Abstract
Robotic Roux en Y gastric bypass (R-RYGB) is becoming more common due to the shifting trend toward robotic gastrointestinal surgery. The goal of this study is to determine if R-RYGB can be safely implemented at a robotic bariatric surgery program in a community hospital with similar results to laparoscopic RYGB (L-RYGB) in a cost-effective manner. A total of 50 R-RYGB procedures were performed with the Xi and the X da Vinci systems and compared with 50 L-RYGB cases by a single surgeon from October 2018 to January 2020 at an acute-care community hospital in a rural setting with a high-volume MBSAQIP-accredited program. A retrospective chart review was conducted with IRB approval and statistical analysis of 30-day morbidity, mortality, re-interventions, and resolution of co-morbidities, with financial analysis of cost reduction. Both groups were similar in age, gender, ASA class, co-morbidities, and body mass index (BMI). There was no mortality or anastomotic leak. The 30-day morbidity for R-RYGB was 10.0% with a re-operation rate of 4.0%. There were no conversions to open, and the mean hospital length of stay was 2.22 ± 1.19 days. There were no statistically significant differences between R-RYGB and L-RYGB with respect to any measured outcome, including intraoperative time (121.94 vs. 113.52, respectively; p = 0.1495). However, when incidences and percentages were used, R-RYGB had improved performance for most of the outcomes measuring safety. There was an average cost reduction of $816.90 per case (total saving of $40,845.00 for 50 cases) in the R-RYGB by transitioning from a hybrid approach to a totally robotic approach. R-RYGB appears to be as safe as L-RYGB and can be performed in a rural community hospital while maintaining a low complication rate, achieving a high co-morbidity resolution rate, and saving costs with a totally robotic approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relevance and practical applications of metabolic and bariatric surgery as a surgical specialty have been long recognized. These benefits have proven to outweigh the risks when the resolution of obesity-related co-morbidities is considered [1,2,3]. Minimally invasive surgical approaches have been validated including the implementation of Advanced Recovery After Surgery (ERAS) protocols to improve outcomes and decrease morbidity [4]. Since its 1994 inception as a laparoscopic procedure, the Roux en Y gastric bypass (RYGB) has become an effective operation that continues to be regarded as the gold standard within the specialty [5].
Medical telerobotic and computer-assisted surgical platforms have been developing for decades, and the current state-of-the-art modalities that are coming to the market reflect a rapidly evolving field [6]. The benefits of robotic surgery have been extensively discussed since the beginning of its wide applications in the surgical specialties in the early 2000s [7]. With such advantages, robotic surgery and its applications have been successfully applied to complex gastrointestinal surgery [8, 9]. This includes metabolic and bariatric surgery, with the RYGB as an ideal procedure where the robotic technology can be responsibly used [10].
Although initial studies reported that the robotic RYGB (R-RYGB) takes longer compared to laparoscopy and its complication rates may be greater, there has not been a uniform way to perform these procedures or to report their outcomes. Such technology has also been successfully applied to less complex operations such as vertical sleeve gastrectomy [11, 12]. More recent studies, mostly based on case series, have shown how effective the R-RYGB is in the adult population including the elderly and those within the super obesity classification, sometimes with a lower rate of complications when compared to laparoscopy [13,14,15]. The totally robotic approach, as opposed to a hybrid approach with laparoscopic stapling, has been beneficial and is associated with lower complication rates [16, 17]. Such observations include revisional bariatric surgery that involves conversions or reconstructions of RYGB [18, 19].
This study seeks to evaluate whether the R-RYGB has a similar safety profile to the laparoscopic approach when performed at a community, non-academic hospital in a rural setting, which is a topic that has not been extensively explored by other publications. As a secondary objective, the study examines whether the hybrid approach (laparoscopic stapling) or the totally robotic approach (robotic stapling) is the most cost-effective option in this context. We hypothesize that regardless of the community hospital environment and the lack of association with a major academic center, it is possible to perform R-RYGB with safe outcomes that are comparable to those of the L-RYGB with mastery of the learning curve for the surgical team. As a result, a retrospective chart review has been conducted with non-inferiority purposes to establish whether the R-RYGB can be performed in a community hospital bariatric program in the rural setting with low complication rates while maintaining a financially responsible attitude.
Methods
Patient selection
A total of 50 patients’ ages 18–65 years who met criteria for metabolic and bariatric surgery and chose to undergo a primary (non-revisional) R-RYGB were included. A retrospective chart review was conducted with Institutional Review Board (IRB) approval at Winchester Medical Center, a 495-bed acute-care community hospital in the Shenandoah Valley of Virginia, USA. All of the 50 R-RYGB procedures were performed by a single metabolic and bariatric surgeon with the Xi and the X da Vinci systems (Intuitive Surgical, Sunnyvale, CA, USA). This study cohort was compared with 50 patients who underwent laparoscopic RYGB (L-RYGB) by the same surgeon from the same time period, October 2018–January 2020. The same institution has a high-volume MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) accredited bariatric program and Bariatric Center of Excellence. A statistical analysis of 30-day morbidity, mortality, re-interventions, and resolution of co-morbidities was conducted. This was complemented with a financial analysis of cost reduction per case corresponding to the 50 cases performed with the robotic technology. The robotic approach was offered to the patients regardless of their prior surgical history. If they were considered to be candidates for a minimally invasive surgery, the robotic approach was offered. However, the robotic platform was not available all the time. In such cases, an L-RYGB was performed with the patient’s approval.
Statistical analysis
Chi-square test or Fisher’s exact test were used for categorical variables. Student’s t test was used for continuous variables. Statistical programming language R and SAS were used to perform the data analysis. Per bio-statistical standards, a p value ≤ 0.05 was chosen to be statistically significant.
Primary outcomes
The primary outcomes correspond to complications from both cohorts including 30-day morbidity, mortality, anastomotic leak, anastomotic strictures, anastomotic ulcers, incidence of post-operative Clostridium difficile infection, pneumonia, and deep venous thrombosis (DVT) or pulmonary embolus (PE), among others. These outcomes were identified and measured according to the Clavien–Dindo classification of surgical complications.
Secondary outcomes
The secondary outcomes identified include readmissions, re-interventions (endoscopic or surgical, including return to the operating room within 30 days), emergency department visits, need for rehydration at the infusion center, total intraoperative time (which includes the non-robotic time, docking time, and console time), and hospital length of stay (LOS), in addition to financial data corresponding to total costs of the procedure for the robotic cases and their corresponding average savings per case.
Surgical technique
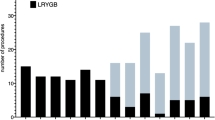
In the R-RYGB group (total of 50 patients), initially, a hybrid robotic approach was applied by performing the entire operation with the robotic platform but with the laparoscopic Powered Echelon 60-mm stapler (Ethicon Endo-Surgery, Johnson & Johnson, Bridgewater, NJ, and Cincinnati, OH, USA), which was fired by the first assistant with guidance from the robotic surgeon. This included the creation of a linear-stapled gastrojejunostomy anastomosis. Group 1 patients underwent the procedure in this fashion (8 patients). Group 2 (2 patients) underwent a totally robotic procedure with the use of the robotic 45-mm stapler (Intuitive Surgical, Sunnyvale, CA, USA) that was also used to construct a linear-stapled gastrojejunostomy. Group 3 (25 patients) underwent a totally robotic procedure with the 60-mm SureForm stapler that was used to create a linear-stapled gastrojejunostomy. Finally, Group 4 (15 patients) had a totally robotic R-RYGB with the 60-mm SureForm stapler, but with a robotic hand-sewn two-layer gastrojejunostomy.
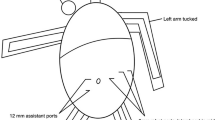
For R-RYGB, the optical trocar entry technique was applied with a 5-mm laparoscopic trocar through a periumbilical incision. The four-arm approach was utilized with three 8-mm trocars and one 12-mm trocar used for the stapler when needed. A Nathanson liver retractor (Cook Medical, Bloomington, IN, USA) was placed through a 5 mm epigastric incision. Insufflation with CO2 at 12-mm Hg facilitated pneumoperitoneum. The peri-gastric dissection technique was used to create a calibrated 15-mL capacity gastric pouch. A 50-cm biliopancreatic limb and a 150-cm alimentary Roux limb were created. As mentioned, Group 1 patients had a linear-stapled gastrojejunostomy created with the laparoscopic Powered Echelon stapler, with the anastomotic opening closed with the robotic intracorporeal suturing technique in two layers with absorbable suture. Groups 2 and 3 had a linear-stapled gastrojejunostomy created with the robotic stapler. Group 4 had a robotic hand-sewn two-layer gastrojejunostomy with absorbable suture. In all cases, the jejunojejunostomy was stapled.
The L-RYGB cohort had a linear-stapled gastrojejunostomy and a linear-stapled jejunojejunostomy, both fashioned with the Powered Echelon laparoscopic stapler. The steps were similar, with the same insufflation parameters and the same gastric pouch volume and intestinal limb measurements. The optical trocar technique was used via a periumbilical incision. However, a total of five 12-mm laparoscopic trocars were used, with the Nathanson liver retractor placed in the epigastric region, too.
Results
There were no incidences of mortality, anastomotic leak, transfusion requirements, or conversions to open in either group.
Table 1 presents the study and control cohort patients’ demographic information along with their most relevant co-morbidities. Both groups were similar in terms of age, gender, pre-operative co-morbidities, American Society of Anesthesiologists (ASA) class, and BMI, with no statistically significant differences. Regarding the demographics, as described, there were no significant differences between the study and the control cohorts. In fact, our patient population in Shenandoah Valley of Virginia is relatively homogeneous in terms of BMI ranges and co-morbidities, which makes it easier for the groups to be compared to each other due to their sharing of co-morbidities and features that would potentially decrease variability. The robotic experience began after most of the laparoscopic experience had already taken place at the institution. In fact, most of the laparoscopic cases were done prior to the robotic cases, with some laparoscopic cases performed intermittently at the end of the first 50 robotic RYGB procedures.
Table 2 shows the presence of additional procedures performed at the same time for both groups when indicated, including ventral or incisional hernia repair without mesh, hiatal hernia repair without mesh, and adhesiolysis. As it was the case with demographic characteristics and co-morbidities, there were no statistical differences between the two groups in terms of performance of additional procedures, and therefore, the likelihood of these procedures affecting the length of the case between both groups was low.
Table 3 provides a comparison between pre-operative and post-operative co-morbidities. Particular attention is noted regarding the co-morbidity resolution or remission. The co-morbidities with the highest rate of success at resolution were OSA, type 2 diabetes mellitus, and gastroesophageal reflux disease (GERD) when compared to hypertension and dyslipidemia for both groups.
Table 4 illustrates the most relevant primary and secondary outcomes for both R-RYGB and L-RYGB. For the R-RYGB cohort, the mean follow-up period was 4.53 ± 3.63 months, while the mean follow-up for the L-RYGB cohort was longer, 7.04 ± 3.19 months. This difference in follow-up duration was statistically significant (p = 0.0004). As a consequence, the mean BMI decrease between the two groups showed statistically significant differences, too, with the R-RYGB patients achieving a decrease of 8.68 ± 5.11 BMI points and the L-RYGB patients losing 11.64 ± 4.47 BMI points (p = 0.002954). However, despite the lack of any other statistical differences, when the outcomes measuring safety were analyzed, the robotic group exhibited lower incidence of complications including marginal ulceration, stricture formation, need for endoscopy, and balloon dilation, among others.
There were no statistically significant differences in rates of resolution of co-morbidities. Although not statistically significant, the R-RYGB group tended to have lower percentages or incidence of complications when compared to the L-RYGB group. These differences were noted in anastomotic marginal ulcer formation (10% vs. 16%, respectively; p = 0.5536) or the need for endoscopic serial balloon dilation over the following weeks or months (2% vs. 6%, respectively; p = 0.6173).
A linear regression analysis of the decrease in BMI was conducted. When the two-sided t test was used to analyze the differences in BMI decrease for the R-RYGB and the L-RYGB groups, initially, there was statistical significance (8.68 ± 5.12 vs. 11.64 ± 4.47 kg/m2, respectively; p = 0.002954) as already shown in Table 4. However, when the linear regression analysis was conducted with the follow-up duration used as a co-variate, this statistical significance was no longer observed (p = 0.3943).
Discussion
The controversy from comparing laparoscopic and robotic surgery across the spectrum of surgical subspecialties seems to be losing momentum. This is due to the surgical community’s realization that robotic surgery is an enhanced extension of laparoscopy, or a refined tool that should be learned and mastered for the benefit of patients and surgeons. This has been supported by large databases such as the MBSAQIP, with studies showing that R-RYGB appears to have lower mortality, transfusion requirements, incidence of bleeding, and surgical site infections (SSI) compared to L-RYGB when executed at experienced centers [20]. A large systematic review and meta-analysis has also found lower incidence of anastomotic leak in robotic bariatric surgery [21]. Retrospective series have continuously reported lower morbidity throughout the years as the R-RYGB continues to improve, and this includes the totally robotic approach and the hand-sewn gastrojejunostomy creation [22,23,24].
Surgeon experience and first assistant level of expertise are important factors for mastery of the team’s learning curve, but the surgeon experience is the most fundamental predictive factor of success [25]. The primordial element is the human component of the equation more than the robotic platform. In our team-training experience, this was concluded after multiple exercises in the operating theater ranging from learning about the technology components, to docking and optimizing port positioning, and to listening to every member’s opinion to perform the operation as safely as possible. This intense training curriculum was also expanded to include quarterly Journal Club education events to discuss the literature and learn from video-based criticism and discussions. At the end, this model yielded excellent results with a low morbidity rate for the robotic cohort that is comparable to the laparoscopic cohort, and with elimination of any statistically significant difference between the intraoperative times for both groups. Although such model of training is ideal in an academic institution environment, it can also be successfully applied in the community hospital arena if support from administration correlates with the surgeon’s initiative and the team’s willingness to learn [26]. Moreover, robotic assistance has been shown to decrease the number of cases that are necessary to master the learning curve most likely due to the ergonomic advantage offered by this platform and its facilitation of the surgeon’s adaptability [27].
This retrospective review of 50 R-RYGB cases by a single robotic metabolic and bariatric surgeon took place at a 495-bed acute-care community hospital in a rural region with a busy MBSAQIP-accredited metabolic and bariatric program. The center regularly performs more than 200 primary RYGB operations per year, with more than 400 bariatric surgeries on an annual basis including other primary procedures such as sleeve gastrectomy, biliopancreatic diversion with duodenal switch, in addition to revisional and emergency bariatric operations. Most of the operations performed at the institution are laparoscopic rather than robotic, mostly due to availability of the robotic platform. As a result, the bariatric surgical team has extensive experience with this approach including the L-RYGB, which is routinely performed not only by the first author, but also by other surgeons in the hospital. The L-RYGB cohort was analyzed as the control population to compare it to the study cohort, i.e., the R-RYGB, due to the principal objective of this study: to evaluate the safety outcomes from the robotic approach as the institution’s first robotic experience with RYGB.
On previous manuscripts by the first author, the first robotic general surgery case-series experience from an American community hospital perspective was presented with 101 cases that culminated with an R-RYGB [28]. This was followed by a second publication that included the first 200 consecutive robotic general and bariatric surgery cases by the same surgeon at that institution prior to starting this hospital’s robotic bariatric program [29]. Both publications were based on a prior community hospital experience with a bariatric program at earlier stages compared to this one. What that series of 200 robotic cases allowed the surgeon to learn was the importance of mastery of the learning curve with an intense team-training model as describe above, with sequential advancement from simple to complex cases. This time, the study of outcomes from the R-RYGB was chosen due to the high incidence of type 2 diabetes and other co-morbidities with high BMI in the area’s patient population.
A large retrospective series published by other authors described no statistically significant differences between R-RYGB and L-RYGB for complications, estimated blood loss (EBL), and length of stay (LOS) [30]. However, in that series, a longer intraoperative time for the robotic group was reported. At the initial phase of this study’s experience, when only 24 R-RYGB procedures had been performed, this group’s longer intraoperative time was statistically significant compared to L-RYGB (139.04 ± 25.47 vs. 114.50 ± 29.98 min, respectively; p = 0.0017). This statistically significant difference was eliminated by the end of our experience after 50 R-RYGB cases had been performed in comparison to 50 L-RYGB procedures (121.94 ± 29.05 vs. 113.52 ± 28.32 min; p = 0.1495). In other words, these differences in length of case were minimized and eventually eliminated during the second half of the study when the learning curve had been mastered by the team. This is a testament to our pursuit of quality through consistency, continuous practice, video review and criticism, team discussion, and learning from our mistakes.
Reoperations occurred in a total of four cases, two in each group (4% incidence). In the R-RYGB group, a patient had to return to the operating room for a missed enterotomy during extensive adhesiolysis at the time of the index procedure. Another patient returned to surgery for significant sinus tachycardia in the recovery unit and otherwise normal workup, with a negative laparoscopy. The two L-RYGB patients who returned to the OR required reintervention on POD#2. One patient returned for sinus tachycardia and fever, but with a negative diagnostic laparoscopy. The other patient returned for a gastrojejunostomy stricture that had to be resected and redone due to a submucosal hematoma. All four patients recovered and were discharged to home.
It has been established that some of the most relevant factors affecting cost variability for bariatric surgery are the complexity of the case, the type of robotic platform used, the length of stay, and the number and nature of co-morbidities [31]. With respect to the financial analysis, it was performed with supervision by the hospital’s financial department, and included the costs associated with the use of the laparoscopic stapler (Group 1), the robotic 45-mm stapler (Group 2), and the robotic 60-mm stapler (Groups 3 and 4). Group 4 increased the length of the case for a few minutes due to the robotic hand-sewn gastrojejunostomy technique. The analysis did not apply to purely laparoscopic cases. The financial analysis or robotic cases also included the difference in length of case contributed by the use of the X da Vinci system, which requires a more meticulous robotic docking technique due to the lack of a rotating boom, which increases the docking time until the learning curve is mastered and the differences are mitigated. Overall, when the transition from a robotic hybrid approach evolved into a totally robotic approach, from Group 1 to Group 4 sequentially, there was an average group cost reduction of US $816.90 per case corresponding to a total cost saving of US $40,845.00 for 50 R-RYGB procedures.
This study has several limitations, starting with its nature as a retrospective cohort study. In addition, it is a single-center and single-surgeon study, which may contribute to limit its reproducibility depending on other surgeons’ and teams’ level of expertise, mastery of their learning curve, techniques, and access to the robotic technology. However, this feature may also contribute to decrease the variability that would be expected from a multi-centric study involving several surgeons whose experience and techniques may vary significantly. On the other hand, the design of the program’s experience by the surgeon by transitioning from a robotic hybrid approach (Group 1) to a totally robotic approach with hand-sewn gastrojejunostomy creation (Group 4) may have created another limitation. This design was based on the need to train as a team to adapt and learn from different levels of difficulty and with different tools as more advanced staplers were available in the hospital. Nevertheless, this variability may have been counteracted by the fact that the same surgeon and the same team performed all of the cases, both robotic and laparoscopic. Finally, the number of patients and the relatively short follow-up period were significant limitations. If a larger number of patients had undergone both the robotic and laparoscopic RYGB procedures over a longer period of time, especially with a more prolonged mean follow-up greater than 1 year, the possibility of encountering statistically significant differences between the two groups could have been enhanced.
The retrospective cohort study presented here provides helpful insight into the successful application of robotic surgery to perform RYGB in a rural community hospital environment. We hope that it may serve as the foundation for a future prospective randomized-controlled trial of totally robotic versus conventional laparoscopic Roux en Y gastric bypass. In such clinical trial, attention would be paid to the incidence of complications such as marginal ulcers and their reduction with the robotic gastrojejunostomy hand-sewn technique compared to the laparoscopic linear-stapled technique.
The relevance of mastering the learning curve while emphasizing team training with continuous quality control and self-improvement cannot be stressed enough. It is possible to achieve excellent outcomes comparable to those of laparoscopy when the surgical team works to accomplish such a task with efficiency and dedication while learning from mistakes and overcoming limitations. The R-RYGB is a safe operation in a community hospital in a rural setting when these elements are present and cultivated. Moreover, a cost-reducing attitude and a financially responsible mentality are important factors to reach the level of support from the hospital leadership that will lead to the program’s success with the patient’s safety and well-being at the core.
Conclusion
By emphasizing quality control, team training, and mastery of the learning curve, R-RYGB can be as safe as L-RYGB when performed at a community hospital in a rural setting while maintaining a low complication rate, achieving a satisfactory co-morbidity resolution rate, and saving costs with a totally robotic approach compared to a hybrid approach.
References
Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults (Review). Cochrane Database Syst Rev 8:1–184
Chang SH et al (2014) Bariatric surgery: an updated systematic review and meta-analysis. JAMA Surg 149(3):275–287. https://doi.org/10.1001/jamasurg.2013.3654
Cirocchi R et al (2013) Current status of robotic bariatric surgery: a systematic review. BMC Surg 13: 53. http://www.biomedcentral.com/1471-2482/13/53
Thorell A et al (2016) Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 40:2065–2083. https://doi.org/10.1007/s00268-016-3492-3
Acquafresca PA, Palermo M, Rogula T, Duza GE, Serra E (2015) Most common robotic bariatric procedures: review and technical aspects. Ann Surg Innov Res 9:9. https://doi.org/10.1186/s13022-015-0019-9
Avgousti S et al (2016) Medical telerobotic systems: current status and future trends. Biomed Eng Online 15:96. https://doi.org/10.1186/s12938-016-0217-7
Shah J, Vyas A, Vyas D (2014) The history of robotics in surgical specialties. Am J Robot Surg 1(1):12–20. https://doi.org/10.1166/ajrs.2014.1006
Lanfranco AR, Castellanos AE, Desai JP, Meyers WC (2004) Robotic surgery: a current perspective. Ann Surg 239(1):14–21. https://doi.org/10.1097/01.sla.0000103020.19595.7d
Antoniou SA, Antoniou GA, Antoniou AI, Granderath FA (2015) Past, present and future of minimally invasive abdominal surgery. JSLS 19(3):e2015.0052. https://doi.org/10.4293/JSLS.2015.00052
Bindal V et al (2015) Review of contemporary role of robotics in bariatric surgery. J Minim Access Surg 11(1):16–21. https://doi.org/10.4103/0972-9941.147673
Fourman MM, Saber AA (2012) Robotic bariatric surgery: a systematic review. Surg Obes Relat Dis 8(4):438–448. https://doi.org/10.1016/j.soard.2012.02.012
Bhatia P et al (2014) Robot-assisted sleeve gastrectomy in morbidly obese versus super obese patients. JSLS 18(3):e2014.00099. https://doi.org/10.4293/JSLS.2014.00099
Bailey GJ, Hayden JA, Davis PJ, Liu RY, Haardt D, Ellsmere J (2014) Robotic versus laparoscopic Roux-en-Y gastric bypass (RYGB) in obese adults ages 18 to 65 years: a systematic review and economic analysis. Surg Endosc 28(2):414–426. https://doi.org/10.1007/s00464-013-3217-8
Buchs NC et al (2013) Robot-assisted Roux-en-Y gastric bypass for super obese patients: a comparative study. Obes Surg 23(3):353–357. https://doi.org/10.1007/s11695-012-0824-8
Stefanidis D, Bailey SB, Kuwada T, Simms C, Gersin K (2018) Robotic gastric bypass may lead to fewer complications compared to laparoscopy. Surg Endosc 32(2):610–616. https://doi.org/10.1007/s00464-017-5710-y
Mohr CJ, Nadzam GS, Curet MJ (2005) Totally robotic Roux-en-Y gastric bypass. Arch Surg 140(8):779–786
Benizri E et al (2013) Perioperative outcomes after totally robotic gastric bypass: a prospective nonrandomized controlled study. Am J Surg 206(2):145–151. https://doi.org/10.1016/j.amjsurg.2012.07.049
Buchs NC, Pugin F, Azagury DE, Huber O, Chassot G, Morel P (2014) Robotic revisional bariatric surgery: a comparative study with laparoscopic and open surgery. Int J Med Robot 10(2):213–217. https://doi.org/10.1002/rcs.1549
Bindal V, Gonzalez-Heredia R, Elli EF (2015) Outcomes of robotic-assisted Roux-en-Y gastric bypass as a reoperative bariatric procedure. Obes Surg 25(10):1810–1815. https://doi.org/10.1007/s11695-015-1632-8
Acevedo E, Mazzei M, Zhao H, Lu X, Soans R, Edwards MA (2019) Outcomes in conventional laparoscopy versus robotic-assisted primary bariatric surgery: a retrospective, case-controlled study of the MBSAQIP database. Surg Endosc. https://doi.org/10.1007/s00464-019-06915-7
Li K, Zou J, Tang J, Di J, Han X, Zhang P (2016) Robotic versus laparoscopic bariatric surgery: a systematic review and meta-analysis. Obes Surg 26:3031–3044. https://doi.org/10.1007/s11695-016-2408-5
Sanchez BR, Mohr CJ, Morton JM, Safadi BY, Alami RS, Curet MJ (2005) Comparison of totally robotic laparoscopic Roux-en-Y gastric bypass and traditional laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 1(6):549–554
Moon RC, Gutierrez JC, Royall NA, Teixeira AF, Jawad MA (2016) Robotic Roux-en-Y gastric bypass, is it safer than laparoscopic bypass? Obes Surg 26:1016–1020. https://doi.org/10.1007/s11695-015-1884-3
Smeenk RM, van’t Hof G, Elsten E, Feskens PG (2016) The results of 100 robotic versus 100 laparoscopic gastric bypass procedures: a single high volume centre experience. Obes Surg 26(6):1266–1273. https://doi.org/10.1007/s11695-015-1933-y
Renaud M et al (2013) Multifactorial analysis of the learning curve for totally robotic Roux-en-Y gastric bypass for morbid obesity. Obes Surg 23(11):1753–1760. https://doi.org/10.1007/s11695-013-1020-1
Chitwood WR et al (2001) Robotic surgical training in an academic institution. Ann Surg 234(4):475–486
Yu SC, Clapp BL, Lee MJ, Albrecht WC, Scarborough TK, Wilson EB (2006) Robotic assistance provides excellent outcomes during the learning curve for laparoscopic Roux-en-Y gastric bypass: results from 100 robotic-assisted gastric bypasses. Am J Surg 192:746–749. https://doi.org/10.1016/j.amjsurg.2006.08.038
Oviedo RJ, Robertson JC, Alrajhi S (2016) First 101 robotic general surgery cases in a community hospital. JSLS 20(3):e2016.00056. https://doi.org/10.4293/JSLS.2016.00056
Oviedo RJ, Brownstein NC, Smith SL, Robertson JC, Nair-Collins S (2018) First 200 robotic general surgery cases in a community hospital: a retrospective cohort study. World J Surg Surgical Res 1:1034
Ahmad A, Carleton JD, Ahmad ZF, Agarwala A (2016) Laparoscopic versus robotic-assisted Roux-en-Y gastric bypass: a retrospective, single-center study of early perioperative outcomes at a community hospital. Surg Endosc 30:3792–3796. https://doi.org/10.1007/s00464-015-4675-y
Khorgami Z et al (2017) Cost of bariatric surgery and factors associated with increased cost: an analysis of national inpatient sample. Surg Obes Relat Dis 13:1284–1289. https://doi.org/10.1016/j.soard.2017.04.010
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Oviedo and Dr. Nayak have no conflicts of interests to report. Ms. Long and Ms. Yan have no conflicts of interests to report.
Consent statement
The retrospective chart review for all patients who belong to both cohorts in this study was approved by the Institutional Review Board at Winchester Medical Center in Winchester, VA, USA. The IRB committee approved the retrospective chart review and consented to its statistical analysis and the writing of this manuscript after ensuring that the patients’ confidential information was fully protected, per ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oviedo, R.J., Nayak, T., Long, Z. et al. Robotic Roux en Y gastric bypass can be safe and cost-effective in a rural setting: clinical outcomes from a community hospital bariatric program. J Robotic Surg 15, 929–936 (2021). https://doi.org/10.1007/s11701-021-01193-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-021-01193-9