (2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19:131–147. doi:10.1105/tpc.106.040782
Brewer PB, Dun EA, Ferguson BJ et al (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150:482–493. doi:10.1104/pp.108.134783
Brewer PB, Dun EA, Gui R et al (2015) Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiol. doi:10.1104/pp.15.00014
Chatfield SP, Stirnberg P, Forde BG, Leyser O (2000) The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 24:159–169
Chen X, Zhou X, Xi L et al (2013) Roles of DgBRC1 in regulation of lateral branching in chrysanthemum (Dendranthema × grandiflora cv. Jinba). PLoS One 8:e61717. doi:10.1371/journal.pone.0061717
Cline MG (1991) Apical dominance. Bot Rev 57:318–358. doi:10.1007/BF02858771
Crawford S, Shinohara N, Sieberer T et al (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137:2905–2913. doi:10.1242/dev.051987
Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12:211–221. doi:10.1038/nrm3088
Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2013) Dynamics of strigolactone function and shoot branching responses in Pisum sativum. Mol Plant 6:128–140. doi:10.1093/mp/sss131
Foo E, Bullier E, Goussot M et al (2005) The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17:464–474. doi:10.1105/tpc.104.026716.1
Foo E, Morris SE, Parmenter K et al (2007) Feedback regulation of xylem cytokinin content is conserved in pea and Arabidopsis. Plant Physiol 143:1418–1428. doi:10.1104/pp.106.093708
Friml J, Vieten A, Sauer M et al (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153. doi:10.1038/nature02085
Gomez-Roldan V, Fermas S, Brewer PB et al (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194. doi:10.1038/nature07271
Katekar GF, Geissler AE (1977) Auxin transport inhibitors. Plant Physiol 60:826–829
Kebrom TH, Mullet JE (2015) Photosynthetic leaf area modulates tiller bud outgrowth in sorghum. Plant Cell Environ n/a n/a. doi:10.1111/pce.12500
Kebrom TH, Brutnell TP, Finlayson SA (2010) Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant, Cell Environ 33:48–58. doi:10.1111/j.1365-3040.2009.02050.x
Kushwah S, Laxmi A (2014) The interaction between glucose and cytokinin signal transduction pathway in Arabidopsis thaliana. Plant Cell Environ 37:235–253. doi:10.1111/pce.12149
Liang J, Zhao L, Challis R, Leyser O (2010) Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J Exp Bot 61:3069–3078. doi:10.1093/jxb/erq133
Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28:465–474. doi:10.1046/j.1365-313X.2001.01173.x
Mashiguchi K, Sasaki E, Shimada Y et al (2009) Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci Biotechnol Biochem 73:2460–2465. doi:10.1271/bbb.90443
Mason MG, Ross JJ, Babst BA et al (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci. doi:10.1073/pnas.1322045111
Mishra BS, Singh M, Aggrawal P, Laxmi A (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS One 4:e4502. doi:10.1371/journal.pone.0004502
Morris SE, Cox MCH, Ross JJ et al (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138:1665–1672. doi:10.1104/pp.104.058743
Ongaro V, Leyser O (2008) Hormonal control of shoot branching. J Exp Bot 59:67–74. doi:10.1093/jxb/erm134
Petrásek J, Friml J (2009) Auxin transport routes in plant development. Development 136:2675–2688. doi:10.1242/dev.030353
Petrásek J, Mravec J, Bouchard R et al (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312:914–917
Rabot A, Henry C, Ben Baaziz K et al (2012) Insight into the Role of Sugars in Bud Burst Under Light in the Rose. Plant Cell Physiol 53:1068–1082. doi:10.1093/pcp/pcs051
Rameau C, Bertheloot J, Leduc N et al (2015) Multiple pathways regulate shoot branching. Front Plant Sci 5:1–15. doi:10.3389/fpls.2014.00741
Sachs T, Thimann KV (1967) The role of auxins and cytokinins in the release of buds from dominance. Am J Bot 54:136–144
Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11:e1001474. doi:10.1371/journal.pbio.1001474
Tanaka M, Takei K, Kojima M et al (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45:1028–1036. doi:10.1111/j.1365-313X.2006.02656.x
Van Minnebruggen A, Roldan-Ruiz I, Van Dingenen J et al (2013) Morphological and molecular characterization of branching in red clover (Trifolium pratense). In: Barth S, Milbourne D (eds) Breeding strategies for sustainable forage and turf grass improvement SE—20. Springer, Netherlands, pp 161–167
Waldie T, McCulloch H, Leyser O (2014) Strigolactones and the control of plant development: lessons from shoot branching. Plant J 79:607–622. doi:10.1111/tpj.12488
Young NF, Ferguson BJ, Antoniadi I et al (2014) Conditional auxin response and differential cytokinin profiles in shoot branching mutants. Plant Physiol 165:1723–1736. doi:10.1104/pp.114.239996
Acknowledgements
This research was funded by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen Grant No. 110771). The authors wish to thank Annemie Van Minnebruggen and Gerda Cnops for demonstrating the split-plate assay in red clover. DVDS acknowledges Ghent University for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by H. Li.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dierck, R., Dhooghe, E., Van Huylenbroeck, J. et al. Response to strigolactone treatment in chrysanthemum axillary buds is influenced by auxin transport inhibition and sucrose availability. Acta Physiol Plant 38, 271 (2016). https://doi.org/10.1007/s11738-016-2292-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2292-6