Abstract
Despite advantages of minimally invasive surgery, many hepatobiliary surgeons are hesitant to offer this approach for major hepatic resection due to concerns of difficulty in liver manipulation, bleeding control, and suboptimal oncologic outcomes. The robotic surgical system has revolutionized the way traditional laparoscopic liver resection is undertaken. Limitations of traditional laparoscopy are being resolved by robotic technology. We aimed to describe aspects of minimally invasive liver surgery and our standardized technical approach. We discussed technical aspects of performing robotic total right hepatic lobectomy and described our standardized institutional method. A 79-year-old man with an 11-cm biopsy-proven hepatocellular carcinoma was taken to the operating room for a robotic total right hepatic lobectomy. Past medical and surgical history was consistent with hypertension and diabetes mellitus. Robotic extrahepatic Glissonean pedicle approach was used to gain inflow vascular control. Right hepatic artery and portal vein were individually dissected and isolated prior to division. An intraoperative robotic ultrasound was utilized to guide liver parenchymal transection, securing negative margins. Robotic vessel sealing device was used as the main energy device during the parenchymal transection. Right hepatic vein was transected intrahepatically using a linear stapler. Operative time was 200 min without intraoperative complications. Estimated blood loss was 100 ml. Postsurgical recovery was uneventful and he was discharged home on postoperative day 4. Minimally invasive robotic total right hepatic lobectomy is feasible with excellent perioperative outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatic resections are performed for both benign and malignant liver pathologies. Outcomes of hepatic resection have evolved over the years with better understanding of the liver anatomy, advancement in surgical instrumentation, and improved perioperative care. Laparoscopic liver resection has become more common since it was first described in the 1990s [1]. An important principle of minimally invasive hepatic surgery is that the indications for resection are similar as those of an open hepatic resection. The INSTALL study published in 2015 has shown that a gradual evolution has occurred since the Louisville Statement in 2008, with expanding indications for laparoscopic approach in terms of tumor size, number of lesion, extent of hepatic resection, level of technical difficulty, and degree of background liver cirrhosis [1, 2]. Advantages of minimally invasive surgery include less intraoperative blood loss, reduced postoperative pain and narcotic requirements, shorter hospital length of stay, lower risk for perioperative complications, fewer days till resumption of oral intake, as well as similar oncologic outcomes when compared to the traditional open approach [3, 4].
However, there are inherent limitations of laparoscopic approach which include limited range of motion, a two-dimensional view, amplification of physiologic tremors, and a steep learning curve. Robotic surgical system provides a solution to these technical limitations by providing a magnified three-dimensional view, tremor filtering, articulating instruments with seven degrees of freedom, and intuitive hand control movements. The first report of robot-assisted liver resection was published in 2006 [5]. It is suggested that robotic surgery can also shorten the minimally invasive learning curve [6,7,8]. A single institution study by Tsung et al. showed only 49.1% of all laparoscopic hepatic resections were completed in a purely minimally invasive approach (i.e., without the need to add a handport or conversion to a minilaparotomy), compared to 93% of cases completed in a purely minimally invasive fashion when performed robotically. Control of bleeding, one of the most difficult aspects of minimally invasive hepatic resection, can be facilitated via the robotic approach at any points during the operation, mostly due to greater degree of instrument movement and ease of suturing even in difficult areas [9, 10]. Herein, we aimed to describe our standardized surgical technique of performing robotic total right hepatic lobectomy.
Materials and methods
Aspects of minimally invasive major hepatic resection are discussed. Our standardized surgical technique of total right hepatic lobectomy using the robotic approach is described.
Patient assessment and operative strategy
The indications and initial work-up for robotic hepatic resection are comparable to those of an open or laparoscopic hepatic resection. Triphasic liver computed tomography (CT) or magnetic resonance imaging (MRI) is the imaging modalities of choice. Occasionally, a percutaneous liver biopsy is necessary in case of diagnostic uncertainty. Future liver remnant volume evaluation is undertaken in a similar fashion to that for an open hepatic resection using a CT volumetric software. Decision to perform a robotic hepatic resection is determined by various factors: tumor size, location, proximity to vital structures, as well as surgeon comfort level in undertaking the operation. The team consisting of the surgeons, surgical technician(s), and anesthesiologist should be familiar with all aspects of the liver operation and effective communication is key. It is imperative that the surgeon at the console and the surgeon assistant at the bedside are skilled in performing both an open and minimally invasive hepatic resection. Ideally, they should be interchangeable throughout the operation. Challenges with liver mobilization, hilar dissection, parenchymal transection, and hemostasis may be minimized by optimal patient positioning, efficient port placement, and proper use of robotic and laparoscopic instruments. The anesthesiologist must maintain a low central venous pressure (< 5 mm Hg) especially during the parenchymal transection phase. Indications to convert to an “open” approach include significant intraoperative bleeding, concerns of oncologic compromise, and prolonged operative time due to difficult dissection or failure to progress [7].
Operative technique
Patient positioning and port placement
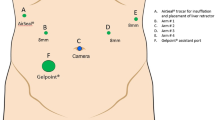
The patient is positioned supine on the operating table, followed by induction and maintenance of general anesthesia. This is routinely followed by the placement of an arterial line, as well as a central venous catheter (usually in the internal jugular or subclavian vein). An orogastric tube is placed for gastric decompression, and a Foley catheter is inserted for urinary bladder decompression. The abdomen is prepped and draped in the standard sterile fashion using alcohol-based solution. A betadine-impregnated plastic drape is applied after 3 min of drying time. The surgeon assistant stands to the patient’s right side and the surgical technician stands to the patient’s left side (Fig. 1). The operating table is placed in reverse Trendelenburg position (up to 13°) with a slight left tilt. The da Vinci Xi® robotic surgical system (Intuitive Surgical, Sunnyvale, CA, USA) is then paired with the operating table to allow for easy re-positioning during the operation without needing to undock the robot. Local anesthetic is injected into the umbilicus prior to making an incision. An 8-mm vertical incision is made in the umbilicus and 15 mmHg of pneumoperitoneum is established with carbon dioxide. The robotic camera is inserted and diagnostic laparoscopy is undertaken.
If diagnostic laparoscopy shows no contraindications to tumor resectability, the remaining trocars are placed. 8-mm trocars are placed in the right mid-clavicular line, the left mid-clavicular line, and left anterior axillary line (all cephalad to the umbilicus). A Gelpoint® Mini Access Platform (Applied Medical, Rancho Santa Margarita, CA, USA) is placed in the right lower quadrant between the umbilicus and right mid-clavicular port (Fig. 2). This allows the surgeon assistant to have an easy access to the target anatomy for suctioning, compression, clipping, and stapling. The ports are generally placed following a curved line to maximize instrument reach to the posterosuperior region, which is necessary during right hemiliver mobilization and dissection of the proximal right hepatic vein at its entrance into the inferior vena cava. This is especially helpful in patients with a large body habitus; otherwise port positioning could be in a straight line, as per intuitive recommendations. The robotic system is then docked. Generally, the fenestrated bipolar is placed in arm #1, camera in arm #2, a hook cautery in arm #3, and non-traumatic bowel grasper in arm #4. A systematic ultrasonographic examination of the liver using a robotic ultrasound probe inserted via the Gelport is undertaken to confirm tumor location and more importantly to rule out the presence of additional tumor(s) that can potentially change the operative plan. The TilePro™ feature is useful since it enables visualization of both robotic camera and ultrasonographic views on one screen.
Liver mobilization
We start the operation by mobilizing the liver. This is done by taking down the falciform ligament close to the anterior abdominal wall all the way from the umbilical vein to the trifurcation of the hepatic veins. The left lobe of the liver is mobilized by dividing the left triangular and coronary ligament. The robotic hook cautery and bipolar device are used in this step. Care should be taken not to injure branches of the phrenic vein, often located in close proximity to the left coronary and triangular ligaments. The liver is then elevated anteriorly by the robotic arm #4, which provides access into the right hepatorenal space. Placing the patient in reverse Trendelenburg position with the bed tilted slightly to the left helps to move the liver towards the left upper quadrant. The right coronary and triangular ligaments are taken down along the right hemidiaphragm with the hook cautery and bipolar energy device as far as possible towards the insertion of the right hepatic vein into the inferior vena cava (IVC). We take down all the ligaments attaching the posterior aspect of the right lobe of the liver to the kidney, adrenal gland and the inferior vena cava. The bare area is entered. The bedside surgeon helps provide continuous dynamic exposure by retracting the proximal transverse colon inferiorly using an atraumatic bowel grasper. It is important to not cause capsular tear or parenchymal laceration while elevating the liver anteriorly using the robotic arm #4, especially in a heavy steatotic liver. The surgeon must also be aware of the short hepatic veins coming off the anterior aspect of the IVC since they can be accidentally avulsed during the liver mobilization. It is necessary to divide these short hepatic veins between robotic WECK® Hem-o-lok® clips when encountered. The entire length of retrohepatic IVC should ideally be visualized when it is safe to do so. At this point, the liver is adequately mobilized.
Portal dissection
The infrahepatic fossa is then exposed to gain access into the porta hepatis. This is done by elevating segment 4b and 5 using the non-traumatic robotic bowel graspers in arm #4. Next, anatomical portal dissection begins by first identifying the common hepatic artery. The peritoneal layer covering the porta hepatis is incised with the robotic hook cautery and fenestrated bipolar. The common hepatic artery is followed distally all the way up to the proper hepatic artery and the hepatic artery bifurcation. The right hepatic artery is isolated. A clamping test is performed routinely to ensure the presence of an intact flow in the left hepatic artery. The right hepatic artery is then clipped with robotic WECK® Hem-o-lok® clips, twice proximally and once distally prior to division with robotic scissors (Fig. 3). If the gallbladder is still present, then cholecystectomy is subsequently undertaken in a standard fashion. The cystic duct and artery are both doubly clipped and ligated prior to division with robotic scissors. Next, the main portal vein is identified posterior to the common hepatic duct. It is followed up to its bifurcation into the right and left branches. The right portal vein is then carefully isolated using the robotic bipolar grasper and hook electrocautery (Fig. 4). A small branch going into hepatic segment 6 posteriorly is sometimes encountered. Division of this small branch can significantly facilitate safe isolation of the right portal vein. Once isolated, the right portal vein is ligated with a 3-0 silk suture followed by placement of robotic WECK® Hem-o-lok® clips prior to division. Anatomical inflow vascular control to the right hepatic lobe is now achieved.
Parenchymal transection and division of right hepatic bile duct
Ispilateral inflow vascular control to the right hepatic lobe should result in a visible demarcation line along the Cantlie’s line separating the right lobe from the left. A systematic ultrasonographic examination of the liver is important to confirm the tumor location, proximity to the major intrahepatic vessels and also to map the intrahepatic vascular anatomy. The line of planned parenchymal transection is drawn on the liver surface under ultrasonic guidance, ensuring at least 1-cm margin of uninvolved tissue if possible. Intrahepatic crossing vessels of the segments 5 and 8 need to be identified and anticipated to achieve a bloodless parenchymal transection. A formal/total right hepatic lobectomy entails removal of hepatic segments 5, 6, 7, and 8. Figure of eight 3-0 silk sutures can be placed on both sides of the transection line to help with retraction. The robotic hook cautery is used to transect the superficial part (1–2 cm deep) of the liver parenchyma. The superficial liver parenchyma is relatively bloodless, since only small capillary vessels are usually encountered. It is very important for the anesthesiologist to maintain a low central venous pressure (CVP) of less than 5 mmHg to reduce back bleeding from the hepatic veins. Deeper parenchymal transection begins with the use of the robotic vessel sealer. The instrument is activated while the jaws are in open position and they are gradually closed as parenchymal coagulation advances (Fig. 5). The intrahepatic vessels need to be identified and properly handled. The vessel sealer can handle < 7 mm vessels securely, while for the larger vessels, we use either the robotic WECK® Hem-o-lok® clips or laparoscopic Endo GIA™ 60-mm linear vascular staplers (Medtronic, Minneapolis, MN, USA) applied by the bedside surgeon, or via a robotic stapler in arm #3 (trocar upsize is necessary). Insertion of the stapler should be done carefully to ensure there is no inadvertent injury/laceration to the intrahepatic vascular structures. The right hepatic bile duct is divided intrahepatically during the liver parenchymal transection using a laparoscopic Endo GIA™ 60-mm linear vascular stapler, which results in an excellent bilestasis. As the parenchymal transection advances cephalad along the lateral aspect of the IVC toward the notch between right and middle hepatic vein, the right hepatic vein is identified intrahepatically near its insertion into the IVC. Another laparoscopic Endo GIA™ 60-mm linear vascular stapler applied by the bedside surgeon is used to divide the right hepatic vein flush to the IVC (Fig. 6). Any bleeding points are either cauterized using the robotic fenestrated bipolar energy device, placement of robotic clips, or stitched using figure of eight 4-0 polyproplene sutures. The bedside surgeon plays an important role in facilitating exposure and tissue hemostasis, as well as maintaining proper orientation of the transection plane during the parenchymal transection.
Hemostasis and specimen removal
Once, the liver specimen is detached from the remaining future liver remnant, it is placed in a laparoscopic specimen retrieval bag and removed from the abdomen via the right lower quadrant GelPoint incision. The transection surface on the remnant liver is carefully inspected. A saline-coupled bipolar sealing device is used to provide effective hemostasis on the large hepatic resection surface. Thermal energy is applied limitedly near the hilum and staple lines. It is a good practice to decrease the insufflation pressure down to 8 mmHg while observing the cut surface of the liver for occult bleeding or biliary leak for about 15 min prior to closure. This is followed by inspection of the right adrenal gland and kidney to ensure no inadvertent injury. Ex vivo, the specimen is grossly examined to confirm adequate margins before being sent to pathology for a frozen section examination. The abdomen is generously irrigated with saline and blood clots were suctioned out. Finally, all the port sites are closed under direct videoscopic visualization.
Results
A 79-year-old man with an 11-cm biopsy-proven hepatocellular carcinoma was taken electively to the operating room for a robotic total right hepatic lobectomy. Body mass index was 35 kg/m2. No history of alcohol abuse or viral hepatitis infection. Past medical and surgical history was only consistent with obesity, hypertension and type 2 diabetes mellitus. Cardiology clearance is routinely obtained prior to any major hepatic resection. CT scan showed a large hepatocellular carcinoma mass predominantly located in segments 7 and 8 with close proximity to the right hepatic vein and inferior vena cava (Fig. 7). A systematic evaluation of the liver using ultrasonography at the beginning of the operation showed no additional hepatic lesion. Robotic extrahepatic Glissonean pedicle approach was used to gain inflow vascular control. Right hepatic artery and portal vein were individually dissected and isolated without any difficulty prior to division. An intraoperative robotic ultrasound was again utilized to guide the liver parenchymal transection, securing negative margins. Robotic vessel sealing device was used as the main energy device during the hepatic parenchymal transection. The right hepatic bile duct was transected intrahepatically using a laparoscopic Endo GIA™ 60-mm linear vascular stapler applied by the bedside surgeon. Toward the end of the parenchymal transection, the right hepatic vein was transected intrahepatically utilizing another load of laparoscopic Endo GIA™ 60-mm linear vascular stapler. The operation went uneventfully. Total operative time was 200 min without intraoperative complications. Estimated blood loss was 100 ml. Intraoperative frozen section showed negative resection margins. He was managed on a regular surgical floor. Intensive care unit admission was not necessary. Clear liquid diet was resumed on postoperative day # (1). Full liquid diet was given on postoperative day # (2). He was able to ambulate without assistance in the evening of postoperative day # 2. The reminder of the postsurgical recovery was uneventful and he was discharged home on postoperative day 4. He was doing very well at 2-week office follow-up.
Discussion
There are numerous recent studies that have shown not only the safety of minimally invasive hepatic resections, but also many of its benefits over the traditional “open” approach. Nguyen et al. conducted a literature review of 31 studies comparing laparoscopic with open hepatectomies (over 2000 patients) and found that the minimally invasive approach was associated with less blood loss, less narcotic requirements, shorter length of hospital stay, with no difference in oncologic outcomes [9]. Another meta-analysis by Croome et al. reviewed 26 articles comparing laparoscopic versus open hepatic resections between 1998 and 2009. The laparoscopic group was again found to be associated with lower operative blood loss, lower relative risk of postoperative complications, shorter length of hospital stay, decreased intravenous narcotic requirements, and fewer days till oral intake [10]. Other study by Beppu et al. showed no statistical difference between the minimally invasive and open group in terms of short-term oncologic outcomes and survivals [11].
One of the major concerns with minimally invasive hepatic resection is intraoperative hemorrhage control. Intraoperative hemorrhage has been clearly shown to correlate with increased morbidity and mortality in patients undergoing hepatic resections [12]. Laparoscopic and robotic approaches are believed to be associated with reduced intraoperative bleeding due to positive pressure exerted by the pneumoperitoneum (10–15 mmHg), in addition to the widely practiced low CVP during parenchymal transection, proper use of hemostatic devices, and anatomical hepatic vascular inflow/outflow control [13]. The magnified three-dimensional view provided by the robotic system theoretically also allows for more precise intrahepatic vessel dissection, thus minimizing the intraoperative bleeding. The ease of vessel and bile duct suturing even in difficult to reach locations, such as in liver segments 7 and 8, is a definitive advantage of the robotic system over the conventional laparoscopy. We utilize the laparoscopic Endo GIA™ 60-mm linear vascular stapler to divide major biliary or vascular structures; however, manual suturing with 4-0 polypropylene stitches is sometimes necessary. Robotic approach in hepatic resection should theoretically lead to further minimalization of intraoperative blood loss and postoperative bile leak.
As previously mentioned, a single institutional study showed 93% of robotic hepatic resections were completed without the need for hand-assist port or conversion into the hybrid (minimally invasive liver mobilization followed by open parenchymal transection via a minilaparotomy incision) technique, while only 49.1% of the laparoscopic hepatic resections were completed without these adjuncts [9, 10]. In this case, robotic system facilitated complete liver mobilization, inflow vessel dissection, and hepatic parenchymal transection without much difficulty. Our early experience convinced us that because of the articulated instruments and seven degrees of freedom, the use of robotic system allows easier, more precise, and safer portal dissection for inflow vascular control, when compared to the conventional laparoscopic technique.
Transient hepatic vascular inflow occlusion, or the Pringle maneuver, is easy to perform and it is very useful in a case of significant blood loss during hepatic resections. However, it is not without adverse effects, such as ischemic-reperfusion injury and splanchnic congestion. Compared with the Pringle maneuver, selective hemihepatic vascular occlusion in cases of partial hepatic lobe resection has the advantage of reducing intraoperative parenchymal ischemia, therefore, improving postoperative hepatic function recovery. In this case, we did not experience significant blood loss; therefore, Pringle maneuver was unnecessary.
We realize that it is crucial for the bedside surgeon to be skilled in open and minimally invasive hepatic resections. The most common reason for immediate conversion to an “open” approach is major intraoperative bleeding, which requires the bedside surgeon to quickly provide temporary hemostasis via effective compression, undock the robot and gain access to the abdomen. Other reasons for conversion to an open approach include difficulty in assessing tumor margins, severe intraabdominal adhesions, difficulty in liver mobilization, failure to progress, and rarely morbid obesity [6, 14].
In this case, the patient had an uneventful postoperative recovery without any complications. He was discharged home on postoperative day # 4. In a recent review article by Ocuin et al., the overall complication rate of hepatic resections was 21% [15]. This included complications specific to the liver (bile leak, liver failure, ascites), those specific to surgery (bleeding, pleural effusions, wound infection, ileus, bladder injury), and those that are general postoperative in nature (deep vein thrombosis, Clostridium difficile colitis). The most common complications are bile leak/biloma, and intra-abdominal fluid collection/abscess formation. In the literature, hospital length of stay after hepatic resections was reported anywhere from 4 to 12 days depending on the country. The shortest length of stay was reported in the USA [15, 16].
We obtained negative resection margins which were confirmed by frozen section examination, despite the medial aspect of the tumor was in a close proximity to the inferior vena cava. He will undergo a serial radiographic surveillance using CT scan every 4 months for the first 2 years postoperatively. There has been no evidence to suggest worse oncologic outcomes, compromised R0 resections, or increased recurrence rates with robotic hepatic resections when compared to the laparoscopic group. The short- and long-term data, however, are still lacking [15, 17].
Thus far, we have undertaken eight cases of robotic total right hepatic lobectomy in our program. Five patients had hepatocellular carcinoma and two patients had metastatic colorectal cancer to the liver. One patient had 13-cm symptomatic giant cavernous hemangioma. The median operative time was 240 min with 150 ml of estimated blood loss. Nasogastric tube was not used after any robotic hepatectomy. All of the patients were placed on oral clear liquid diet on postoperative day 0 and they were advanced to full liquid diet by postoperative day 2 or 3. Median length of hospital stay was 4 days. None of the patients required admission to the intensive care unit or received blood transfusion. One patient developed postoperative fluid collection and leukocytosis which was managed with CT guided percutaneous drain placement by interventional radiology service in addition to intravenous antibiotic.
Although this technique is feasible, it requires adequate training in a high-volume tertiary hepatobiliary center with high expertise. Practice on robotic simulation machine is a recommended starting point for robotic hepatectomy training. The surgeon must have qualification in general surgery with full competency in open liver surgery, in addition to experience in laparoscopic surgery. Atlases of liver anatomy and liver surgery technique, lectures on procedures from experts in the field, detailed review of video recordings, and attending hands-on workshops formulate the initial phase of training. The surgeon should first start to participate as a bedside-assistant surgeon. After a certain number of operations as a bedside-assistant surgeon, she/he begins to perform robotic hepatectomy as a console surgeon, starting with minor non-anatomical anteroperipheral resections and left lateral sectionectomy prior to undertaking major resection.
In conclusion, we believe that the use of robotic technology increases the technical feasibility and safety of major hepatic resections, such as total right hepatic lobectomy. This technique is feasible, easy to learn, and safe with minimal perioperative morbidity and mortality. This approach should be integrated into the armamentarium of modern hepatobiliary surgeons.
References
Buell JF, Cherqui D, Geller DA et al (2009) The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 250(5):825–830
Hibi T, Cherqui D, Geller DA et al (2016) Expanding indications and regional diversity in laparoscopic liver resection unveiled by the International Survey on Technical Aspects of Laparoscopic Liver Resection (INSTALL) study. Surg Endosc 30(7):2975–2983
Nguyen KT, Marsh JW, Tsung A et al (2011) Comparative benefits of laparoscopic versus open hepatic resection: a critical appraisal. Arch Surg 146(3):348–356
Croome KP, Yamashita MH (2010) Laparoscopic vs open hepatic resection for benign and malignant tumors: an updated meta-analysis. Arch Surg 145(11):1109–1118
Ryska M, Fronek J, Rudis J et al (2006) Manual and robotic laparoscopic liver resection. Two case-reviews. Rozhl Chir 85(10):511–516
Choi GH, Choi SH, Kim SH et al (2012) Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc 26(8):2247–2258
Giulianotti PC, Bianco FM, Daskalaki D, Gonzalez-Ciccarelli LF, Kim J, Benedetti E (2016) Robotic liver surgery: technical aspects and review of the literature. Hepatobiliary Surg Nutr 5(4):311–321
Chandra V, Nehra D, Parent R et al (2010) A comparison of laparoscopic and robotic assisted suturing performance by experts and novices. Surgery 147(6):830–839
Tsung A, Geller DA (2015) Reply to letter: “Does the robot provide an advantage over laparoscopic liver resection?” Ann Surg 262(2):e70–e71
Tsung A, Geller DA, Sukato DC et al (2014) Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 259(3):549–555
Beppu T, Wakabayashi G, Hasegawa K et al (2015) Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 22(10):711–720
Jarnagin WR, Gonen M, Fong Y et al (2002) Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 236:397–407
Tranchart H, O’Rourke N, Van Dam R et al (2015) Bleeding control during laparoscopic liver resection: a review of literature. J Hepatobiliary Pancreat Sci 22(5):371–378
Qiu J, Chen S, Chengyou D (2016) A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc 30(3):862–875
Ocuin LM, Tsung A (2015) Robotic liver resection for malignancy: current status, oncologic outcomes, comparison to laparoscopy, and future applications. J Surg Oncol 112(3):295–301
Ji WB, Wang HG, Zhao ZM et al (2011) Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg 253:342–348
Lai EC, Yang GP, Tang CN (2013) Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg 205(6):697–702
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Sucandy, Author Durrani, Author Ross, and Author Rosemurgy declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Sucandy, I., Durrani, H., Ross, S. et al. Technical approach of robotic total right hepatic lobectomy: How we do it?. J Robotic Surg 13, 193–199 (2019). https://doi.org/10.1007/s11701-018-0881-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-018-0881-7