Abstract
Studies comparing right (RC) and left colectomies (LC) show higher rates of ileus in RC and higher wound infection and anastomotic leak rates in LC. However, prior studies did not include robotic procedures. We compared short-term outcomes of laparoscopic and robotic RC and LC for cancer, with sub-analysis of robotic procedures. In a retrospective review of a prospective database, preoperative factors, intraoperative events, and 30-day postoperative outcomes were compared. Student’s t tests and Chi-square tests were used for continuous and categorical variables, respectively. A logistic binomial regression was performed to assess whether type of surgery was associated with postoperative complications. Between January 2014 and August 2020, 115 patients underwent minimally invasive RC or LC for cancer. Sixty-eight RC [30 (44.1%) laparoscopic, 38 (55.9%) robotic] and 47 LC [13 (27.6%) laparoscopic, 34 (72.4%) robotic] cases were included. On univariate analysis, RC patients had significantly higher overall postoperative complications but no differences in rates of ileus/small bowel obstruction, wound infection, time to first flatus/bowel movement, length of hospital stay, and 30-day readmissions. On multivariate analysis, there was no significant difference in overall complications and laparoscopic surgery had a 2.5 times higher likelihood of complications than robotic surgery. In sub-analysis of robotic cases, there was no significant difference among all outcome variables. Previously reported outcome differences between laparoscopic RC and LC for cancer may be mitigated by robotic surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In analyzing post-colectomy outcomes, right (RC) and left (LC) colectomies are generally grouped together despite previous studies showing disparities in outcome [1,2,3,4,5,6,7]. Differences in anatomical and physiological sections of the colon may lead to differences in outcomes among patients undergoing resection. It is unknown how much of the difference is due to surgical technique rather than disease process. In comparing RC and LC, most studies group open and laparoscopic surgeries together while a few focus solely on laparoscopy [1,2,3,4,5,6,7]. Moreover, several of the studies include varieties of pathology and indication (i.e., colon cancer, diverticular disease, inflammatory bowel disease) [1, 7, 8]. Past comparative studies have shown that left colectomy (LC) for cancer has higher rates of anastomotic leak and wound infections, whereas right colectomy (RC) has higher rates of ileus [1, 2, 9]. No studies to our knowledge have included robotic procedures when comparing RC and LC [1,2,3,4,5,6,7]. It is not known whether the surgical limitations of laparoscopic and open techniques may result in different outcomes for different types of colectomies. Therefore, we sought to compare outcomes of minimally invasive right and left colectomies for cancer with sub-analysis of robotic RC versus LC to determine whether robotic techniques mitigate disparities.
Materials and methods
A retrospective review of a prospective colorectal surgery database was conducted between January 2014 and August 2020. Consecutive patients over 18 years old undergoing laparoscopic or robotic right and left colonic resection for tumors were included. The right colectomy group included those undergoing ileocolic resection or right hemicolectomy for benign or malignant lesions in the cecum, ascending colon, hepatic flexure, and proximal transverse colon. The left colectomy group included those undergoing left hemicolectomies or sigmoidectomies for benign and malignant lesions in the distal transverse, splenic flexure, descending, sigmoid, and rectosigmoid colon. Colonic resections for inflammatory and infectious indications, such as diverticulitis, ischemic colitis, and Crohn’s disease, were excluded to maintain homogeneity between groups in our sample. Decisions to perform surgery laparoscopically or robotically were based on the availability of robotic systems rather than the complexity of the operation, with increased access to the robot beginning in 2017. The same oncological guidelines and parameters were followed during either laparoscopic or robotic surgery as applicable to oncological bowel resection, vascular ligation, and lymphadenectomy. All procedures were performed by two double-board certified surgeons with a combined 18 years of experience in laparoscopic surgery and 14 years of experience in robotic surgery. Each surgeon has performed over 200 robotic colorectal cases. The study was approved by Cedars-Sinai Medical Center’s institutional review board (Pro00039937).
In robotic RC, the dissection, resection, and anastomosis were performed intracorporeally. The incision was made solely for extraction of the specimen. In laparoscopic RC, the entire dissection and mesenteric ligation were done intracorporeally while bowel resection/stapling and anastomosis were performed extracorporeally. In both robotic and laparoscopic LC, the entire dissection, mesenteric ligation, and resection were performed intracorporeally. Then, the colocolonic or colorectal anastomosis was performed with the use of the EEA stapler. The anvil of the stapler was introduced into the proximal colon extracorporeally following extraction of the specimen. The EEA spike and anvil were then engaged laparoscopically.
RC and LC patients were compared by analyzing patients’ preoperative, intraoperative, and postoperative records. Preoperative factors included age, sex, BMI, ASA (scored dichotomously as 1–2 and 3–4), Charlson Comorbidity Score [10], and smoking status (never smoked, prior smoker, and current smoker). Intraoperative variables analyzed were total operative time, laparoscopic time, robotic console time, estimated blood loss, rate of conversion to an open operation, and intraoperative complications (bladder injury, enterotomy, hemorrhage, liver injury, ureter damage, splenic injury, anastomotic leak, and death). Robotic console time was defined as the total time the surgeon spent operating on the console. Docking and undocking time were not included, as they are dependent on various factors including room setup and experience of operating room staff as opposed to console time, which is more reflective of complexity of the operation. Laparoscopic time describes the total time the surgeon was operating using a laparoscope. Postoperative variables recorded were overall complications, medical complications (deep venous thrombosis, pneumonia, pulmonary embolism, urinary infection, acute renal failure, myocardial infarction, urinary retention, and c. diff), surgical complications (wound infection, anastomotic leak, abdominal/pelvic abscess, and ileus or small bowel obstruction), time to first flatus or bowel movement, length of hospital stay, and 30-day readmissions. Ileus was defined as abdominal distention with vomiting or necessitating insertion of a nasogastric tube without a clear transition point on imaging. Small bowel obstruction (SBO) was defined as intestinal blockage with a transition point on imaging. Wound infections included both superficial and deep incisional infections. Overall complications were also categorized according to the Clavien–Dindo classification [11]. Time to first flatus or bowel movement was measured in postoperative days.
Sub-analysis including only robotic cases was conducted. Variables included were age, sex, Charlson Comorbidity Score, total operative time, estimated blood loss, overall postoperative complications, overall medical and surgical postoperative complications, ileus or SBO, wound infection, time to first flatus or bowel movement, length of hospital stay, and 30-day readmissions. Clavien–Dindo classification was utilized for robotic complications. Sub-analysis of laparoscopic cases was not performed due to the small number of cases.
A Student’s t test and Chi-square test were run for continuous and categorical variables, respectively. Lastly, a logistic binomial regression was performed to assess whether type of surgery was associated with postoperative complications. All statistics were run in SPSS Statistics 27.0. P values less than 0.05 were considered significant.
Results
Demographics and preoperative variables (Table 1)
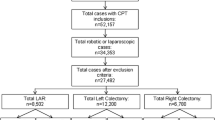
Our cohort included a total of 115 patients who underwent minimally invasive RC or LC for cancer. The RC group (n = 68) consisted of 30 laparoscopic and 38 robotic cases, whereas the LC group (n = 47) consisted of 13 laparoscopic and 34 robotic cases. The RC group had a significantly higher mean age than the LC group (71.1 vs. 63.9, p = 0.007). There was no difference between the two groups in sex, BMI, ASA, Charlson Comorbidity Score, smoking status, and surgical technique.
Intraoperative events (Table 2)
Patients undergoing RC had a higher mean robotic console time (153.5 vs. 92.9, p < 0.0001) likely due to intracorporeal anastomosis in RC. Total operative times were found to be nearly the same in both groups (212 vs. 224 min, p = 0.48). The only intraoperative complication found was hemorrhage— one in each group. There were no significant differences in laparoscopic time, estimated blood loss, conversion to open operation, and overall intraoperative complications.
Postoperative events (Tables 3, 4)
The overall complication rate was significantly higher in the RC group than the LC group (50.0% vs. 29.8%, p = 0.032). Nevertheless, there was no significant difference in either overall medical (27.9% vs. 14.9%, p = 0.10) or surgical complications (38.2% vs. 23.4%, p = 0.09). More specifically, there were no significant differences in rates of ileus/SBO (27.9% vs. 19.1%, p = 0.28) and wound infections (9.0% vs. 4.3%, p = 0.34). Although RC had a higher length of hospital stay than LC, the difference was not significant (median: 5 vs. 4 days, p = 0.47). There were no significant differences in time to first flatus or bowel movement (3.0 vs. 2.7 days, p = 0.38) and 30-day readmissions (9% vs. 8.5%, p = 0.72). There were no significant differences among each Clavien–Dindo subclass.
Multivariate analysis (Table 5)
A binomial regression showed that RC and LC are not significantly different in overall postoperative complications when adjusting for age, gender, and surgical technique (p = 0.260). Furthermore, laparoscopic surgery was 2.5 times more likely to have complications than robotic surgery when adjusting for age, gender, and side (Odds Ratio 2.54, p = 0.029).
Robotic sub-analysis (Tables 6,7)
A sub-analysis on outcomes of robotic cases was conducted. Robotic RC (n = 38) patients were significantly older than robotic LC (n = 34) patients (72.1 vs. 64.1, p = 0.0083). There were no differences between sex, Charlson comorbidity score, operation length, estimated blood loss, overall postoperative complications, overall medical and surgical complications, small bowel obstruction or ileus, wound infection, time to first flatus or bowel movement, length of hospital stay, and 30-day readmissions. No significant difference was observed between Clavien–Dindo subgrades.
Discussion
Our retrospective review of a prospective database showed similar outcomes between minimally invasive RC and LC for cancer. Both procedures were shown to be safe with low rates of conversion and comparable complications despite differences in technique. Right colectomy was associated with older patient age and a higher rate of overall postoperative complications. However, multivariate analysis revealed no differences in postoperative complications between RC and LC when adjusting for age, gender, and surgical technique. Interestingly, multivariate analysis did reveal a 2.5 times higher likelihood of complications in laparoscopic versus robotic cases when adjusting for age, gender, and side. A sub-analysis of robotic cases showed no differences between RC and LC in all outcome variables.
This is the first study to directly compare outcomes following minimally invasive RC and LC for cancer with incorporation of robotics. Only three other studies—Campana et al., Turrado-Rodriguez et al., and Nfonsam et al.—have compared minimally invasive RC versus LC for cancer, but with the inclusion of only laparoscopic cases [2, 3, 7]. Campana’s series included a retrospective review of a prospective database of 547 patients from a tertiary hospital operated on by staff and residents between 2004 and 2014 [2]. Turrado-Rodriguez retrospectively analyzed 881 cancer patients from their own single-center prospective laparoscopic database between 1998 and 2012 [7]. Nfonsam’s study consisted of 2512 patients from the American College of Surgeons NSQIP database between 2005 and 2010 [3]. Each study reported on preoperative, intraoperative, and short-term postoperative variables. Other studies comparing RC and LC have included both open and laparoscopic methods and grouped together colectomies for various indications [1, 4,5,6, 8]. As such, we will mainly limit our discussion to the three pertinent papers mentioned above.
Similar to our study, others have shown that patients undergoing RC are older than LC [2, 5, 6]. Several papers found RC patients to have more comorbidities and higher ASA scores [2, 5, 12]. Although we saw a trend towards higher comorbidities and ASA scores in our RC group, this did not reach significance which is likely reflective of our relatively small sample size.
In regard to differences in intraoperative complications, our results mirror those reported in the literature. Campana et al. revealed no significant differences between RC and LC [2]. Turrado-Rodriguez’s series reported greater operative complexity in the RC group as manifested by the higher rate of adhesions and difficulty of dissections [7]. These differences were likely due to their inclusion of a higher proportion of patients in the RC group with previous abdominal surgeries. Otherwise, in their series, there were no differences between RC and LC in intraoperative complications, such as hemorrhage and hollow viscus perforation. Nfonsam et al. only found significantly higher rates of ureteral injury in LC versus RC (0.6% vs. 0.4%; p < 0.04) [3]. We did not have any ureteral injuries in our series, likely reflective of our sample size and the rarity of this complication.
Rates of conversion to an open operation have been a subject of interest due to its association with increased postoperative complications [13]. Turrado-Rodriguez et al. and Campana et al. found no differences in conversion between the two groups [2, 7]. Nfonsam and other studies that combined open and laparoscopic procedures found higher conversion rates in LC than RC [3, 11, 14]. We found 4 (5.9%) conversions in RC vs. 3 (7.5%) in LC (p = 0.91). Of the overall conversions, 5 were in laparoscopy and 2 in robotics for a rate difference of 11.6% vs. 2.8% (p = 0.055), respectively. This is consistent with several comparative studies favoring robotics over laparoscopic in terms of colorectal surgery conversion rates [15,16,17].
Following multivariate analysis, we found no difference in overall postoperative complications between RC and LC. Past studies have revealed opposing results for overall postoperative complication rates with some being higher in RC [6], others in LC [1, 7], and the rest being equal [2,3,4,5, 8, 14]. The three aforementioned laparoscopic studies have also produced mixed results. While Campana et al. and Nfonsam et al. found no difference in overall postoperative complications, Turrado-Rodriguez et al. found LC to have a higher rate of overall complications [2, 3, 7].
Previous minimally invasive comparative papers revealed differences in postoperative ileus and surgical site infection (SSI) [2, 3]. Campana et al. showed RC to have a fourfold higher rate of ileus than LC [2]. While Nfonsam et al. did not find a specific difference in rates of ileus or SBO, they and other papers did report higher rates of wound infections in LC [1, 3, 5]. Nfonsam hypothesized this to be due to a higher bacterial load in the left colon [3]. In our study, we found no significant difference between RC and LC in either ileus/SBO or SSI.
We hypothesized that no outcome differences were found between minimally invasive RC and LC in our series because of several factors: (1) the surgeries were performed by two skilled and experienced laparoscopic and robotic colorectal surgeons in a (2) large, tertiary hospital with (3) experienced laparoscopic and dedicated robotic operative staff, and (4) the added benefits of robotic surgery. Previous studies comparing outcomes of robotic versus laparoscopic colon resections have favored robotics. Duan et al. published a meta-analysis of robotic versus laparoscopic colectomy for cancer consisting of fourteen studies and 125,998 patients (4924 robotic and 121,055 laparoscopic). They found significantly lower blood loss, conversion rate, hospital length of stay, postoperative complication rate, and faster return to bowel function in robotics [18]. Another meta-analysis by Trastulli et al. of the robotic and laparoscopic approaches to malignant and benign colonic diseases consisting of 12 papers with a total of 4148 patients (744 robotic and 3404 laparoscopic) similarly found significantly lower estimated blood loss, overall postoperative complications, wound infections, hospital length of stay, and shorter time to first flatus in the robotic group [19]. In our series, the majority of cases performed were robotic (63%). This may have in turn dampened differences in outcome between RC and LC, as seen in previous papers reporting on laparoscopic colectomies.
Robotic surgery in our series may have further mitigated differences in outcome between RC and LC as it facilitates intracorporeal suturing and anastomosis. Robotics may achieve this through its benefits of 3D vision, articulating instruments, and ease of suturing [20,21,22]. Campana et al. partially attributed their lower rate of ileus in LC to intracorporeal anastomosis in LC versus extracorporeal anastomosis in RC in their series. Previous studies have also shown better outcomes in intracorporeal versus extracorporeal anastomoses. Two meta-analyses comparing the anastomotic techniques in RC showed intracorporeal anastomosis to be associated with significantly lower conversion to open-surgery rate, hospital length of stay, anastomotic leak, surgical site infections, total complications, and earlier bowel recovery rates [23, 24]. There are fewer studies on the intracorporeal technique in LC, however, a meta-analysis showed intracorporeal anastomosis to be associated with fewer conversions, faster recovery of bowel function, decreased postoperative complications, and shorter length of stay when compared to extracorporeal anastomosis [25]. These benefits are likely due to less bowel manipulation and smaller extraction site incisions [25].
Our study has several strengths. To our knowledge, this is the first study comparing outcomes of RC and LC to incorporate robotic surgeries. The inclusion of robotics fills an important gap in the literature of RC versus LC comparative studies as it is becoming increasingly more utilized and offers added advantages. We only focused on minimally invasive surgeries specifically for cancer, minimizing further confounding variability. Furthermore, by only having two highly skilled surgeons perform each case, we reduced the variability that may otherwise be seen in reports using large databases or resident surgeons. Importantly, our database was collected by the surgeons themselves, minimizing errors commonly seen in larger administrative databases.
Our study contains several limitations. We included a small number of patients. The two surgeons in the study were skilled, experienced minimally invasive surgeons operating in a large tertiary center which may not reflect well the makeup of the surgical community and experience at large. There was a variable rate of robotic surgery in each cohort which may have further confounded findings.
Conclusion
When comparing minimally invasive RC and LC for cancer, we found no differences in intraoperative or postoperative outcomes. Robotic surgery may help improve colectomy outcomes and in turn level differences between RC and LC seen in previous reports.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Hinojosa MW, Konyalian VR, Murrell ZA, Varela JE, Stamos MJ, Nguyen NT (2007) Outcomes of right and left colectomy at academic centers. Am Surg. https://doi.org/10.1177/000313480707301002
Campana JP, Pellegrini PA, Rossi GL, Ojea Quintana G, Mentz RE, Vaccaro CA (2017) Right versus left laparoscopic colectomy for colon cancer: does side make any difference? Int J Colorectal. https://doi.org/10.1007/s00384-017-2776-x
Nfonsam V, Aziz H, Pandit V, Khalil M, Jandova J, Joseph B (2016) Analyzing clinical outcomes in laparoscopic right vs. left colectomy in colon cancer patients using the NSQIP database. Cancer Treat Commun. https://doi.org/10.1016/j.ctrc
Lavazza M, Rausei S, Lianos GD, Pappalard VO, Frattini F, Dionigi G, Iovino D, Rovera F, Boni L (2017) Right-sided versus left-sided colectomies for cancer: surgical outcomes and novel considerations. Surg Technol Int 31:111–116
Kwaan MR, Al-Refaie WB, Parsons HM, Chow CJ, Rothenberger DA, Habermann EB (2013) Are right-sided colectomy outcomes different from left-sided colectomy outcomes?: Study of patients with colon cancer in the ACS NSQIP database. JAMA Surg. https://doi.org/10.1001/jamasurg.2013.1205
Masoomi H, Buchberg B, Dang P, Carmichael JC, Mills S, Stamos MJ (2011) Outcomes of right vs. left colectomy for colon cancer. J Gastrointest Surg. https://doi.org/10.1007/s11605-011-1655-y
Turrado-Rodriguez V, Targarona Soler E, Bollo Rodriguez JM, Balagué Ponz C, Hernández Casanovas P, Martínez C, Trías Folch M (2016) Are there differences between right and left colectomies when performed by laparoscopy? Surg Endosc. https://doi.org/10.1007/s00464-015-4345-0
Rana AR, Cannon JA, Mostafa G, Carbonell AM, Kercher KW, Norton HJ, Heniford BT (2007) Outcomes of right- compared with left-side colectomy. Surg Innov. https://doi.org/10.1177/1553350607303209
Veyrie N, Ata T, Muscari F, Couchard AC, Msika S, Hay JM, Fingerhut A, Dziri C (2007) Anastomotic leakage after elective right versus left colectomy for cancer: prevalence and independent risk factors. J Am Coll Surg. https://doi.org/10.1016/j.jamcollsurg.2007.06.284
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, Colon/Rectum Carcinomas (Primary Tumor) Study Group (2010) Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. https://doi.org/10.1007/DCR.0b013e3181c703a4
Masoomi H, Moghadamyeghaneh Z, Mills S, Carmichael JC, Pigazzi A, Stamos MJ (2015) Risk factors for conversion of laparoscopic colorectal surgery to open surgery: does conversion worsen outcome? World J Surg. https://doi.org/10.1007/s00268-015-2958-z
Tekkis PP, Senagore AJ, Delaney CP, Fazio VW (2005) Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. https://doi.org/10.1097/01.sla.0000167857.14690.68
Wells LE, Smith B, Honaker MD (2020) Rate of conversion to an open procedure is reduced in patients undergoing robotic colorectal surgery: a single-institution experience. J Minim Access Surg. https://doi.org/10.4103/jmas.JMAS_318_18
Tam MS, Kaoutzanis C, Mullard AJ, Regenbogen SE, Franz MG, Hendren S, Krapohl G, Vandewarker JF, Lampman RM, Cleary RK (2016) A population-based study comparing laparoscopic and robotic outcomes in colorectal surgery. Surg Endosc. https://doi.org/10.1007/s00464-015-4218-6
Dolejs SC, Waters JA, Ceppa EP, Zarzaur BL (2017) Laparoscopic versus robotic colectomy: a national surgical quality improvement project analysis. Surg Endosc. https://doi.org/10.1007/s00464-016-5239-5
Duan BS, Zhao GH, Yang H, Wang Y (2016) A pooled analysis of robotic versus laparoscopic surgery for colon cancer. Surg Laparosc Endosc Percutan Tech. https://doi.org/10.1097/SLE.0000000000000359
Trastulli S, Cirocchi R, Desiderio J, Coratti A, Guarino S, Renzi C, Corsi A, Boselli C, Santoro A, Minelli L, Parisi A (2015) Robotic versus laparoscopic approach in colonic resections for cancer and benign diseases: systematic review and meta-analysis. PLoS One. https://doi.org/10.1371/journal.pone.0134062
Vallribera F, Kraft M, Pera M, Vidal L, Espín-Basany E (2021) Outcomes of intra- versus extra-corporeal ileocolic anastomosis after minimally invasive right colectomy for cancer: an observational study. J Clin Med. https://doi.org/10.3390/jcm10020307
Zelhart M, Kaiser AM (2018) Robotic versus laparoscopic versus open colorectal surgery: towards defining criteria to the right choice. Surg Endosc. https://doi.org/10.1007/s00464-017-5796-2
Yeo HL, Isaacs AJ, Abelson JS, Milsom JW, Sedrakyan A (2016) Comparison of open, laparoscopic, and robotic colectomies using a large national database: outcomes and trends related to surgery center volume. Dis Colon Rectum. https://doi.org/10.1097/DCR.0000000000000580
Emile SH, Elfeki H, Shalaby M, Sakr A, Bassuni M, Christensen P, Wexner SD (2019) Intracorporeal versus extracorporeal anastomosis in minimally invasive right colectomy: an updated systematic review and meta-analysis. Tech Coloproctol. https://doi.org/10.1007/s10151-019-02079-7
van Oostendorp S, Elfrink A, Borstlap W, Schoonmade L, Sietses C, Meijerink J, Tuynman J (2017) Intracorporeal versus extracorporeal anastomosis in right hemicolectomy: a systematic review and meta-analysis. Surg Endosc. https://doi.org/10.1007/s00464-016-4982-y
Brown RF, Cleary RK (2020) Intracorporeal anastomosis versus extracorporeal anastomosis for minimally invasive colectomy. J Gastrointest Oncol. https://doi.org/10.21037/jgo.2019.12.02
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BC and EK were responsible for statistical analyses. All authors contributed to literature review, manuscript drafting and revising, and review prior to submission.
Corresponding author
Ethics declarations
Conflict of interest
Yosef Nasseri declares that he has no conflict of interest. Eli Kasheri declares that he has no conflict of interest. Kimberly Oka declares that she has no conflict of interest. Brian Cox declares that he has no conflict of interest. Jason Cohen declares that he has no conflict of interest. Joshua Ellenhorn declares that he has no conflict of interest. Moshe Barnajian declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasseri, Y., Kasheri, E., Oka, K. et al. Minimally invasive right versus left colectomy for cancer: does robotic surgery mitigate differences in short-term outcomes?. J Robotic Surg 16, 875–881 (2022). https://doi.org/10.1007/s11701-021-01310-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-021-01310-8