Abstract
To construct patient-specific physical three-dimensional (3D) models of renal units with materials that approximates the properties of renal tissue to allow pre-operative and robotic training surgical simulation, 3D physical kidney models were created (3DSystems, Rock Hill, SC) using computerized tomography to segment structures of interest (parenchyma, vasculature, collection system, and tumor). Images were converted to a 3D surface mesh file for fabrication using a multi-jet 3D printer. A novel construction technique was employed to approximate normal renal tissue texture, printers selectively deposited photopolymer material forming the outer shell of the kidney, and subsequently, an agarose gel solution was injected into the inner cavity recreating the spongier renal parenchyma. We constructed seven models of renal units with suspected malignancies. Partial nephrectomy and renorrhaphy were performed on each of the replicas. Subsequently all patients successfully underwent robotic partial nephrectomy. Average tumor diameter was 4.4 cm, warm ischemia time was 25 min, RENAL nephrometry score was 7.4, and surgical margins were negative. A comparison was made between the seven cases and the Tulane Urology prospectively maintained robotic partial nephrectomy database. Patients with surgical models had larger tumors, higher nephrometry score, longer warm ischemic time, fewer positive surgical margins, shorter hospitalization, and fewer post-operative complications; however, the only significant finding was lower estimated blood loss (186 cc vs 236; p = 0.01). In this feasibility study, pre-operative resectable physical 3D models can be constructed and used as patient-specific surgical simulation tools; further study will need to demonstrate if this results in improvement of surgical outcomes and robotic simulation education.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rise of robot-assisted surgery in the past decade has significantly reshaped surgical practice and subsequently surgical education [1, 2]. Residents, especially those in urology, now, actively seek programs that have robust robotic exposure and high robotic case volume [2]. However, the increased cost of training including greater operative time, maintenance costs, and learning curves for residents is among the new challenges programs must face [3]. Training modules using inanimate tasks and dry labs have been shown to decrease the initial learning curve, but are unable to provide realistic surgical experience [4]. In comparison in vivo simulation using porcine or cadaver models are perhaps the most effective learning modules due to anatomic accuracy and living tissue perfusion. However, these platforms are limited by high costs [4, 5]. Ex vivo tissue labs bridge these modalities and provide realistic surgical exercises while also keeping costs low [6]. Herein lies the benefit of 3D printing, a modality that allows for creation of anatomic models that can mimic clinically realistic organ systems and be personalized for individual cases.
To date, the major limitations of all 3D-printed models to aid surgeons and trainees is that the models are fixed and do not approximate the normal tissue texture. Most anatomical models are fabricated using a firm opaque monocolor structure, with utility restricted to orthopedic and maxillofacial procedures, where these models are recapitulated bony structures [7, 8]. Previously, we reported on the use of 3D physical models to aid in the understanding and subsequent expiration of in situ soft-tissue renal malignancies [9]. Our initial models incorporated clear translucent resin to represent normal renal parenchyma and a red translucent resin to delineate tumor, renal vasculature, collecting system, and proximal ureter. The variation in color allowed the surgeon to appreciate the depth and dimensions of the tumor in relation to both normal parenchyma and critical renal structures. Other groups have also used similar firm multicolor kidney models to aid in surgical planning [10, 11].
The purpose of this study was to develop a novel 3D-printed kidney model using materials that closely approximated normal kidney. We aimed to demonstrate the feasibility of creating a soft-tissue 3D model of renal tumors using images imported from the standard computerized tomography for the purpose of pre-surgical resection and future incorporation into simulation labs.
Materials and methods
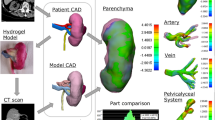
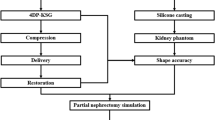
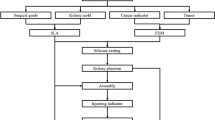
3D physical kidney models were created from the standard contrasted diagnostic computerized tomography (CT) images from various scanners using 3–5 mm sections. No additional specialized or additional imaging was needed. The images were converted to models with the assistance of a 3D printing and manufacturing company (3DSystems, Rock Hill, SC). First, the contrasted studies were segmented to identify structures of interest, delayed imaging allowed for better segmentation of the collection system from multiple series within the patient study. The segmented two-dimensional image data from the CT scans were then converted to a 3D triangulated surface mesh file (STL) suitable for fabrication (Fig. 1). Structures of interest arising from multiple imaging series were combined into one file using alignment markers common to all the imaging series, such as parenchyma and tumors. The segmentation and design of each model were reviewed for accuracy by the surgeon and segmenting engineer prior to model fabrication.
Models were constructed using multi-jet 3D printers that selectively deposit photopolymer material that is cured immediately after deposition with an ultraviolet lamp. 3D printer materials are jetted through a print head in tiny droplets, and the process allows for multiple materials to be blended and deposited together creating a composite flexible structure. A firm but malleable renal capsule was created using this technique with a hollow cavity. Next, an agarose gel solution was prepared and injected into the cavity of the kidney shell creating an accurate model of the patient-specific pathology with texture that approximated normal anatomic tissue. Multiple contrasting materials and colors allowed delineation of the enhancing renal mass, collecting system and renal vasculature from the normal parenchyma.
After obtaining institutional review board approval, six patients with seven enhancing renal lesions (one patient had bilateral renal masses) found on the standard cross-sectional CT imaging had 3D physical models of their complete renal unit with in situ lesion printed using the 3D printing techniques described above prior to intended partial nephrectomy. Investigators prospectively resected tumors on the models using the da Vinci® robotic platform followed by sliding clip renorrhaphy the week prior to the patient’s surgery (Figs. 2, 3). All patients then proceeded to robot-assisted partial nephrectomy (RAPN).
To explore potential demonstrate differences between the study population included in this manuscript and other patients undergoing the same procedure, we compared the data from the seven unique tumors included in this study to our prospectively maintained, IRB approved, robot-assisted partial nephrectomy database.
Results
Seven separate renal units with enhancing renal masses were constructed as described using 3D printing techniques. The da Vinci surgical platform was used to simulate RAPN with sliding clip renorrhaphy on all renal units. Following this surgical simulation, all six patients, seven renal tumors, successfully underwent robot-assisted partial nephrectomy with complete excision of the suspicious renal mass. One patient underwent bilateral retroperitoneal RAPN in a staged approach, and the remaining patients underwent transperitoneal RAPN. Surgical margins were negative in all cases, and there were no intraoperative or peri-operative complications observed. Pre-operative patient demographics, tumor characteristics, operative and peri-operative data, as well as histopathologic findings are described in Table 1.
The cohort of patients having undergone 3D construction of their kidney consisted of four men and two women with a median age of 64 years (49–70). The median Charlson comorbidity index score for the population was 5 (2–6). The median body mass index for the group was 29.1 kg/m2 (23.6–35.9), and the median tumor diameter was 4.7 cm (2.8–5.5). Tumors ranged in complexity with a median numerical RENAL nephrometry score of 8 (5–9) indicating an intermediate anatomic complexity.
The median serum creatinine and estimated glomerular filtration rate (GFR) for the cohort were 0.93 mg/dl (0.76–1.2) and 72 ml/min (64.8–101), respectively. Post-operative renal function assessment revealed a median serum creatinine of 1.2 mg/dl (0.99–1.4) and GFR of 63 ml/min (39–75.2). Only one patient progressed to stage III chronic kidney disease (CKD) following surgery, and this patient underwent bilateral RPN with an initial pre-operative GFR of 65 ml/min. Two patients (33%) experienced a one-stage increase in their CKD, while no patients had a two-stage progression of their CKD. No patients progressed to dialysis post-operatively.
When compared to our prospectively maintained RAPN database, we found that the patients within this study have some differences with respect to their demographics, tumor characteristics, operative data, peri-operative data, and pathologic data. While patients in this study had longer WIT (25 vs 21.6 min), they also had more complex tumors as demonstrated by the higher mean nephrometry score (7.4 vs 6.9), larger average tumor size (4.3 vs 3.4), and higher pathologic stage (pT1b 57 vs 30%): of note, none of these differences reached significance. Despite these differences, patients included in this study had fewer complications, fewer positive surgical margins, and shorter hospitalization, again without significance. The only significant differences found in these comparisons were that patients with a pre-operative surgical model experienced a lower estimated blood loss at the time of resection (186 cc vs 236; p = 0.01).
Discussion
In this study, we have demonstrated the utility of a novel surgical technology that allows surgeons the ability to perform surgical simulation on a 3D-printed soft-tissue physical model that is specific to their individual patient. To our knowledge, this is the first report of 3D printing of soft-tissue models for pre-surgical simulation. The advantages of these models are that they are patient specific, have tissue properties that approximate that of the kidney, and allow robotic resection and renorrhaphy of the model prior to operating on the patient, especially helpful in the education of the urological resident. In this pilot trial, our primary goal has been to highlight our novel methodology. We also compared the seven tumors with pre-surgical 3D modeling to our large institutional database to find any changes in surgical outcomes. The lack of differences is most likely secondary to the small sample size and limitations of the study design. However, we found a significant difference in the estimated blood loss in patients with pre-surgical modeling. Likewise, subjectively, our surgeons felt that the 3D model felt similar to actual kidney and provided useful insight prior to actual patient procedure. In the future, a larger series with more controls will allow for a more accurate comparison of cohorts, but few can doubt the ability to teach trainees on a replica that is highly similar in shape and material to their upcoming surgery will result in a flattening of the early learning curve.
RAPN is a persistently challenging operation with a steep learning curve, especially in the training of resident surgeons [12]. First, the kidney receives approximately 20% of the body’s cardiac output per minute (range 982–1209 mL/min), making intraoperative bleeding a significant possibility despite renal artery clamping [13]. Second, there is evidence to support that every minute of warm ischemia can have detrimental effects on post-operative renal function and thus efforts are made to keep warm ischemia to a minimum [14]. Therefore, renorrhaphy is commonly the most difficult portion of the surgery for young surgeons. Furthermore, each case is unique in the location, depth, and orientation of the tumor and its relation to surrounding structures creating greater variability than other commonly performed robotic surgery. Finally, oncologic outcomes of RAPN hinge on complete resection of the tumor while avoiding any violation of the capsule, and concurrently preserving maximal uninvolved renal parenchyma.
Surgical educators’ primary duty to their trainees is the transmission of surgical skill and proficiency while optimizing patient outcome. While these potentially disparate goals are not new, they may be particularly challenging for robotic procedures because of the inability of the mentor to directly guide a trainee through an operation with hands-on instruction as with open operations [15]. As a result, surgical simulators have provided an alternative to pre-clinical resident robotic training. There are multiple robotic surgical simulators that are available and have undergone previous validation, including the da Vinci Skills Simulator (Intuitive Surgical, Sunnyvale CA) [16], Mimic dV-Trainer (Mimic Technologies, Seattle WA) [17], and Robotic Surgery Simulator (RoSS, Simulated Surgical Systems, Williamsville NY) [18]. Although these surgical simulators have circumvented some of the logistic problems with pre-clinical resident training, they come at a significant expense and rely on virtual reality technology. Virtual reality simulators primarily use basic virtual tasks to help with robotic skill acquisition at the cost of realistic surgical experience and procedure-specific correlations.
In an attempt to offset these disadvantages of virtual reality, simulators have been developed with augmented reality (AR) capabilities to provide more relevant and procedure-specific training [19, 20]. With augmented reality, simulators can superimpose live images with synthetic computer-generated images, allowing for more realistic tasks for skill acquisition. Taking surgical simulation one step beyond procedure-specific training, we have created a novel method of ex vivo surgical simulation using 3D printers and standard CT scans to create patient-specific individualized models that can be used for soft-tissue solid organ surgical simulation. AR platforms are limited in the number of pre-programmed tasks and they are coded with whereas endless number of patient-specific models can be created each with unique nuances. In addition, while there is no “tactile feedback” on the da Vinci platform, there is resistance that is experienced when using the machine to resect a model, which is more similar to the actual procedure than many AR platforms.
Previously, both our group and others have demonstrated that the construction of patient-specific 3D models is possible and models have high fidelity to patients’ anatomy [9, 10]. We have also demonstrated that these physical models improved trainees’ anatomic understanding of renal tumors by improving nephrometry scoring compared to cross-sectional imaging [21]. The outcomes of this pilot study demonstrate relatively low estimated blood loss, no transfusions, short clamp times, all negative margins, no complications, and good short-term renal functional outcomes on complex renal masses. Importantly, these outcomes are within the context of an ACGME approved training environment in which resident physicians were the primary surgeons on all these procedures. As surgeries and surgical tools become more complex, education and teaching tools also must evolve and these studies are demonstrating how 3D printing can be used to achieve this goal.
The limitations in our previous study include the lack of soft-malleable 3D modeling that would provide not only an understanding of patient anatomy, but allow the surgeon to mimic the procedure on an ex vivo model. The 3D soft-tissue model developed in this project aimed to meet these obstacles. In the original development of a soft-tissue kidney model, our group looked at different materials and using an outer shell. Prior iterations were found to be too dense and, therefore, unable to be manipulated in the simulation session. By incorporating both the hollowed out shell and a new type of gel agarose material, our group was able to create a model that subjectively felt-like normal renal tissue. Though it is difficult to quantify the accuracy of tissue handling, both senior level faculty and upper level residents with ample experience on robotic console for RAPN felt that tissue handling, cutting, and suturing were similar to actual surgery. Likewise, to note, our models were made from stable materials that allowed for strong durability and shelf life.
Partial nephrectomy is underutilized and this may be in large part to limitations in the training of young surgeons. A potential remedy may be for surgeons to assess the feasibility of a partial nephrectomy on a patient-specific model before touching the actual patient. Kidney masses are particularly suited for the task of creating these models in that the tumors are variable in size and location, and often the goal of the procedure is to perform extirpation of the tumor while preserving normal tissue. However, similar models could be constructed for other solid organ malignancies and aid any type of proceduralist.
In an attempt to demonstrate more than feasibility, we compared the patients included in this study, to our prospectively maintained database of 323 patients who underwent robotic partial nephrectomy without pre-surgical simulation. The outcomes of these data (Table 1) suggest that there may be important differences between the groups; while the WIT was longer in 3D surgical simulation group and they also had more complex tumors and better peri-operative and pathologic data. The only significant difference we found was in lower estimated blood los in patients with pre-surgical modeling. However, with only seven renal units, it is highly unloss that any differences observed are attributable to the models when a multitude of factors is known to influence outcomes. To properly perform this type of a comparison, a randomized trial with a much greater number of patients, accounting for a variety of patient-specific, oncologic specific, and surgeon specific factors, will need to be performed. Such a trial is intended in the near future.
While to our knowledge, this is the first report of its kind certain limitations of the models and the current study design warrant mention. The models are complex and involved to construct, requiring skilled technologists at significant cost. Likewise, at this time, the high initial start up cost for advanced machinery and materials makes an in house development of 3D models unlikely. However, the standard fixed monocolor 3D models can be manufactured for dollars; therefore, our group believes as this technology becomes readily available and we anticipate the cost of resectable soft-tissue models used in this study to drop dramatically in the near future. While these models are excellent replicas of the actual patient’s anatomy and texture, further refinement may be possible. Currently, the renal parenchyma in these models lack small arterial and venous branches that may be encountered during RAPN, the models do not currently actively exsanguinate, and the models also lack peri-nephric fat which can make identification and dissection of the vasculature challenging. These shortcomings of peri-nephric fat and active blood supply are the next developments; our group is exploring to provide the most realistic ex vivo RAPN simulation to date. Our models were based on CT scans that had 3–5 mm cuts and although they had good fidelity, “smoothing programs” were used to account for any gaps between slices on the imaging, and developments in finer cuts on the imaging would also allow for more precise and more detailed models with inclusion of the intra-parenchymal vasculature. We did not objectively compare the tensile material of these models to actual renal parenchyma, although subjectively, we do feel that they are similar. We intend to formally compare tissue properties of the resectable 3D models to that of an animal model in the near future. A larger case control study in which we account for both the trainees’ level of experience and the complexity of the resection/renorrhaphy will be needed to convincingly prove outcomes. During this further study, face and construct validity of the models and the pre-surgical simulation they allow will also be assessed.
Conclusion
3D printing is likely to change the way medicine is practiced, and advances in this technology continue to expand capabilities and applications within Urology. We have demonstrated the feasibility of creating high-fidelity 3D physical models that recapitulate the texture of renal parenchyma. For trainees and young surgeons, pre-operative patient-specific surgical simulation using these models may decrease the slope of the learning curve and improve patient outcomes; further evaluation is requisite and currently ongoing.
Attributions
3D physical models were constructed in conjunction with 3DSystemsInc. (Rock Hill, SC).
Abbreviations
- 3D:
-
Three-dimensional
- CT:
-
Computerized tomography
- STL:
-
Surface mesh file
- RAPN:
-
Robot-assisted partial nephrectomy
- GFR:
-
Glomerular filtration rate
- CKD:
-
Chronic kidney disease
- AR:
-
Augmented reality
References
Atug F, Castle EP, Woods M et al (2006) Robotics in urologic surgery: an evolving new technology. Int J Urol 13:857–863
Rashid HH, Leung YM, Rashid MJ et al (2006) Robotic surgical education: a systematic approach to training urology resident to perform robotic-assisted laparoscopic radical prostatectomy. J Urol 68:75–79
Grover S, Tan GY, Srivastava A et al (2010) Residency training program paradigms for teaching robotic surgical skills in urology residents. Curr Urol Rep 11:87–92
Schreuder HW, Wolswijk R, Zweemer RP et al (2012) Training and learning robotic surgery, time for a more structured approach: a systematic review. BJOG 119:137–149
Liss MA, McDougall EM (2013) Robotic surgical simulation. Cancer J 19:124–129
Sun AJ, Arong M, Hung AJ (2014) Novel training methods for robotic surgery. Indian J Urol 30(3):333–338
Mehra P, Miner J, D’Innocenzo R et al (2011) Use of 3-d stereolithographic models in oral and maxillofacial surgery. J Maxillofac Oral Surg 10:6–13
Starosolski Z, Kan JH, Rosenfeld SD et al (2014) Application of 3-D printing (rapid prototyping) for creating physical models of pediatric orthopedic disorders. Pediatr Radiol 44:216–221
Silberstein JL, Maddox MM, Dorsey P et al (2014) Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: a pilot study. Urology 84:268–272
Cheung CL, Looi T, Lendvay TS et al (2014) Use of 3-dimensional printing technology and silicone modeling in surgical simulation: development and face validation in pediatric laparoscopic pyeloplasty. J Surg Educ 71(5):762–767
Zhang Y, Ge HW, Li NC et al (2016) Evaluation of three-dimensional printing for laparoscopic partial nephrectomy of renal tumors: a preliminary report. World J Urol 34(4):533–537
Petros F, Sukumar S, Haber G-P et al (2012) Multi-institutional analysis of robot-assisted partial nephrectomy for renal tumors > 4 cm versus ≤4 cm in 445 consecutive patients. J Endourol 26:642–646
Dworkin DL, Brenner BM (2004) Brenner and Rector's: the kidney, 7th edn. Elsevier, Philadelphia, PA
Thompson RH, Lane BR, Lohse CM et al (2010) Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 58(3):340–345
Liss MA, McDougall EM (2013) Robotic surgical simulation. Cancer J 111(2):124–129
Hung AJ, Zehnder P, Patil MB et al (2011) Face, content and construct validity of a novel robotic surgery simulator. J Urol 186:1019–1024
Korets R, Mues AC, Graversen JA et al (2011) Validating the use of the Mimic dV-trainer for robotic surgery skill acquisition among urology residents. Urology 78:1326–1330
Seixas-Mikelus SA, Kesavadas T, Srimathveeravalli G et al (2010) Face validation of a novel robotic surgical simulator. Urology 76:357–360
Kumar A, Smith R, Patel VR (2015) Current status of robotic simulators in acquisition of robotic surgical skills. Curr Opin Urol 25(2):168–174
Hung AJ, Shah SH, Dalag L et al (2015) Development and validation of a novel robotic procedure-specific simulation platform: partial nephrectomy. J Urol 194(2):520–526
Knoedler M, Feibus A, Lange A et al (2015) Individualized physical 3-dimensional kidney tumor models constructed from 3-dimensional printers result in improved trainee anatomic understanding. Urology 85(6):1257–1262
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
None.
Ethical approval
This article does not contain any studies with animals or human participants performed by any authors.
Informed consent
Informed consent was obtained from all individual participants included in this study to use cross-sectional imaging as template for 3D models.
Rights and permissions
About this article
Cite this article
Maddox, M.M., Feibus, A., Liu, J. et al. 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: a feasibility study. J Robotic Surg 12, 27–33 (2018). https://doi.org/10.1007/s11701-017-0680-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-017-0680-6