Abstract
In spite of difficult anatomic access for tumors of mediastinum, surgical resection remains the best diagnostic and therapeutic approach. Widespread acceptance of video-assisted thoracoscopy (VATS) is restricted by the limiting nature of instruments and suboptimal visualization. Robotic assisted minimally invasive surgery seems to hold most promise in remote, narrow anatomical regions. After obtaining approval from Institutional Review Board (IRB), a retrospective review of prospectively collected database on patients that underwent Robotic VATS between 2009 and 2013 was conducted. Forty-eight patients underwent RVATS resection of mediastinal tumor. One procedure (2.1%) was converted to open. The size of the mass ranged from 0.6 to 12.5 cm in greatest dimension (mean 5.16 cm). The mean duration of procedure was 127.96 min (60–240 min). Five patients (10.4%) had early postoperative complications including chylothorax (1 patient), new onset atrial fibrillation (1 patient), pleural effusion (1 patient), empyema (1 patient), and bleeding (1 patient). Mean follow-up time was 186 days (10–1300 days). Two patients (4%) with invasive thymoma developed local recurrence. The present study documents the feasibility of RVATS in the management of mediastinal tumors irrespective of the location in various mediastinal compartments. The role for careful and complete excision of the tumor, and surveillance afterward on invasive thymoma, was noted in our study, as in literature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tumors of the mediastinum represent a wide diversity of disease states ranging from thymoma to congenital cysts to metastatic disease from other primaries. Although imaging studies such as CT, MRI, and PET scan are useful in diagnostic workup of a mediastinal mass, management of these rare tumors is guided by pathology [1, 2]. Moreover, these lesions are frequently symptomatic mandating surgical resection to control symptoms. Surgical resection, therefore, remains the best diagnostic and therapeutic approach in the management of these rare tumors [3]. The choice of surgical technique depends on the location of the lesion, other associated clinical factors such as body habitus, previous surgery, extent of disease, and the availability of special techniques and equipment, such as availability of video-assisted thoracoscopy or robotic instruments and staff familiar with those equipments. Median sternotomy is regarded as standard of care, especially for the management of anterior mediastinal tumors including thymoma [4]. Following introduction of video-assisted thoracoscopy (VATS) as a minimally invasive alternative to open procedures, there has been an increasing volume of data showing improved surgical outcome secondary to decreased surgical trauma, decreased length of hospital stay, and deceased overall complications [5,6,7]. But its widespread acceptance is restricted by the limiting nature of instruments and suboptimal visualization to operate in constrained space in mediastinum, and proximity to vital vascular structures [7,8,9]. Robotic assisted surgery has been developed as an adjunct to offset the limitations of minimally invasive techniques due to its intuitive ergonomic range of motion and increased degree of freedom, non-transfer of surgeon’s hands tremor, and improved three-dimensional optics. It design makes its use most promising in remote, narrow anatomical regions such as the mediastinum [7, 10, 11]. We present a single surgeon’s experience with robotic assisted thoracoscopy for the resection of mediastinal tumor over a period of 4 years.
Methods and materials
Surgical method
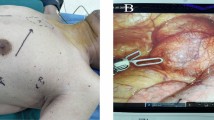
The surgical approach to these lesions is variable and is dictated by the surgical anatomy. The DaVinci Si system (Surgical Intuitive, Mountain View, CA) with three arms was universally utilized. For a typical anterior or posterior mediastinal mass, patient positioning included a roll vertically placed beneath the scapular on the surgical side and with the patient’s arm tucked at the side. In female patients the breast was elevated towards the contralateral shoulder with Ioban 2(3M, United Kingdom plc) during the draping process. The robot was docked from the contralateral side at 90 degree angle. Double lumen tubes or bronchial blockers were used at the discretion of the surgeon and anesthesiologist. The procedure was started with a single 12 mm port in the 4th or 5th interspace along the anterior axillary line. Low flow carbon dioxide insufflation (8 mmHg) was routinely used. A 30° Stryker high-definition handheld camera was used to assess the surgical anatomy and guide optimal placement of the 8-mm metallic ports. One of the secondary ports was placed in the 2nd intercostal space and the other port was placed in the 8th or 9th intercostal space in anterior axillary line. A 12-mm assistant port was used in selected cases around the 6th interspace in the mid axillary line. Thirty degree up and down cameras was utilized depending on the anatomy. Once all the vital structures were identified and preserved, careful dissection was accomplished utilizing the Cardiere forceps (Surgical Intuitive, Mountain View, CA), Curved Monopolalar shear (Surgical Intuitive, Mountain View, CA) and Hemolock clips® (Weck Surgical Instruments, Teleflex Medical, Durham, NC).
Stapling devices and suturing were less commonly utilized. Specimens were removed with endocatch bags. Soft flexible Blake® (Ethicon, USA) or Jackson Pratt® drains (Cardinal Health, Dublin, Ohio, USA) were used to drain the space. A single chest tube was used to drain pleural cavity. Local intercostal blocks were performed at the port interspaces prior to resuming ventilation. On-Q pain pump catheters (On-Q Pain Relief System, Halyard Health, Inc., Irvine, CA, USA) were placed along the posterior para-spinal space. Posterior mediastinal tumors were approached utilizing the lung resection technique or occasionally with prone positioning.
Data collection and analysis methods
A prospective database of patients undergoing robotic assisted thoracoscopy between years 2009 and 2013 by a single surgeon for the management of mediastinal tumors was maintained. After obtaining approval from Institutional Review Board (IRB) at Mount Sinai Medical center, a retrospective review was conducted. Age, gender, co-morbidities, length of surgery, estimated blood loss (EBL), length of ICU and hospital stay, early and late post-operative complications, conversion to open technique, tumor recurrence rate, and follow-up were reviewed. Descriptive analysis of data was done calculating mean, median, and comparison using by student’s t test where applicable.
Results
Forty-eight patients underwent robotic video-assisted thoracosopic resection of mediastinal tumor during the study period. This group included 22 females (45.8%) and 26 males (54.2%). The mean age of patients was 54.19 years. All patients had pre-operative imaging, without pathologic diagnosis. Forty-one cases (85.4%) underwent total resection of mediastinal mass, and seven cases (14.6%) underwent biopsy of unresectable mediastinal mass (unresectable secondary to type of tumor or invasion of vital organs).
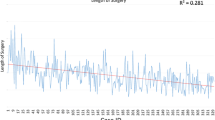
Thirty-one cases (64.6%) were performed via Left side access, while 17 (35.4%) cases were performed via right side. In 40 patients (83.3%) mass was located in anterior-middle mediastinum, and 8 (16.7%) patients had the tumor in posterior mediastinum. The size of the mass ranged from 0.6 to 12.5 cm in greatest dimension (mean 5.16 cm). The most common pathology was thymoma (including thymus hyperplasia), followed by mediastinal cyst (Table 1). Two patients were found to have invasive thymoma (Masaoka stage III). One procedure (2.1%) had to be converted to open procedure secondary to locally invasive nature of tumor, while the rest were completed using RVATS as planned (97.9%). Average blood loss was 45.94 ml (5–500 ml). Mean ICU stay was 1.13 days while the mean hospital stay was 3.73 days. The mean duration of procedure was 127.96 min (60–240 min). When we analyzed the mean operative time between the first 24 patients and second group of 24 patients, we found that the mean operative time decreased from 147.5 to 108.42 min (P < 0.002). Mean EBL for the first 24 cases was 58.54, and 33.33 ml for second half, although this difference was not statistically significant (P < 0.2).

Five patients (10.4%) had early postoperative complications including chylothorax (1 patient), new onset atrial fibrillation (1 patient), pleural effusion (1 patient) empyema (1 patient), and bleeding (1 patient). The most common late complication (more than 30 days post-operative) was chronic pain (2 patients, 4.2%) and one patient (2.1) developed late pericardial tamponade (Table 2). Three patients needed reoperation secondary to complications (6.2%), due to bleeding (Post-op day one), empyema (which occurred secondary to infected mediastinal cyst, post-op day 3), and pericardial tamponade (Post-op. day 65). Mean follow-up time was 186 days, with median follow-up of 60 days (10–1300 days). Two patients (4%) with invasive thymoma developed local recurrence. There was no operative and post-operative mortality.
One patient (2.1%) with recurrent invasive thymoma required re-operation via sternotomy later in follow-up.
Discussion
Management of a mediastinal mass is challenging due to a wide range of disease process ranging from benign thymic cyst to lymphoma, or metastatic malignancies from other primaries and limited anatomic work space. Imaging studies such as CT scan, MRI, or PET scan are helpful in the workup of the mass [1, 2], but adequate diagnosis and treatment need tissue diagnosis. Moreover, these lesions are frequently symptomatic and require resection for treatment. There are different ways to obtain biopsy for tissue diagnosis such as mediastinoscopy, or CT scan guided needle biopsy, but very frequently the tissue sample from these modalities is inadequate for pathologic diagnosis [3]. For many years, median sternotomy or open thoracotomy had beenthe preferred surgical approach for resection of these tumors [4, 8, 12]. Video-assisted thoracoscopic surgery was proposed as an minimally invasive alternative approach with the advantages of decreased surgical trauma, decreased length of stay, and decreased surgical complications [5,6,7]. Nowadays, VATS procedure is regarded as standard of care for these tumors except for invasive thymoma (Masaoka’s stage III–IV) [5,6,7,8,9]. Despite this trend, VATS has relative limitations, secondary to steep learning curve, bulky nature of instruments, less than ideal range of motion, transferring the surgeon’s hand tremors to surgical site, and less than ideal 2-D visual instruments [7, 9,10,11]. For all of these reasons, the technique has not been widely practiced and its use is limited to specialized centers [10, 11].
Robotic assisted surgery has been developed to overcome limitations proposed by conventional minimally invasive techniques. 7° of articulation with intuitive range of motion, 3-D visual scopes, and not-transferring surgeon’s hands tremor to instrument make this technique ideal for working in limited anatomic space such as mediastinum [7, 10,11,12]. Yoshino et al. [13, 14] first reported case series of Robotic video-assisted thoracoscopic procedures done on mediastinal tumors, demonstrating feasibility of this technique. Cakar et al. [16], and Weksler et al. [17] have demonstrated decreased length of stay, decreased post-operative complications, and better outcome of RVATS in comparison with open mediastinal tumor resection, while Balduyck et al. [18] have shown improved early post-operative quality of life in patients undergoing RVATS in comparison with open surgery. There have been subsequent studies by Bonder et al. [10], Melfi et al. [7], Marulli et al. [15], and Seong et al. [11] demonstrating improved outcomes of RVATS. Our study also compares favorably with the available literature on RVATS on management of mediastinal tumors (Table 3).
It is not practical to perform a randomized control trial, to compare this technique with VATS or open. It is not ethical or possible to randomize people to more invasive techniques while there is potential evidence for its outcome equalities with more minimally invasive technique, plus surgeon and patient’s preference would make randomization impossible. The increasing body of evidence proving the feasibility and safety of robotic technique in comparison with VATS and open could make this technique standard of care in near future. There are some critics on RVATS technique, such as increased cost of procedure. Some studies have shown with decreased length of stay and complication rate with this technique, the overall cost may decrease, despite more cost of device per case. The cost effectiveness of this technique was not in scope of this study and we realize the need for studies regarding this issue.
Limitations
The retrospective nature of our study and lack of a control group are the main limitations of this series. The data collection in these studies could be less than ideal. As mentioned earlier, cost of procedure was not in scope of this study, which is one of main contributors in decision making regarding surgical technique.
Conclusion
The present study demonstrates the feasibility of Robotic assisted thoracoscopy in the management of mediastinal tumors irrespective of the location in different mediastinal compartments. A brief review of literature suggests that results from our study are comparable with the historical results. The only recurrence in our study was noted in patients with invasive thymoma underlining a role for careful and complete excision of the tumor, and surveillance afterward. The need for more studies to compare this technique with VATS and open technique is inevitable.
References
Toker A, Erus S, Kaba E, Tanju S, Ozkan E (2014) Has there been a paradigm shift in mediastinal surgery from open to minimally invasive, and from magnetic resonance imaging (MRI) to positron emission tomography–computerized tomography (PET–CT) in the last decade? Surg Endosc 28:861–865
Davis RD, Oldham HN, Sabiston DC (1987) Primary cysts and neoplasms of the mediastinum: recent changes in the clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg 44:229–237
Yim APC (1995) Video assisted thoracoscopic management of anterior mediastinal masses, preliminary experience and results. Surg Endosc 9:1184–1188
Rea F, Marulli G, Girardi R, Bortolotti L, Favaretto A, Galligioni A et al (2004) Longterm survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg 26:412–418
Cheng YJ, Wu HH, Chou SH, Kao EL (2001) Video-assisted thoracoscopic management of mediastinal tumors. J Soc Laparosc Surg 5:241–244
Chetty GK, Khan OA, Onyeaka CVP, Ahmad F, Rajesh PB, Waller DA (2004) Experience with video-assisted surgery for suspected mediastinal tumours. Eur J Surg Oncol 30:776–780
Melfi F, Fanucchi O, Davini F, Viti A, Lucchi M, Ambrogi MC, Mussi A (2012) Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 41:847–851
Roviaro G, Rebuffat C, Varoli F, Vergani C, Maciocco M, Scalambra SM (1994) Video-thoracoscopic excision of mediastinal masses: indications and technique. Ann Thorac Surg 58:1679–1683
Hazelrigg SR, Landreneau RJ, Mack MJ, Acuff TE (1993) Thoracoscopic resection of mediastinal cysts. Ann Thorac Surg 56:659–660
Bodner J, Wykypiel H, Greiner A, Kirchmayr W, Freund MC, Margreiter R et al (2004) Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 78:259–265 (discussion 65–6)
Seong YW, Kang CH, Choi JW, Kim HS, Jeon JH, Park IK, Kim YT (2014) Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 45(3):e68–e73
Davenport E, Malthaner RA (2008) The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 86:673–684
Yoshimo I, Hashizume M, Shimada M, Tomikawa M, Tomiyasu M, Suemitsu R, Sugimachi K (2001) Thoracoscopic thymomectomy with the DaVinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 122(4):783–785
Yoshino I, Hashizume M, Shimada M, Tomikawa M, Sugimachi K (2002) Video-assisted thoracoscopic extirpation of a posterior mediastinal mass using the DaVinci computer enhanced surgical system. Ann Thorac Surg 74:1235–1237
Marulli G, Rea F, Melfi F, Schmid TA, Ismail M, Fanucchi O, Augustin F, Swierzy M, DiChiara F, Mussi A, Rueckert JC (2012) Robot-aided thoracoscopic thymectomy for early stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 144:1125–1132
Cakar F, Werner P, Augustin F, Schmid T, Wolf-Magele A, Sieb M et al (2007) A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 31:501–504 (discussion 04–5)
Weksler B, Tavares J, Newhook TE, Greenleaf CE, Diehl JT (2012) Robot-assisted thymectomy is superior to transsternal thymectomy. Surg Endosc 26:261–266
Balduyck B, Hendriks JM, Lauwers P, Mercelis R, Ten Broecke P, Van Schil P (2011) Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 39:543–548
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Pejman Radkani declares that he has no conflict of interests. Devendra Joshi declares that he has no conflict of interests. Tushar Barot declares that he has no conflict of interests. Roy Williams declares that he has no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This is a retrospective study, and “for this type of study formal consent is not required.”
Rights and permissions
About this article
Cite this article
Radkani, P., Joshi, D., Barot, T. et al. Robotic video-assisted thoracoscopy: minimally invasive approach for management of mediastinal tumors. J Robotic Surg 12, 75–79 (2018). https://doi.org/10.1007/s11701-017-0692-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-017-0692-2