Abstract
The objective of this study was to investigate the effects of decreasing insufflation pressure during robotic gynecologic surgery. The primary outcomes were patient-reported postoperative pain scores and length of stay. Secondary outcomes include surgical time, blood loss, and intraoperative respiratory parameters. This is a retrospective cohort study of patients undergoing robotic surgery for benign gynecologic conditions by a single minimally invasive surgeon at an academic hospital between 2014 and 2017. Patients were categorized by the maximum insufflation pressure reached during the surgery as either 15, 12, 10, or 8 mmHg. Continuous variables were compared using analysis of variance and χ2 test was used for categorical variables. 598 patients were included in this study with no differences in age, BMI, race, prior abdominal surgeries, or specimen weight between the four cohorts. When comparing cohorts, each decrease in insufflation pressure correlated with a significant decrease in initial pain scores (5.9 vs 5.4 vs 4.4 vs. 3.8, p ≤ 0.001), and hospital length of stay (449 vs 467 vs 351 vs. 317 min, p ≤ 0.001). There were no differences in duration of surgery (p = 0.31) or blood loss (p = 0.09). Lower operating pressures were correlated with significantly lower peak inspiratory pressures (p < 0.001) and tidal volumes (p < 0.001). Surgery performed at lower-pressure pneumoperitoneum (≤ 10 mmHg) is associated with lower postoperative pain scores, shorter length of stay, and improved intraoperative respiratory parameters without increased duration of surgery or blood loss. Operating at lower insufflation pressures is a low-cost, reversible intervention that should be implemented during robotic surgery as it results in the improved pain scores and shorter hospital stays.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gynecologic surgery has rapidly evolved over recent years, embracing the use of minimally invasive surgery (MIS) and “Enhanced Recovery After Surgery” (ERAS) protocols to improve perioperative recovery time and transform major surgeries into outpatient procedures. Traditional laparoscopy and robot-assisted laparoscopic surgery are safe and feasible alternatives to laparotomy, with improved outcomes in length of hospital stay, blood loss, analgesic requirements, return to baseline activities, and patient-reported pain and quality of life [1,2,3,4,5]. ERAS protocols incorporate multimodal perioperative pain control with local or regional anesthesia, non-narcotic pharmacotherapy, maintaining euvolemia, early ambulation, and minimizing postoperative drains, resulting in reduced narcotic use, patient-reported pain, hospital costs, and length of stay [6,7,8,9,10,11].
ERAS protocols originally intended for laparotomy are now employed routinely in MIS and have improved perioperative outcomes [8, 9, 12,13,14,15,16]. However, components of MIS, including insufflation and steep Trendelenburg, are unique to laparoscopy, and current ERAS models do not address these physiologic changes directly. During laparoscopic procedures, carbon dioxide (CO2) gas is insufflated into the body cavity, and an electronic insufflator controls the insufflation pressure. Routinely, pressure during gynecologic surgery ranges between 8 and 15 mmHg and is typically higher than 10 mmHg [17, 18]. Intraperitoneal insufflation and steep Trendelenburg positioning increase overall physiologic stress, catecholamine activation, peritoneal stretching, and diaphragmatic irritation which increases postoperative pain [17]. Therefore, an integrated ERAS protocol for MIS must target the pathophysiology of visceral and nerve pain related to pneumoperitoneum, which is distinct from the somatic pain of laparotomy.
Various studies have evaluated methods to reduce postoperative pain specifically related to MIS, with mixed results. One way to decrease postoperative pain and add synergistic value to an ERAS protocol is to operate at low (≤ 10 mmHg) insufflation pressure. In general surgery, systematic reviews concluded that low-pressure insufflation (< 12 mmHg) is safe and associated with significantly lower pain scores compared to standard-pressure groups (12–15 mmHg) [19,20,21]. Other strategies with low-quality evidence include evacuation of intraperitoneal CO2, warming and humidifying CO2, or warm saline lavage [22, 23]. To date, there is limited evidence analyzing the association between insufflation pressure and postoperative pain scores in gynecologic laparoscopic surgery with and with no studies in robotic surgery. Furthermore, prior studies were performed in the absence of an ERAS protocol, and thus, it is unknown if the benefit of low-pressure pneumoperitoneum is sustained in the setting of an enhanced recovery pathway.
The objective of this study was to investigate the effects of decreasing insufflation pressure on intraoperative and postoperative parameters during robotic-assisted gynecologic surgery with an ERAS protocol in place. We hypothesize that low-pressure pneumoperitoneum will lead to lower postoperative pain scores and shorter length of hospital stay. If we are correct, operating at lower insufflation pressure offers a feasible, safe and effective addition to MIS-oriented ERAS protocols.
Methods
In this retrospective cohort study, we identified women undergoing robotic-assisted laparoscopic surgery for benign gynecologic conditions at New York University (NYU) Langone Medical Center. With the approval of the NYU School of Medicine Institutional Review Board, patients were identified through EPIC electronic medical records. All women who underwent robotic surgery for benign gynecologic conditions by a single minimally invasive gynecologic surgeon between January 1, 2014 and December 31, 2017 were included. We excluded women who had a combined procedure with an additional surgeon and women with incomplete medical records.
The primary outcomes were patient-reported postoperative pain scores and length of hospital stay. Pain scores were determined by a validated 10-point verbal numerical rating scale (VNRS) and documented in the electronic medical record during routine postoperative care. PACU nurses asked the patient to indicate their pain level at multiple times during recovery. Patients rated the intensity of their pain on a 0–10 scale with 0 representing no pain and 10 representing the worst possible pain. Length of stay in the hospital was calculated as the time in minutes from PACU arrival to discharge from the hospital.
Secondary outcomes were duration of surgery, estimated blood loss, and intraoperative respiratory parameters. Duration of surgery was defined as the time from incision to skin closure, in minutes. Estimated blood loss was determined by the surgeon at the conclusion of each case. Intraoperative respiratory parameters included peak inspiratory pressure (PIP), plateau airway pressure (Pplat), tidal volume (TV), positive end-expiratory pressure (PEEP), and end tidal CO2 (EtCO2). PIP, Pplat, TV, and PEEP were collected at three times. T0 represents the respiratory parameters 1 min prior to insufflation as a baseline measure. T1 refers to 5 min after insufflation initiation and represents the immediate effect of increased abdominal pressure on the cardiopulmonary system. T2 represents the midway point of the insufflation time to reflect the average effect of increased abdominal pressure on the cardiopulmonary system. EtCO2 was obtained at two times, at the T1 and end of insufflation (T3). All anesthesia data were extracted by the investigators from the anesthesia record.
Demographic information, preoperative clinical characteristics, surgical data, intraoperative anesthesia respiratory parameters, and PACU data were collected for all patients who met inclusion criteria. Women were categorized into one of four groups according to the maximum insufflation pressure used during the surgery: 15, 12, 10, or 8 mmHg, regardless of time point at which this was achieved. All women were positioned in steep Trendelenburg throughout their surgery. Abdominal entry was gained using a Veres needle in the umbilicus and the abdomen was insufflated to the desired pressure. The DaVinci SI or XI robotic platform was used in all cases. All patients were treated according to New York University’s Enhanced Recovery After Surgery (ERAS) protocol (Appendix 1), which was in place during the study period.
During the study time period, the surgeon lowered the standard operating pressure after 100 consecutive cases from 15 mmHg to 12, 10, and finally to 8 mmHg. The timeframe of 100 consecutive cases was chosen as part of a quality improvement initiative and was thought to represent a wide sample of cases performed.
Characteristics of all women in this study were summarized and compared using analysis of variance for continuous variables after ascertaining normality of data distribution by Shapiro–Wilk test. This was followed by Bonferroni correction to adjust for multiple comparisons. Categorical variables were analyzed using χ2 test. All analyses were conducted using IBM SPSS for Windows, Version 20.0 (IBM Corp., Armonk, NY) and the two-sided significance level was set at p < 0.05.
Results
A total of 623 women underwent robotic-assisted laparoscopic surgery by a single gynecologic surgeon during the study period. Of these, 25 were excluded for combined surgical procedures or insufficient data in the medical record. The final cohort of 598 women had similar demographics across the 4 insufflation cohorts. On average, they were overweight and represented multiple racial groups (Table 1). The average age was 41 years and there were no significant differences in prior abdominal surgery, American Society of Anesthesiologists (ASA) status, or specimen weight between the cohorts. There was a statistically significant difference in the types of surgery performed as more patients in the higher-pressure groups (15, 12, and 10 mmHg) underwent hysterectomy compared to the lower-pressure group (8 mmHg), which had a higher percentage of women undergoing endometriosis or adnexal surgery (Table 1).
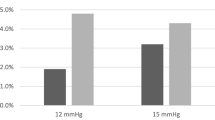
When comparing the four cohorts, each decrease in insufflation pressure correlated with a statistically significant decrease in initial pain scores (p < 0.001) and length of hospital stay (p < 0.001, Table 2). Surgeries lasted an average of 70 min without any significant differences between insufflation pressure groups (p = 0.31, Table 2). Estimated blood loss did not differ across all cohorts (p = 0.09). When comparing maximum pain level reported in the PACU, there was a statistically significant difference between the groups. For this measure, the 10 mmHg group had the lowest maximum pain score at 5.4, compared to the highest score of 7.3 in the 15 mmHg group (Table 2).
To determine which insufflation pressure results in optimal patient outcomes, the 10 mmHg and 8 mmHg cohorts were directly compared. Compared to women in the 10 mmHg group, those in the 8 mmHg group had lower length of hospital stay and blood loss with the same duration of surgery (Table 3). When comparing these two cohorts, there is no difference in initial pain scores (4.4 vs 3.8, p = 0.12); however, the maximum pain score is significantly higher in the 8 mmHg group (p = 0.005, Table 3).
Lower operating pressure correlated with significantly lower peak inspiratory pressures (PIP) (p < 0.001) across all time points. The magnitude of difference was larger after insufflation (T1 and T2) compared to prior to insufflation (T0) (Table 4). Pre-insufflation tidal volumes did not differ between the four cohorts (p = 0.09). However, there was a significant difference in tidal volumes immediately after insufflation (T1) and midway through insufflation (T2) with decreasing volumes appreciated as insufflation pressure decreased (p < 0.001, Table 4). There were no differences in positive end-expiratory pressure. End tidal CO2 values were significantly different between the four cohorts; however, all values for end tidal CO2 remained within the normal range of 35–45 mmHg.
Discussion
Our results show that lower intra-abdominal insufflation pressure is associated with lower postoperative pain scores and shorter length of hospital stay. We found no differences in blood loss and operative time between the four cohorts, suggesting that visualization and procedure difficulty were not compromised at lower insufflation pressures. Operating at lower insufflation pressure reduces the peak inspiratory pressure and tidal volumes needed during anesthesia, without higher positive end-expiratory pressures or clinically meaningful elevations in end tidal CO2. In the literature, both 8 and 10 mmHg are considered “low pressure,” whereas 12–15 mmHg defines “standard pressures” [19, 21, 24,25,26]. To determine the optimal “low” insufflation pressure, we directly compared the 10 mmHg and 8 mmHg cohorts. The 10 mmHg cohort had lower maximum pain scores with no differences in initial pain scores or duration of surgery. On average, the 10 mmHg group stayed in the hospital for 34 min longer than the 8 mmHg group.
In laparoscopy, pneumoperitoneum stretches the peritoneal cavity, stimulating the vagal nerve and triggering a pain response. Increased intraperitoneal pressure also irritates the diaphragm, exacerbating postoperative shoulder pain. It is plausible that operating at lower insufflation pressures helps reduce pain through these two mechanisms. Our study demonstrates a 2.1-point reduction in initial pain scores and a 1.2-point reduction in maximum PACU pain scores between the 15 mmHg and 8 mmHg cohorts. The cohort with the lowest maximum pain score was the 10 mmHg group, which was 1.9 points lower compared to the 15 mmHg cohort. This reduction in pain scores reflects an improvement in perioperative care as the evidence suggests that even a reduction of 1.0–1.5 points on a 0–10 pain scale is clinically significant [27,28,29].
Robust evidence demonstrates that low-pressure pneumoperitoneum reduces pain scores immediately following a wide range of laparoscopic procedures [21, 26, 30,31,32]. While our study did not investigate pain scores beyond the recovery room, the literature also demonstrates a clinically relevant decrease in pain scores at 24 h [19, 21, 24, 26, 31,32,33]. In a meta-analysis and systematic review, Ozdemir-van Brunschot et al. concluded that low-pressure pneumoperitoneum reduced postoperative pain scores and this difference was clinically significant at 2 and 3 days [26]. Another systematic review of 22 randomized control trials comparing laparoscopic cholecystectomies performed at low pressure (7–10 mmHg) to standard pressure (12–15 mmHg) reports significantly lower pain scores in the low-pressure group immediately and 12–24 h postoperatively, in addition to a lower incidence of shoulder pain [21]. In the field of minimally invasive gynecology, similar outcomes have been reported, though the level of evidence to date is mixed and no studies within robotics exist. Topcu et al. prospectively randomized patients undergoing minor gynecologic laparoscopy and concluded that compared to standard and high insufflation, low insufflation reduces postoperative pain at 6, 12, and 24 h. One small study by Bogani et al. showed decreased shoulder pain in the low-pressure pneumoperitoneum group at 1 and 3 h postoperatively, with no differences at 24 h [34]. In addition to lower patient-reported pain scores, low insufflation pressure reduces the amount of postoperative analgesic used by patients [32, 33].
Another benefit of MIS is shorter hospital stays which translates into lower costs, infection rates, VTE incidence, and faster return to normal activity. Our study showed a shorter hospital stay with decreasing pneumoperitoneum pressures. Hospital length of stay decreased by 132 min between the highest and lowest pressure cohorts. The proposed mechanisms include decreased pain requirements in PACU due to decreased pneumoperitoneal irritation, and improved cardiopulmonary status at lower insufflation pressures. Our findings are consistent with other published data. In two randomized trials in general surgery, patients with low insufflation pressures were discharged home faster than those with standard or high pressures [30, 33]. In a systematic review, Hua et al. concluded that the low-pressure group had significantly shorter length of stay (weighted mean difference = − 0.27; p = 0.01) [21]. The 2014 Cochrane review showed a trend toward shorter hospital stays in the low-pressure group (mean difference = − 0.3, (95% CI − 0.63 to 0.02); however, this did not reach statistical significance [20]. Operating at lower insufflation pressures could reduce the economic burden of healthcare by decreasing the length, and therefore the cost, of hospital stays. As the body of literature on cost saving interventions in surgery grows, additional studies investigating low-pressure pneumoperitoneum should be performed as a potentially cost-effective intervention.
In addition to pain, postoperative pulmonary complications may impact perioperative morbidity and mortality, and contribute to an increased length of stay in PACU. Insufflation increases intra-abdominal pressure, which elevates the diaphragm, decreases functional residual capacity, and causes ventilation perfusion ratio mismatch and intrapulmonary shunting of blood [35, 36]. These physiologic changes result in hypoxemia that is overcome by mechanical ventilation. The physiologic effects of pneumoperitoneum are further aggravated by patient positioning of 30 degrees Trendelenburg during robotic surgery, decreasing chest wall and lung compliance and increasing upper airway edema [17]. Recent evidence suggests that lower tidal volumes and lower plateau pressures during mechanical anesthesia can reduce postoperative pulmonary complications when compared to conventional ventilation strategies in patients who are undergoing general anesthesia [37, 38]. Our data show that a reduction in abdominal insufflation pressure improves peak inspiratory pressures throughout surgery, with lower tidal volumes and plateau pressure immediately following insufflation.
From a surgeon’s perspective, when weighing the benefits of low-pressure pneumoperitoneum, the primary concern is compromised visibility and space, potentially leading to less-efficient, more technically challenging procedures. However, our findings did not validate this concern. Our results showed no difference in blood loss or operative time between cohorts. Of the few randomized controlled trials assessing the risks and benefits of lower insufflation pressures in MIS gynecology, it was concluded that in expert hands low-pressure pneumoperitoneum is safe and feasible without additional complications [19, 24, 31, 32, 34]. This is corroborated in the general surgery literature, with a Cochrane review of low-pressure pneumoperitoneum in laparoscopic cholecystectomies, indicating no difference in operative times between low- and high-pressure pneumoperitoneum cohorts [20]. Hua et al. also showed no differences in complication rates or conversions to laparotomy, with an increase of 2 min in operative time in the lower-pressure cohort, of which the clinical and financial implications are arguably negligible [21]. Specific to gynecology, a systematic review of three randomized control trials, including a total of 238 patients, found no differences in blood loss or duration of surgery between low- and high-pressure cohorts [25]. Importantly, the option to increase pneumoperitoneal pressures is always at the discretion of the surgeon and anesthesiologist and should be undertaken if visualization is compromised and the safety of the patient is in question. If a surgeon decides his or her visualization is compromised, increasing the pneumoperitoneum pressure is fast and easy intervention with no associated cost increases.
Enhanced recovery after surgery (ERAS) implements evidence-based interventions with the goal of improving postoperative recovery through a reduction in surgical stress response [39]. One of the pillars of ERAS is multimodal treatment of pain, which incorporates oral pain medications, locoregional analgesia, and incisional injections resulting in decreased postoperative opioid use [39,40,41]. Current intraoperative recommendations include minimally invasive surgical technique, avoiding long acting opioids, maintaining fluid balance, restrictive use of drains, removal of nasogastric tubes, and control of body temperature [41]. Our study uniquely examines postoperative pain and length of stay using a standardized ERAS protocol and low-pressure pneumoperitoneum and has found an added benefit with decreasing pneumoperitoneum. We suggest adding low insufflation pressure as an inexpensive and reversible intervention that could amplify the effects of an ERAS program and contribute to lower postoperative pain scores and length of stay in laparoscopic surgery.
Strengths of this study include a large sample size of ethnically diverse patients with a variety of advanced gynecologic pathology. Our study is the largest published within gynecology and uniquely includes complex procedures and pathology. This is the first study investigating the effect of decreased pneumoperitoneum during robotic surgery. We used objective measures of pain, surgical time, blood loss, length of stay, and respiratory parameters in a realistic clinical environment. Additionally, a consistent ERAS protocol was implemented for all patients as part of their perioperative care, highlighting the potential additive effect-reduced pneumoperitoneum pressure can have on perioperative outcomes. The study is limited by the use of retrospective, non-randomized data, which can introduce confounding bias. One potential confounder is the learning curve of the surgeon, which could impact the collected measures over time. However, that learning curve would exist within each cohort as the surgeon adapts to operating at lower insufflation pressures. Another limitation is our patient population, which was premenopausal and overweight, and may impede the generalizability of our results. While the patients were not obese, the specimen weight (median weight of > 250 g) and percentage of patients with prior abdominal surgery suggest the procedures were technically challenging. This is in comparison to prior studies in gynecology which exclude patients with prior surgeries and only examined those undergoing minor procedures [19]. Finally, there was a significant difference in the types of surgeries performed between the high-pressure cohort and the low-pressure cohort. This may reflect a change in referral patterns over time, as during the study period a dedicated endometriosis center was opened and patients were included in this study cohort, potentially resulting in more endometriosis resections occurring later in the study period when lower pressures were more routinely employed. Despite the difference in surgery type across cohorts, there were no significant differences in median specimen weight and percentage of patients with prior abdominal surgery.
Prospective randomized studies are needed to validate the benefits of operating at low insufflation pressures during robotic surgery for both the patients and hospital systems. Further research aimed at identifying the ideal insufflation pressure, which we hypothesize is between 8 and 10 mmHg, should include subjects with higher BMIs as well as those having traditional straight-stick laparoscopic procedures. Operating at low insufflation pressures is an intervention that could have cost-saving implications in health care. Cost-effective analyses should be performed to understand the relative savings that may be gained from a relatively simple change in technique. Finally, studying the impact of insufflation pressure on postdischarge pain medication use, specifically opioid intake, will help understand the long-term implications of low-pressure pneumoperitoneum on patients’ postoperative pain.
Increased intra-abdominal pressure can adversely affect perioperative physiology and postoperative recovery. Operating at lower insufflation pressure is a low-cost, reversible intervention that should be incorporated into enhanced recovery pathways for all patients undergoing robotic surgery. The European Association for Endoscopic Surgery recognizes the benefits of low-pressure insufflation and recommends “using the lowest intra-abdominal pressure allowing adequate exposure of the operative field, rather than using a routine pressure” [42]. Despite increasing evidence supporting decreased postoperative pain with lower pneumoperitoneum pressure, no professional or societal guidelines exist within the gynecologic community. This retrospective cohort study demonstrates that operating at low insufflation pressures improves postoperative pain, decreases length of stay, and is feasible and safe in complex robotic gynecologic surgery. Our study establishes a platform of safety and feasibility that additional research studies can expand on to understand the added effect of low-pressure pneumoperitoneum and minimally invasive surgery to decrease lengths of stay and healthcare costs, improving the care of surgical patients.
References
Zechmeister JR, Pua TL, Boyd LR, Blank SV, Curtin JP, Pothuri B (2015) A prospective comparison of postoperative pain and quality of life in robotic assisted vs conventional laparoscopic gynecologic surgery. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2014.08.003
Advincula AP, Xu X, Goudeau S IV, Ransom SB (2007) Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. https://doi.org/10.1016/j.jmig.2007.06.008
Martino MA, Berger EA, McFetridge JT, Shubella J, Gosciniak G, Wejkszner T, Kainz GF, Patriarco J, Thomas MB, Boulay R (2014) A comparison of quality outcome measures in patients having a hysterectomy for benign disease: robotic vs. non-robotic approaches. J Minim Invasive Gynecol. https://doi.org/10.1016/j.jmig.2013.10.008
Obermair A, Janda M, Baker J, Kondalsamy-Chennakesavan S, Brand A, Hogg R, Jobling TW, Land R, Manolitsas T, Nascimento M, Neesham D, Nicklin JL, Oehler MK, Otton G, Perrin L, Salfinger S, Hammond I, Leung Y, Sykes P, Ngan H, Garrett A, Laney M, Ng TY, Tam K, Chan K, Wrede DH, Pather S, Simcock B, Farrell R, Robertson G, Walker G, McCartney A, Gebski V (2012) Improved surgical safety after laparoscopic compared to open surgery for apparent early stage endometrial cancer: Results from a randomised controlled trial. Eur J Cancer. https://doi.org/10.1016/j.ejca.2012.02.055
Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, Spiegel G, Barakat R, Pearl ML, Sharma SK (2009) Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. https://doi.org/10.1200/JCO.2009.22.3248
Kalogera E, Dowdy SC (2016) Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin N Am. https://doi.org/10.1016/j.ogc.2016.04.006
Steinberg AC, Schimpf MO, White AB, Mathews C, Ellington DR, Jeppson P, Crisp C, Aschkenazi SO, Mamik MM, Balk EM, Murphy M (2017) Preemptive analgesia for postoperative hysterectomy pain control: systematic review and clinical practice guidelines. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2017.03.013
Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, Antrobus J, Huang J, Scott M, Wijk L, Acheson N, Ljungqvist O, Dowdy SC (2016) Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery after Surgery (ERAS®) Society recommendations-part I. Gynecol Oncol. https://doi.org/10.1016/j.ygyno.2015.11.015
Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, Antrobus J, Huang J, Scott M, Wijk L, Acheson N, Ljungqvist O, Dowdy SC (2016) Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery after Surgery (ERAS®) Society recommendations-part II. Gynecol Oncol. https://doi.org/10.1016/j.ygyno.2015.12.019
Dickson E, Argenta PA, Reichert JA (2012) Results of introducing a rapid recovery program for total abdominal hysterectomy. Gynecol Obstet Invest. https://doi.org/10.1159/000328713
Miller EC, McIsaac DI, Chaput A, Antrobus J, Shenassa H, Lui A (2015) Increased postoperative day one discharges after implementation of a hysterectomy enhanced recovery pathway: a retrospective cohort study. Obstet Gynecol Surv. https://doi.org/10.1007/s12630-015-0347-6
Modesitt SC, Sarosiek BM, Trowbridge ER, Redick DL, Shah PM, Thiele RH, Tiouririne M, Hedrick TL (2016) Enhanced recovery implementation in major gynecologic surgeries: effect of care standardization. Obstet Gynecol. https://doi.org/10.1097/AOG.0000000000001555
Keil DS, Schiff LD, Carey ET, Moulder JK, Goetzinger AM, Patidar SM, Hance LM, Kolarczyk LM, Isaak RS, Strassle PD, Schoenherr JW (2019) Predictors of admission after the implementation of an enhanced recovery after surgery pathway for minimally invasive gynecologic surgery. Anesth Analg. https://doi.org/10.1213/ANE.0000000000003339
Lee J, Asher V, Nair A, White V, Brocklehurst C, Traves M, Bali A (2018) Comparing the experience of enhanced recovery programme for gynaecological patients undergoing laparoscopic versus open gynaecological surgery: a prospective study. Perioper Med. https://doi.org/10.1186/s13741-018-0096-5
Carey ET, Moulder JK (2018) Perioperative management and implementation of enhanced recovery programs in gynecologic surgery for benign indications. Obstet Gynecol. https://doi.org/10.1097/AOG.0000000000002696
Trowbridge ER, Dreisbach CN, Sarosiek BM, Dunbar CP, Evans SL, Hahn LA, Hullfish KL (2018) Review of enhanced recovery programs in benign gynecologic surgery. Int Urogynecol J. https://doi.org/10.1007/s00192-017-3442-0
Badawy M, Béïque F, Al-Halal H, Azar T, Akkour K, Lau SK, Gotlieb WH (2011) Anesthesia considerations for robotic surgery in gynecologic oncology. J Robot Surg. https://doi.org/10.1007/s11701-011-0261-z
Lestar M, Gunnarsson L, Lagerstrand L, Wiklund P, Odeberg-Wernerman S (2011) Hemodynamic perturbations during robot-assisted laparoscopic radical prostatectomy in 45° trendelenburg position. Anesth Analg. https://doi.org/10.1213/ANE.0b013e3182075d1f
Topçu HO, Cavkaytar S, Kokanali K, Guzel AI, Islimye M, Doganay M (2014) A prospective randomized trial of postoperative pain following different insufflation pressures during gynecologic laparoscopy. Eur J Obstet Gynecol Reprod Biol 182:81–85. https://doi.org/10.1016/j.ejogrb.2014.09.003
Gurusamy KS, Vaughan J, Davidson BR (2014) Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006930.pub3
Hua J, Gong J, Yao L, Zhou B, Song Z (2014) Low-pressure versus standard-pressure pneumoperitoneum for laparoscopic cholecystectomy: a systematic review and meta-analysis. Am J Surg 208:143–150. https://doi.org/10.1016/j.amjsurg.2013.09.027
Kaloo P, Armstrong S, Kaloo C, Jordan V (2019) Interventions to reduce shoulder pain following gynaecological laparoscopic procedures. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD011101.pub2
Joshi GP, Bonnet F, Kehlet H, Bonnet F, Camu F, Fischer HBJ, Joshi GP, Neugebauer EAM, Rawal N, Schug SA, Simanski CJP, Kehlet H (2013) Evidence-based postoperative pain management after laparoscopic colorectal surgery. Color Dis. https://doi.org/10.1111/j.1463-1318.2012.03062.x
Sroussi J, Elies A, Rigouzzo A, Louvet N, Mezzadri M, Fazel A, Benifla JL (2017) Low pressure gynecological laparoscopy (7 mmHg) with AirSeal ® System versus a standard insufflation (15 mmHg): A pilot study in 60 patients. J Gynecol Obstet Hum Reprod 46:155–158. https://doi.org/10.1016/j.jogoh.2016.09.003
Kyle EB, Maheux-Lacroix S, Boutin A, Laberge PY, Lemyre M (2016) Low vs standard pressures in gynecologic laparoscopy: a systematic review. J Soc Laparoendosc Surg. https://doi.org/10.4293/JSLS.2015.00113
Özdemir-van Brunschot DMD, van Laarhoven KCJHM, Scheffer G-J, Pouwels S, Wever KE, Warlé MC (2016) What is the evidence for the use of low-pressure pneumoperitoneum? A systematic review. Surg Endosc 30:2049–2065. https://doi.org/10.1007/s00464-015-4454-9
Kendrick DB, Strout TD (2005) The minimum clinically significant difference in patient-assigned numeric scores for pain. Am J Emerg Med 23:828–832. https://doi.org/10.1016/j.ajem.2005.07.009
Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB (2003) What decline in pain intensity is meaningful to patients with acute pain? Pain 105:151–157. https://doi.org/10.1016/s0304-3959(03)00176-3
Bijur PE, Latimer CT, Gallagher EJ (2003) Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. https://doi.org/10.1197/aemj.10.4.390
Wallace DH, Serpell MG, Baxter JN, O’Dwyer PJ (1997) Randomized trial of different insufflation pressures for laparoscopic cholecystectomy. Br J Surg 84:455–458. https://doi.org/10.1002/bjs.1800840408
Warlé MC, Berkers AW, Langenhuijsen JF, van der Jagt MF, Dooper PM, Kloke HJ, Pilzecker D, Renes SH, Wever KE, Hoitsma AJ, van der Vliet JA, D’Ancona FCH (2013) Low-pressure pneumoperitoneum during laparoscopic donor nephrectomy to optimize live donors’ comfort. Clin Transpl. https://doi.org/10.1111/ctr.12143
Vijayaraghavan N, Sistla SC, Kundra P, Ananthanarayan PH, Karthikeyan VS, Ali SM, Sasi SP, Vikram K (2014) Comparison of standard-pressure and low-pressure pneumoperitoneum in laparoscopic cholecystectomy: a double blinded randomized controlled study. Surg Laparosc Endosc Percutaneous Tech 24:127–133. https://doi.org/10.1097/SLE.0b013e3182937980
Joshipura VP, Haribhakti SP, Patel NR, Naik RP, Soni HN, Patel B, Bhavsar MS, Narwaria MB, Thakker R (2009) A prospective randomized, controlled study comparing low pressure versus high pressure pneumoperitoneum during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutaneous Tech 19:234–240. https://doi.org/10.1097/SLE.0b013e3181a97012
Bogani G, Uccella S, Cromi A, Serati M, Casarin J, Pinelli C, Ghezzi F (2014) Low vs standard pneumoperitoneum pressure during laparoscopic hysterectomy: prospective randomized trial. J Minim Invasive Gynecol 21:466. https://doi.org/10.1016/j.jmig.2013.12.091
Hayden P, Cowman S (2011) Anaesthesia for laparoscopic surgery. Contin Educ Anaesthesia Crit Care Pain. https://doi.org/10.1093/bjaceaccp/mkr027
Srivastava A, Niranjan A (2010) Secrets of safe laparoscopic surgery: Anaesthetic and surgical considerations. J Minim Access Surg. https://doi.org/10.4103/0972-9941.72593
Güldner A, Kiss T, Serpa Neto A, Hemmes SNT, Canet J, Spieth PM, Rocco PRM, Schultz MJ, Pelosi P, Gama de Abreu M (2015) Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications. Anesthesiology. https://doi.org/10.1097/aln.0000000000000754
Ladha K, Vidal Melo MF, McLean DJ, Wanderer JP, Grabitz SD, Kurth T, Eikermann M (2015) Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 351:h3646. https://doi.org/10.1136/bmj.h3646
Wijk L, Udumyan R, Pache B, Altman AD, Williams LL, Elias KM, McGee J, Wells T, Gramlich L, Holcomb K, Achtari C, Ljungqvist O, Dowdy SC, Nelson G (2019) International validation of Enhanced Recovery After Surgery Society guidelines on enhanced recovery for gynecologic surgery. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2019.04.028
Meyer LA, Lasala J, Iniesta MD, Nick AM, Munsell MF, Shi Q, Wang XS, Cain KE, Lu KH, Ramirez PT (2018) Effect of an enhanced recovery after surgery program on opioid use and patient-reported outcomes. Obstet Gynecol 132:281–290. https://doi.org/10.1097/AOG.0000000000002735
Ljungqvist O, Scott M, Fearon KC (2017) Enhanced recovery after surgery: a review. JAMA Surg 152:292–298. https://doi.org/10.1001/jamasurg.2016.4952
Neudecker J, Sauerland S, Neugebauer E, Bergamaschi R, Bonjer HJ, Cuschieri A, Fuchs KH, Jacobi C, Jansen FW, Koivusalo AM, Lacy A, McMahon MJ, Millat B, Schwenk W (2002) The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg Endosc Other Interv Tech 16:1121–1143
Funding
The authors have no financial support to disclose. The data that support the findings of this study are available from the corresponding author, [CF] upon reasonable request.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CEF and ER. The first draft of the manuscript was written by CEF, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Christine E. Foley has no conflicts of interest to disclose. Erika Ryan has no conflicts of interest to disclose. Jian Qun Huang is a consultant for ConMed, Intuitive Surgical and Ethicon.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of New York University School of Medicine approved this study. IRB Date and Number: 4/3/19: i18-00606, 6/12/15: i15-00360.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: Enhanced recovery protocol for ambulatory robot-assisted gynecological surgery
Appendix 1: Enhanced recovery protocol for ambulatory robot-assisted gynecological surgery
Preoperative on day of surgery:
-
1.
Follow Departmental guidelines regarding nil per os (NPO) status. This allows for clear liquids up until 2 h prior to surgery.
-
2.
Tylenol 1000 mg per os (PO) in holding area.
Intraoperative:
-
1.
Use propofol-based total intravenous anesthesia (TIVA) as the primary anesthetic
-
2.
Antibiotic prophylaxis as per protocol.
-
3.
Orogastric (OG)/nasogastric (NG) tube and Foley to be placed and removed prior to ex-tubation unless directed by surgical team.
-
4.
Zofran 4 mg intravenous push (IVP) (if not contraindicated).
-
5.
Decadron 10 mg IVP (if not contraindicated).
-
6.
Hydromorphone 1 mg intramuscular (IM) approximately 30 min prior to emergence (consider less for smaller individuals).
-
7.
Toradol 30 mg IM prior to emergence (if not contraindicated).
-
8.
Fluid Management: 15 cc/kg/h + replacement for blood loss for the first two hours for surgery and then continue at 5 cc/kg/h.
-
9.
Local infiltration of surgical sites with 0.5% bupivacaine per surgical team.
Postoperative in postoperative anesthesia unit (PACU):
-
1.
Incentive spirometry to begin in PACU.
-
2.
IV fluids at 15 cc/kg/h for the first hour; then 5 cc/kg/h.
-
3.
Tea or coffee as preferred PO intake.
-
4.
Rescue medications in PACU:
-
a.
Antiemetics (choice of)
-
i.
Haldol 1 mg IM × 1.
-
ii.
Tigan 200 mg IM × 1.
-
(b)
Breakthrough analgesics (choice of)
-
i.
Fentanyl in titrated doses of 25 mcg IV per dose.
Percocet/Vicodin when necessary (PRN).
Rights and permissions
About this article
Cite this article
Foley, C.E., Ryan, E. & Huang, J.Q. Less is more: clinical impact of decreasing pneumoperitoneum pressures during robotic surgery. J Robotic Surg 15, 299–307 (2021). https://doi.org/10.1007/s11701-020-01104-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-020-01104-4