Abstract
This study presents an exploration into the relationship between the molecular structure of oil-soluble fluorinated surfactants and their surface properties across a variety of organic solvents. A series of oil-soluble surfactants with fluorocarbon/hydrocarbon hybrid chains, N-methyl-N-alkyl-4-perfluoroalkylsulfoxybenzylamines (FmHn), were designed and synthesized, and their surface activity was evaluated in 12 organic solvents with different polarities. The surface parameters, including CMC, \({\gamma }_{CMC}\), \({\Gamma }_{max}\) and \({A}_{min}\), were measured in n-hexadecane, m-xylene, and DMSO, allowing for an in-depth analysis of the influence of molecular structure on these surface properties. Results indicate that an increase in the length of the fluorocarbon chain generally enhances surface activity, leading to a reduction in the CMC value and an increase in the effectiveness of surface tension reduction. However, the impact of the hydrocarbon chain length on surface activity is more complex and dependent on the polarity of the organic solvents. In low-polarity solvents, surface activity is improved with a longer hydrocarbon chain, whereas in high-polarity solvents, a shorter hydrocarbon chain is more beneficial. Consequently, a “polarity–directionality” strategy was proposed to tailor the molecular structure of surfactants to optimize performance in solvents with varying polarities, resulting in a significant reduction in surface tension. Specifically, F8H12 was identified as particularly effective in low-polarity n-alkanes and cycloalkanes, F6H8 was most effective in medium polarity aromatics, and F8H4 or F6H4 was ideal for larger polar solvents. These findings enrich the understanding of the structure–activity relationship in oil-soluble fluorinated surfactants and offer new perspectives for the development of high-performance surfactants with reduced environmental impact.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fluorocarbon moiety exhibits exceptional hydrophobicity, enabling fluorinated surfactant molecules to adsorb at the water/air interface by escaping from the aqueous phase. This superior capacity to reduce surface tension of water far surpasses that of hydrocarbon surfactants. Typically, fluorinated surfactants can reduce water surface tension to approximately 15–20 mN/m, whereas hydrocarbon surfactants can only achieve a reduction to about 30–40 mN/m (Krafft and Riess 2009). Consequently, fluorinated surfactants can be employed in harsh conditions which are too severe for hydrocarbon surfactants, such as applications in aqueous systems including the oil and gas industry (Hussain et al. 2022), energy storage and transportation (Cao et al. 2020), aqueous fire-fighting foam (Wu et al. 2023), and evaporation suppressant (Wu et al. 2021). However, their applications in non-aqueous systems may cause the entire molecule to acquire an oleophobic structure, leading to an increase in the surface energy of the organic solvent, which in turn may reduce surface activity at the oil/air interface (Li et al. 2010).
Oil-soluble surfactants possess amphiphilic properties that allow for dense adsorption at the oil/air interface, reducing surface tension of organic solvents. This characteristic provides application potential in non-aqueous systems, serving as efficient foam stabilizers (Mustan et al. 2022), reducing CO2-oil minimum miscible pressure to release miscible displacement increasing oil recover (Guo et al. 2017), acting as a viscosity-reducing agent for super heavy oil (Pan et al. 2017), employing as charging agents in the development of electrophoretic inks for electronic image displays or toners for electrostatic lithographic printers (Parent et al. 2011), preventing ultrafine carbon black particle accumulation in engine lubrication (Shen and Duhamel 2008), and effectively controlling asphaltene deposition during petroleum production and transportation (Atta and Elsaeed 2011). Fluorocarbon chains possess strong oleophobic properties in addition to high hydrophobicity, enabling them to escape from the oil phase. The hydrophilic head groups of surfactants can be replaced with lipophilic alkyl or aryl groups through appropriate molecular design to yield oil-soluble fluorinated surfactants. These surfactants play a vital role in fields including petrochemical, biomedicine, and colloidal catalysis, such as enhancing the solubility of compounds in dense carbon dioxide (Hoefling et al. 1992), as antiwear and antifriction additives for boundary lubrication (Basset et al. 1984), as a novel excipient in lipid-based drug delivery systems (Holm et al. 2011), and as an organocatalyst for aldol condensation of different ketones with various aldehydes (Zhu et al. 2011). Nevertheless, the variety of organic solvents and their differing polarities and solubilities can make it challenging to find a single oil-soluble fluorinated surfactant suitable for all solvents. Thus, the development of a class of oil-soluble fluorinated surfactants and understanding the relationship between their molecular structure and surface tension in various organic solvents has significant theoretical and practical implications.
In recent years, there have been relatively few up-to-date reports on the synthesis and properties of oil-soluble fluorinated surfactants, with most previous studies dating back a decade or more. For instance, a series of oil-soluble fluorine-containing surfactants were synthesized using Wittig and Wittig–Horner reactions, and their surface tensions in toluene, n-hexane, and nitromethane were measured (Zhang et al. 2019). The fundamental physicochemical properties of N, N-dialkyl perfluoroalkane sulfonamides, including melting point, density, viscosity, and dielectric constant, were determined (Fu et al. 2013). The structure–function relationships of surface tension changes of m-xylene in the presence of fluorous 1H-1,2,3-triazole and tetrazole were discussed (Read and Wang 2012). A variety of N-alkyl perfluorooctanesulfonamides was prepared, and their surface activities in a wide variety of organic solvents, involving alkanes (n-dodecane, n-tetradecane, n-hexadecane, and liquid paraffin), cyclanes (cyclohexane), aromatics (toluene and m-xylene), polar solvents (ethyl acetate, 2-butanone, nitromethane, DMF, and DMSO), were explored (Li et al. 2009), particularly their aggregation behavior in DMSO, which were also investigated (Li et al. 2010). Perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid ester fluorocarbon surfactants were confirmed to reduce the surface tension of organic solvents by 40–83% (Han et al. 2009). Perfluoropolyalkylether N, N-diphenylamide exhibited significant surface tension reduction in organic solvents even at low concentrations (Gu et al. 2007). Ether-linked hydrocarbon-fluorocarbon surfactants with single and double tails were synthesized to evaluate the effect of molecular structure on foamability and aggregate formation in organic solvents (Huang et al. 2004). A novel fluoroalkylated benzene was verified to effectively reduce the surface tension of m-xylene (Abe et al. 1992).
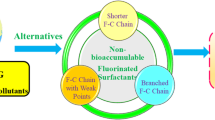
The literature mentioned above reports various structures of oil-soluble fluorinated surfactants, including poly/perfluoroalkyl chains with different lengths of oleophilic chains and perfluoroether chains. The linking group contains flexible ether bonds, rigid sulfonamide groups, and semi-rigid alkyl triazole or tetrazole rings. The oleophilic chains consist of alkyl, cycloalkyl, and aryl groups, all of which exhibit surface activity in organic solvents. However, there exist two deficiencies in the literature: (1) The fluorocarbon chains of oil-soluble fluorinated surfactants are limited to long perfluoroalkyl chains when dealing with a wide range of organic solvents, but such derivatives are persistent, toxic, and bioaccumulative in the environment, which have been designated as persistent organic pollutants (POPs) and restricted in production and utilization in many fields under the Stockholm convention in 2009 (Zhou et al. 2021); (2) while oil-soluble surfactants containing short poly/perfluoroalkyl and perfluoroether chains have relatively minimal environmental impact and have yet to be categorized under international law (Peshoria et al. 2020), their applicability to a broad range of organic solvents is constrained.
Henceforth, the objective of this research document is to meticulously investigate the structure–activity relationships between the oil-soluble fluorinated surfactant molecules, particularly the fluorocarbon chain length, and their surface activity in organic solvents via molecular architecture engineering, with an aspiration to identify a category or subclass of oil-soluble surfactants featuring short fluorocarbon chains, capable of mitigating the environmental ramifications while considerably reducing the surface tension of diverse organic solvents. In order to achieve this goal, a class of oil-soluble fluorocarbon/hydrocarbon hybrid surfactants, N-methyl-N-alkyl-4-perfluoroalkylsulfoxybenzylamines (FmHn, m = 4, 6, 8; n = 4, 8, 12, 16), with different fluorocarbon and hydrocarbon chain lengths were designed and synthesized. The surface tension reduction method was employed to investigate their surface activity in various polar solvents such as alkanes, cycloalkanes, aromatics, and large polar solvents. The correlation between the length of the fluorocarbon chain, the length of the hydrocarbon chain, and the polarity of the solvents was analyzed to establish the “polarity–directionality” strategies, which guides the selection of appropriate length of the fluorocarbon and hydrocarbon chains for the surfactants based on the polarity of the organic solvent. Furthermore, the adsorption behavior of these hybrid surfactants at the air/n-cetane, m-xylene, and DMSO interfaces was systematically studied using the tension method. The interface parameters were calculated based on the Gibbs adsorption curve to elucidate the properties of these compounds in different polar solvents at a molecular level.
Experimental
Materials and characterization techniques
Perfluoroalkylsulfonyl (including butyl, hexyl, and octyl) fluoride (industrial grade) were purchased from Hubei Hengxing Chemical Co., Ltd. (Yingcheng, China). Sodium borohydride (AR grade), 4-hydroxybenzaldehyde (AR grade), n-butylamine (AR grade), n-octylamine (99% grade), n-dodecylamine (CP grade), n-cetylamine (95% grade), paraformaldehyde (AR grade), and decalin (AR grade) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Other reagents and organic solvents were of AR grade and used without further purification.
1H NMR spectra were performed on a Mercury-Plus 400 MHz spectrometer by using tetramethylsilane (TMS) as an internal reference and CDCl3 or DMSO-d6 as the solvent. 19F NMR spectra were recorded on a Mercury-Plus 400 MHz spectrometer equipped with a pulse field gradient module (Z axis) using a 5-mm BBO probe operating at 376 MHz. 1H NMR and 19F NMR measurement were performed at 25 °C. Mass spectra (MS) were obtained on a Shimadzu GCMS-QP2020 spectrometer using an electron impact (EI) source. Oil/air interface tension was measured on Contact Angle System (OCA 20) and record three times to ensure a reproducibility for each solution.
Genernal synthesis of N-methyl-N-alkyl-4-perfluoroalkylsulfoxybenzylamines (FmHn)
The target oil-soluble fluorocarbon/hydrocarbon hybrid surfactants, namely N-methyl-N-alkyl-4-perfluoroalkylsulfoxybenzylamines, were synthesized by two steps (Scheme 1). Initially, 4-hydroxybenzaldehyde reacts with perfluoroalkyl sulfonyl fluoride yielding intermediates (FmCHO), subsequently engaged in a second stage with alkylamines to synthesize the final products (FmHn). The structures of the intermediates and target compounds were characterized by FT-IR, 1H, 19F NMR, and MS/HRMS. Detailed synthetic methods, yields, melting points, and structural characterization data and spectra (Fig. S1–S45) of all intermediates and target compounds are described in the Supplementary Materials.
Selection of organic solvents
In comparison with aqueous solutions, organic solvents possess diverse structural components and exhibit a vast range of polarities. To perform an all-encompassing, systematic analysis of the surface activity of the oil-soluble fluorocarbon/hydrocarbon hybrid surfactants synthesized in this work, a range of representative organic solvents was selected with the intention of covering as many polar ranges as possible, including small polar n-alkanes (n-hexane, n-decane, and n-hexadecane), cycloalkanes (cyclohexane and decalin), middle polar aromatics (toluene and m-xylene), along with middle to large polar solvents (ethyl acetate, ethanol, acetone, DMSO, and DMF).
Surface activity of the synthesized oil-soluble fluorocarbon/hydrocarbon hybrid surfactants in organic solvents
The surface activity of the oil-soluble fluorinated surfactant in organic solvents can be evaluated by their surface tension reduction value (\(\Delta \gamma\)) (Li et al. 2009). This value represents the difference between the surface tension of a pure organic solvent (\({\gamma }_{solvent}\)) and the surface tension of an organic solvent solution after the addition of a specific quantity of surfactant (\({\gamma }_{solution}\)). The formula to calculate this value is given by Eq. (1).
Equation (1) reveals that the surface activity of the compounds increases with higher \(\Delta \gamma\) values and vice versa. The present study aims to determine \(\Delta \gamma\) values by measuring the surface tension of the compounds in 12 organic solvents, thereby assessing their surface activity in diverse solvents. These oil-soluble fluorocarbon/hydrocarbon hybrid surfactants possess differing fluorocarbon and hydrocarbon chain lengths and solvency in organic solvents. Therefore, preliminary exploration experiments were conducted which showed that these surfactants demonstrate favorable solubility in small and middle polar n-alkanes, cycloalkanes, and aromatics. At a concentration of 200 mM, the surface tension of these surfactants can approach or reach the value at the critical micelle concentration. Conversely, their solubility in large polar solvents is poor, and 200-mM concentrations are already supersaturated. To facilitate accurate comparison of the surface activity of these surfactants in different organic solvents, we standardized the concentration of surfactants at 200 mM, measured the surface tension of the compounds in various organic solvents at room temperature (\({\gamma }_{solution}\)) and calculated the surface tension reduction value using Eq. (1).
Measurement of oil/air interface parameters
The adsorption behavior of oil-soluble fluorocarbon/hydrocarbon hybrid surfactants at the oil/air interface of can be described by several parameters, including critical micelle concentration (CMC), surface tension (\({\gamma }_{CMC}\)) at the CMC, maximum surface excess concentration (\({\Gamma }_{max}\)), and the area occupied by a single surfactant molecule (\({A}_{min}\)) (Padoan et al. 2015). Previous investigations into the effect of reducing surface tension demonstrated conclusively that these molecules substantially reduced the surface tension of n-hexadecane, m-xylene, and DMSO, suggesting that these solvents are ideal models for studying the adsorption behavior of these compounds at the surface of small polar n-alkanes, middle polar aromatics, and large polar solvents like DMSO. The wide solubility range of these surfactants in alkanes and aromatics allows for a sample concentration range between 50 and 250 mmol, whereas their limited solubility in DMSO restricts the concentration range between 0.1 and 15 mmol.
To investigate the adsorption behavior of oil-soluble fluorocarbon/hydrocarbon hybrid surfactants, surface tension measurements are performed at various concentrations, with each concentration measured three times and the average value used for constructing the Gibbs isothermal adsorption curve. This curve illustrates the relationship between the surface tension and the concentration change of the surfactants and tends toward equilibrium at a certain concentration, which corresponds to the CMC with a surface tension of \({\gamma }_{CMC}\). \({\Gamma }_{max}\) and \({A}_{min}\) can be calculated using Eq. (2) and (3) after a first-order exponential descending nonlinear fitting of the Gibbs isothermal adsorption curve (Dai et al. 2013).
where C is the concentration of surfactant, n = 1 for non-ionic surfactant, (\(d\gamma /dC\))T is the slope in the Gibbs adsorption isotherm, R is the gas constant (8.314 J mol−1 K−1), T is the absolute temperature (298 K) and N is the Avogadro constant (6.02 × 1023).
Results and discussion
Design and synthesis
As can be seen in Scheme 1, the target compounds FmHn lack ionic heads yet are comprised of three structural parts: an oleophobic perfluoroalkyl chain, a lipophilic alkyl chain, and a bridging benzyl moiety. By manipulating the molecular design, a diverse array of non-ionic oil-soluble hybrid surfactants featuring variable fluorocarbon and hydrocarbon chains can be synthesized.
Application of varying perfluoroalkyl chain lengths as oleophobic moieties heightens surfactant resistance to oil, facilitating their release from the oil phase and boosting their occupancy at the oil/air interface (Krafft and Goldmann 2003). This approach profoundly reduces the surface tension of organic solvents, essentializing analysis of the effect of fluorocarbon chain length on surface activity. Various alkyl chains augment surfactant solubility in organic solvents, offering an avenue for studying the role of hydrocarbon chain length on surface activity. Specifically, utilizing benzyl as a linker yields a semi-rigid backbone that supports vertical arrangement at the interface while preserving rotational freedom, yielding a tightly packed structure and high surface activity (Zhu et al. 2012). Furthermore, the π–π interaction presents between benzene rings and mitigates the mutual repulsion force between fluorocarbon and hydrocarbon chains (Jung et al. 2002), fostering dense monolayer formation, thereby contributing to improved surface activity (Fukushima et al. 1999). Amine groups can enhance surfactant solubility in polar organic solvents. The molecular structure design mentioned above can effectively address the surface activity requirements of these compounds in various organic solvents.
Analysis of surface tension reduction
Surface activities of an oil-soluble fluorocarbon/hydrocarbon hybrid surfactant were evaluated by measuring its surface tension across diverse organic solvents at room temperature, employing a 200-mM solution. A total of 12 solvents, encompassing small polar (n-hexane, n-decane, n-hexadecane, cyclohexane, and decalin), middle polar aromatic (toluene and m-xylene), and intermediate to large polar (ethyl acetate, ethanol, acetone, DMSO, and DMF) types, were employed. \(\Delta \gamma\) values for these solvents are provided in Table S1-S4 (Supplementary Materials). To analyze the influence of fluorocarbon and hydrocarbon chain lengths on surface tension in organic solvents, three-dimensional plots were prepared showing \(\Delta \gamma\) variations versus the number of carbon atoms in respective chains (Fig. 1). These aids in understanding the surfactant behavior across different organic media.
\(\Delta \gamma\) values versus the number of carbon atoms m and n in fluorocarbon chain and hydrocarbon chain of oil-soluble fluorocarbon/hydrocarbon hybrid surfactants in small polar n-alkanes (a: n-hexane, b: n-decane, and c: n-hexadecane), cycloalkanes (d: cyclohexane and e: decalin), middle polar aromatics (f: toluene and g: m-xylene), and middle to large polar solvents (h: ethyl acetate, i: ethanol, j: acetone, k: DMSO, and l: DMF), respectively
Generally, increases in the fluorocarbon chain tend to increase \(\Delta \gamma\) values in organic solvents independent of the solvent types, signifying enhancement in surface activity of the compound via extending the fluorocarbon chain. Similar pattern exists for fluorinated surfactants in water, indicating solvophobic augment promotes adsorption at the solvent/air interface. Reductions in solvent surface tension follow consequently (Eastoe et al. 2001). Remarkably, the \(\Delta \gamma\) values for all organic solvents are lower than 19 mN/m, which is much smaller than that of the aqueous solution of fluorinated surfactant (more than 50 mN/m). Two main reasons contribute to this result. First, the organic solvents themselves exhibit low surface tensions of 19–30 mN/m, while the surface tension of water is 72.75 mN/m at 25 °C, which can be reduced by a greater margin (Li et al. 2009). Second, the free energy required to transfer the − CF2 − group from pure fluorocarbon to an alkane solution is approximately 1.4 kJ/mol, while the free energy required to transfer − CF2 − group from fluorocarbon to water is 6.5 kJ/mol (Binks et al. 1997), reflecting that fluorinated surfactants can be more easily adsorbed at the water/air interface, and their effect in lowering the surface tension of water is more apparent.
The variation of \(\Delta \gamma\) with the hydrocarbon chain length exhibits a complex pattern, with different trends observed in different polar solvents. In n-alkanes, with the exception of F4Hn in n-hexane, which shows a decrease in \(\Delta \gamma\) with increasing hydrocarbon chain (e.g., 2.12 → 1.97 → 1.85 → 1.73 mN/m, Fig. 1a), and F8Hn in n-alkanes, which exhibits a tendency to increase and then decrease with increasing hydrocarbon chain (e.g., F8H16 in n-hexadecane with a \(\Delta \gamma\) of 4.41 → 5.15 → 7.29 → 3.96 mN/m, Fig. 1c), all other compounds tend to show an increase in \(\Delta \gamma\) with increasing hydrocarbon chain (e.g., \(\Delta \gamma\) of F6H16 in n-decane is 2.21 → 2.28 → 2.80 → 2.87 mN/m, Fig. 1b). The \(\Delta \gamma\) of the FmHn in cycloalkanes is broadly analogous to that in n-alkanes, where \(\Delta \gamma\) increases in proportion to the elongation of the hydrocarbon chain (e.g., for F4Hn in cyclohexane, \(\Delta \gamma\) range from 1.90 → 1.99 → 2.08 → 2.52 mN/m, Fig. 1d) or exhibits an initial ascent followed by a subsequent decline (e.g., for F6Hn in decalin, \(\Delta \gamma\) range from 1.37 → 1.61 → 0.97 → 0.80 mN/m, Fig. 1e; for F8Hn in cyclohexane, \(\Delta \gamma\) range from 3.96 → 5.22 → 6.84 → 4.23 mN/m, Fig. 1d). \(\Delta \gamma\) of FmHn in aromatics varies with the length of the hydrocarbon chain in two distinct patterns. The first scenario is an increase in \(\Delta \gamma\) followed by a decrease in \(\Delta \gamma\) with a shorter length of fluorocarbon chain (e.g., the \(\Delta \gamma\) of F4Hn in toluene vary from 2.24 → 2.53 → 2.34 → 2.03 mN/m, Fig. 1f, and that of F6Hn in m-xylene range from 3.47 → 7.93 → 2.99 → 2.47 mN/m, Fig. 1g). The second scenario is a decline in \(\Delta \gamma\) with an extension of the hydrocarbon chain when the fluorocarbon chain is longer (e.g., the \(\Delta \gamma\) of F8Hn in toluene decreases from 5.13 → 5.08 → 4.95 → 4.00 mN/m, Fig. 1f). In intermediate to large polar solvents, \(\Delta \gamma\) displays a gradual decrease with the increase of the hydrocarbon chain (e.g., the \(\Delta \gamma\) of F4Hn in ethyl acetate decreases from 1.34 → 1.33 → 1.30 → 1.26 mN/m, Fig. 1h, the \(\Delta \gamma\) of F6Hn in acetone decreases from 3.12 → 2.94 → 2.78 → 2.50 mN/m, Fig. 1j and that of F8Hn in DMF decreases from 14.54 → 13.28 → 12.04 → 11.64 mN/m, Fig. 1l).
A scrutiny of the data presented above indicates three correlations between \(\Delta \gamma\) and hydrocarbon chain length: (1) \(\Delta \gamma\) progressively intensify with the increase of the hydrocarbon chain; (2) \(\Delta \gamma\) augments initially, followed by a decline as the hydrocarbon chain increases; and (3) \(\Delta \gamma\) progressively diminishes with the increase of the hydrocarbon chain. Despite the relatively straightforward trend exhibited by \(\Delta \gamma\) as the hydrocarbon chain length elongation, the rationale behind such trend presents a more multifaceted conundrum, potentially influenced by factors like the inherent polarity of the employed organic solvent, the oleophobic–lipophilic balance and the solubility of the compounds FmHn in various organic solvents.
The molecular architecture of the compounds FmHn incorporates both alkyl chains and benzene rings, rendering the molecule with minimal polarity and pronounced lipophilicity, hence exhibiting excellent solubility in small polar n-alkanes, cycloalkanes, and middle polar aromatic solvents. Surfactants in the solvent must initially possess a certain solubility, ensuring enough molecules are adsorbed at the interface, thereby noticeably diminishing the surface tension of the solvent. This elucidates why the \(\Delta \gamma\) of most compounds in n-alkane, cycloalkane, and aromatic solvents progressively intensifies with the increase of the hydrocarbon chain; it is precisely due to the increase of the hydrocarbon chain that the solubility of these compounds progressively improves, and more surfactant molecules are accessible for adsorption at the oil/air interface (Scheme 2a). Nevertheless, the hydrocarbon chain should not be excessively long, as the hydrocarbon chain exhibits a Zigzag configuration in the solvent, and an excessively long hydrocarbon chain is prone to bending, which escalates the steric hindrance between the hydrocarbon chains (Cormanich et al. 2017), and results in the surfactant molecules packing loosely during the interfacial adsorption (Scheme 2b), culminating in the reduction of the surface activity, which is why \(\Delta \gamma\) increases initially and subsequently diminishes with the increase of the hydrocarbon chain. Significantly, due to the minimal polarity of these compounds, robust lipophilic properties, and favorable solubility within low-polarity n-alkanes and cycloalkanes, a potent oleophobic perfluorooctyl chain is required for efficient interfacial adsorption. As solvent polarity escalates, the solvophilicity of these lower polarity compounds declines; yet, owing to their shared aromatic rings with the solvent, the compatibility remains excellent, permitting continued good solubility in aromatics. Hence, an intense oleophobic fluorocarbon is not necessary to promote satisfactory interfacial adsorption of these compounds, accounting for the reason why the compound F8H12 exhibits the most superior surface activity among n-alkanes and cycloalkanes, while in aromatics, it is the compound F6H8 that exhibits the pinnacle of surface activity.
Presence of two scenarios exists wherein the increment in the hydrocarbon chain results in a decrease in \(\Delta \gamma\), notably for F4Hn n-hexane solutions, which might correlate with the excessive solubilization of F4Hn within n-hexane and enhanced mutual miscibility and compatibility between the fluorocarbon chain and short chain alkanes (Binks et al. 1995). Moreover, the significantly diminished solvophobic characteristic of the perfluorobutyl chain enhances the likelihood that an increase of the hydrocarbon chain would simply increase the solubility of surfactant molecules instead of promoting their adsorption at the interface (Scheme 2c). Similarly, FmHn solutions in ethyl acetate, ethanol, acetone, DMSO, and DMF also exhibit this phenomenon, likely attributed to the decreased solubility of FmHn in these middle to large polar solvents. The longer the hydrocarbon chain, the less polar the FmHn become, thereby reducing their solubility in highly polar organic solvents and subsequently decreasing the number of molecules adsorbed at the interface, thereby manifesting a diminished surface activity (Scheme 2d). Hence, FmH4 demonstrates superior surface activity in such solvents. Furthermore, an intriguing phenomenon emerges. Intuitive comprehension presumes that the solubilities of low-polarity compounds within the high-polarity DMSO and DMF should be exceedingly minute, leading to their \(\Delta \gamma\) comparable with those present in ethyl acetate, methanol, or acetone. Instead, it transpires that these values exhibit the greatest degree in the DMSO and DMF solvents. Such disparity may result from two aspects: First, the surface tensions inherent in DMSO and DMF are notably higher among various organic solvents, therefore showing potential for significant reduction; second, both DMSO and DMF have garnered reputations as “all-purpose solvents” (Li et al. 2009), displaying exceptional dissolving capability of compounds FmHn, in conjunction with pronounced solvophobic tendencies, contributing to a superior \(\Delta \gamma\) compared to other solvents.
Following the analysis of \(\Delta \gamma\) values in relation to hydrocarbon chain length, it can be known that the variation in \(\Delta \gamma\) with hydrocarbon chain length is influenced by several factors, including the solvation effect, molecular packing at the interface, and the inherent polarity of the solvents. In solvents with low polarity, an increase in the hydrocarbon chain length generally enhances the solubility of the surfactant, leading to a more pronounced reduction in surface tension due to improved adsorption at the oil/air interface. However, as the hydrocarbon chain extends beyond a certain point, steric hindrance can disrupt the compact molecular arrangement, reducing the efficiency of surface coverage and thus the \(\Delta \gamma\) values. Conversely, in highly polar solvents, the longer hydrocarbon chains may not be as beneficial due to decreased solubility. Here, the surfactants with shorter hydrocarbon chains exhibit greater surface activity, as they can more readily adsorb at the interface without the hindrance associated with longer chains. This behavior underscores the delicate balance between hydrocarbon chain length and solvent polarity in dictating the surface properties of fluorinated surfactants.
From the foregoing examination, even though an ideal oil-soluble fluorinated surfactant suitable across the gamut of organic solvents remains elusive, certain guidelines along the lines of “polarity–directionality” strategies can be established. (1) Within low-polarity n-alkanes and cycloalkanes, fluorinated surfactants possessing longer fluorocarbon and hydrocarbon chains exhibit superior surface activity (e.g., F8H12); (2) within middle polarity aromatics, the one featuring shorter fluorocarbon and hydrocarbon chain demonstrate enhanced surface activity (e.g., F6H8); and (3) within high-polarity solvents, compound with prolonged fluorocarbon and shorted hydrocarbon chain display elevated surface activity (e.g., F8H4), while those with shorted fluorocarbon chain length combined with hydrocarbon (e.g., F6H4) also qualify, given the ecological implications of long fluorocarbon chains. Moreover, constituents having perfluorobutyl chain typically manifest diminished surface activity in organic solvents, while incorporating benzene ring into the molecular framework of surfactants does amplify the surface activity, corroborating the soundness of our molecular structure design philosophy.
Adsorption behavior at oil/air interface of surfactant molecules
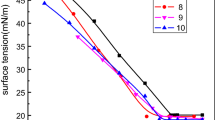
The self-assembly of hydrocarbon/fluorocarbon amphiphiles in organic solvents offers a great potential for applications in low interfacial energy fields such as coating materials in microelectromechanical systems (Huang et al. 2004). In this paper, oil-soluble fluorocarbon/hydrocarbon hybrid surfactants are designed and synthesized to exhibit surface activity in organic solvents of various polarities. Studying the adsorption behavior of these surfactants at the oil/air interface provides theoretical support for comprehending their structure–activity relationships and self-assembly properties in organic solvents. To this end, small, middle, and large polar solvents (n-hexadecane, m-xylene, and DMSO, respectively) were chosen as representatives, and the critical micelle concentration (CMC), surface tension (\({\gamma }_{CMC}\)) at the CMC, maximum surface excess concentration (\({\Gamma }_{max}\)), and the area occupied by a single surfactant molecule (\({A}_{min}\)) interfacial parameters were determined and calculated by the tensile method to investigate the adsorption behavior of the surfactants at the oil/air interface (Fig. 2 and Tables 1, 2 and 3). These three solvents were selected due to their larger \(\Delta \gamma\), making them conducive to plotting Gibbs isothermal adsorption curves, and their frequent use in previously published literature on surface activity and aggregation behaviors of oil-soluble fluorinated surfactants (Li et al. 2009, 2010; Read and Wang 2012).
The CMC is not immediately correlated with the \({\gamma }_{CMC}\) but instead to the molecular architecture, polarity, oleophobic-oleophilic balance, solubility within the organic solvents, and polarity of the solvents, resulting in an intricate oscillation of the CMC with increasing fluorocarbon and hydrocarbon chain length. Typically, an increase in the fluorocarbon chain length is associated with improved surface activity due to enhanced solvophobicity, which would lead to a decrease in CMC. However, the results indicate a contrary trend, which may be attributed to the unique molecular design of the surfactants used in this study. The presence of a benzyl group as a linker could influence the amphiphilic properties of the surfactants, affecting their solvation and interfacial behavior in the selected solvents. Consequently, this may lead to an increased CMC despite the extended fluorocarbon chain. Furthermore, the increased lipophobicity with longer fluorocarbon chains might also reduce the solubility of the surfactants in certain solvents, necessitating higher concentrations for micelle formation. Nonetheless, it is unequivocally noted that the CMC for these compounds in DMSO is three orders of magnitude less than that present in n-hexadecane or m-xylene (for instance, the CMC of the compound F8H4 in DMSO is 7.8 × 10–4 mol/L, while its counterpart in n-hexadecane or m-xylene has a CMC of 1.56 × 10–1 and 1.97 × 10–1 mol/L, respectively). This unequivocally implies that the compounds exhibit exceptional ability in generating micelles within DMSO. Such capabilities could potentially correlate with the adsorption behavior of these compounds at the oil/air interface.
The values of \({\Gamma }_{max}\) and \({A}_{min}\) reflect the degree of packing and orientation of the adsorbed surfactant molecules at the interface. Larger \({\Gamma }_{max}\) and smaller \({A}_{min}\) means that there are more surfactant molecules adsorbed on the surface of the solution, which also means a lower surface tension (Li et al. 2009). In DMSO solution, these compounds exhibit superior \({\Gamma }_{max}\) values while simultaneously exhibiting diminished \({A}_{min}\) values when compared with their counterparts in n-hexadecane and m-xylene. For example, compound F8H4 displays a \({\Gamma }_{max}\) value of 5.30 × 10–6 mol/m2 and an \({A}_{min}\) value of 0.31 nm2 in DMSO; however, its \({\Gamma }_{max}\) and \({A}_{min}\) values in n-hexadecane and m-xylene measure 7.66 × 10–7 and 8.57 × 10–6 mol/m2, 2.17 and 1.94 nm2, respectively. These findings corroborate that these surfactants possess a higher number of molecules adsorbed at the DMSO/air interface and are arranged more densely, a trait directly linked to their suitable oleophobic-oleophilic balance in DMSO. Consequently, it rationalizes the significant deviation in CMC values across DMSO. In DMSO, these surfactants exhibit pronounced dissolution and adsorption properties at the interface, giving rise to a disproportionately stronger dissolution propensity compared to adsorption predilection in n-hexadecane and m-xylene.
A discernible correlation exists between \({\Gamma }_{max}\) and \({A}_{min}\) parameters along with the lengths of the fluorocarbon and hydrocarbon chain. Within the context of smaller polar n-hexadecane, substances possessing longer fluorocarbon and hydrocarbon chain exhibit increased \({\Gamma }_{max}\) and diminished \({A}_{min}\) (e.g., compound F8H12, Table 1), attributed to the superior solvophobicity facilitated by longer fluorocarbon chain and augmented solubility by longer hydrocarbon chain, culminating in an elevated and dense arrangement of surfactant molecules at the n-hexadecane/air interface. Nonetheless, the hydrocarbon chain length should be judiciously controlled, as excessive kinking imparts steric hindrance, thereby inhibiting the dense packing of surfactant molecules (e.g., compound F8H16, Table 1). In m-xylene, as solvent polarity intensifies, the solvophobic force of the compounds escalates; hence, the fluorocarbon chain length might not mandate elongation for providing adequate solvophobic force. Pertinently, the introduction of benzene ring ensures retention of good solubility in aromatics. Here, substances with shorter, inflexible hydrocarbon chain exhibit the highest \({\Gamma }_{max}\) and lowest \({A}_{min}\) values (e.g., compound F6H8, Table 2). As the solvent polarity amplifies further, the solubility of compounds in methanol, acetone among other solvents plummets, the propensity to bend diminishing with the increase of the hydrocarbon chain. Nevertheless, in the so-called “all-purpose solvents”, DMSO, solubility remains robust, even at a concentration of 200 mM, thus necessitating an elongated fluorocarbon chain to sustain adequate solvophobic force. Hence, substances with a protracted fluorocarbon chain and shorter hydrocarbon chain exhibit the highest \({\Gamma }_{max}\) and lowest \({A}_{min}\) values (e.g., compound F8H4, Table 3).
From the insights derived from the aforementioned data, it can be ascertained that the adsorption profile of oil-soluble fluorocarbon/hydrocarbon hybrid surfactant molecules at the oil/air interface is fundamentally consistent with those derived from the surface tension reduction analysis, providing a corroboration for the “polarity–directionality” strategies proposed previously. For the oil-soluble fluorocarbon/hydrocarbon hybrid surfactants devised in this study, it is feasible to screen the compounds with appropriate structures contingent upon the magnitude of solvent polarity, to maximize the reduction in surface tension of organic solvents.
Conclusions
Twelve oil-soluble fluorocarbon/hydrocarbon hybrid surfactants constructed from various fluorocarbon chain types (inclusive of perfluorobutyl, hexyl, and octyl) and hydrocarbon chain lengths (including butyl, octyl, dodecyl, and hexadecyl), accompanied by benzyl bridge groups, have been synthesized, subsequent to which their respective surface activity was meticulously evaluated via the surface tension reduction approach across a comprehensive range of organic solvents (spanning small, middle, and middle-to-large polar organic solvents). The “polarity–directionality” strategies have subsequently been postulated. As per solvent polarity characteristics, appropriate molecules could be tailored to drastically reduce the surface tension of various organic solvents, such as compound F812 in small polar n-alkanes and cycloalkanes, compound F6H8 in middle polar aromatics, and compound F8H4 or F6H4 among middle to large polar solvents. Furthermore, the absorption tendencies of the substances across the interface between the solvent and air were scrutinized utilizing specific organic solvents (n-hexadecane, m-xylene, and DMSO) encompassing diverse polarity levels, thereby validating the effectiveness of the “polarity–directionality” strategies. These outcomes align with those derived from surface tension reduction evaluations. Although an impeccable “panacea substance” applicable for all organic solvents has not surfaced, the “polarity–directionality” strategies alleviate this disadvantage, concurrently proffer a novel perspective for the formulation and creation of oil-soluble fluorinated surfactants. Reducing the surface tension of organic solvents surpasses that of water, and substances comprising lengthy perfluorocarbon chains typically exhibit robust surface activity in organic solvents. Nonetheless, judicious molecular framework design makes that certain short perfluorohexyl compounds exhibit commendable surface activity in aromatic hydrocarbons and large polar organic solvents, representing a minor milestone toward synthesizing oil-soluble surfactants with shorted perfluorocarbon chains.
References
Abe M, Morikawa K, Ogino K, Sawada H, Matsumoto T, Nakayama M (1992) Reduction of surface tension of pure m-xylene by novel fluorinated surfactants. Langmuir 8:763–764. https://doi.org/10.1021/la00039a005
Atta AM, Elsaeed AM (2011) Use of rosin-based nonionic surfactants as petroleum crude oil sludge dispersants. J Appl Polym Sci 122:183–192. https://doi.org/10.1002/app.34052
Basset D, Hermant M, Martin JM (1984) Oil-soluble fluorinated compounds as antiwear and antifriction additives. ASLE Trans 27:380–388. https://doi.org/10.1080/05698198408981584
Binks BP, Fletcher PDI, Sager WFC, Thompson RL (1995) Adsorption of semifluorinated alkanes at hydrocarbon-air surfaces. Langmuir 11:977–983. https://doi.org/10.1021/la00003a047
Binks BP, Fletcher PDI, Kotsev SN, Thompson RL (1997) Adsorption and aggregation of semifluorinated alkanes in binary and ternary mixtures with hydrocarbon and fluorocarbon solvents. Langmuir 13:6669–6682. https://doi.org/10.1021/la970408i
Cao Q, Xu D, Xu H, Luo S, Guo R (2020) Efficient promotion of methane hydrate formation and elimination of foam generation using fluorinated surfactants. Front Energy 14:443–451. https://doi.org/10.1007/s11708-020-0683-2
Cormanich RA, O’Hagan D, Bühl M (2017) Hyperconjugation is the source of helicity in perfluorinated n-alkanes. Angew Chem Int Ed 129:7975–7978. https://doi.org/10.1002/ange.201704112
Dai C, Du M, Zhao M, You Q, Guan B, Wang X, Liu P (2013) Study of micelle formation by fluorocarbon surfactant N-(2-hydroxypropyl)perfluorooctane amide in aqueous solution. J Phys Chem B 117:9922–9928. https://doi.org/10.1021/jp404387d
Eastoe J, Paul A, Rankin A, Wat R, Penfold J, Webster JRP (2001) Fluorinated nonionic surfactants bearing either CF3- or H–CF2- terminal groups: adsorption at the surface of aqueous solutions. Langmuir 17:7873–7878. https://doi.org/10.1021/la010958n
Fu ST, Liao SL, Nie J, Zhou ZB (2013) N, N-dialkyl perfluoroalkanesulfonamides: synthesis, characterization and properties. J Fluorine Chem 147:56–64. https://doi.org/10.1016/j.jfluchem.2013.01.009
Fukushima S, Machida M, Akutsu T, Shimizu K, Tanaka S, Okamoto K, Mashiba H, Yokoyama M, Okano T, Sakurai Y, Kataoka K (1999) Roles of adriamycin and adriamycin dimer in antitumor activity of the polymeric micelle carrier system. Colloids Surf B 16:227–236. https://doi.org/10.1016/S0927-7765(99)00073-9
Gu G, Yu M, Meng W, Qing FL (2007) Surface activity of perfluoropolyalkylether N, N-diphenylamide (PFPEA). J Mater Sci 42:8537–8543. https://doi.org/10.1007/s10853-007-1756-x
Guo P, Hu Y, Qin J, Li S, Jiao S, Chen F, He J (2017) Use of oil-soluble surfactant to reduce minimum miscibility pressure. Pet Sci Technol 35:345–350. https://doi.org/10.1080/10916466.2016.1259630
Han L, Zhang Y, Li H, Li L (2009) The synthesis and surface activity of perfluoro-2,5-dimethyl-3,6-dioxanonanoic acid ester fluorocarbon surfactants. Colloids Surf A 334:176–180. https://doi.org/10.1016/j.colsurfa.2008.10.020
Hoefling T, Stofesky D, Reid M, Beckman E, Enick RM (1992) The incorporation of a fluorinated ether functionality into a polymer or surfactant to enhance CO2-solubility. J Supercrit Fluids 5:237–241. https://doi.org/10.1016/0896-8446(92)90013-A
Holm R, Jørgensen EB, Harborg M, Larsen R, Holm P, Müllertz A, Jacobsen J (2011) A novel excipient, 1-perfluorohexyloctane shows limited utility for the oral delivery of poorly water-soluble drugs. Eur J Phar Sci 42:416–422. https://doi.org/10.1016/j.ejps.2011.01.007
Huang W, Jin C, Derzon DK, Huber TA, Last JA, Provencio PP, Gopalan AS, Dugger M, Sasaki DY (2004) Synthesis of ether-linked fluorocarbon surfactants and their aggregational properties in organic solvents. J Colloid Interf Sci 272:457–464. https://doi.org/10.1016/j.jcis.2003.11.038
Hussain SMS, Adewunmi AA, Mahboob A, Murtaza M, Zhou X, Kamal MS (2022) Fluorinated surfactants: a review on recent progress on synthesis and oilfield applications. Adv Colloid Interf Sci 303:102634. https://doi.org/10.1016/j.cis.2022.102634
Jung HT, Lee SY, Kaler EW, Coldren B, Zasadzinski JA (2002) Gaussian curvature and the equilibrium among bilayer cylinders, spheres, and discs. Proc Natl Acad Sci 99:15318–15322. https://doi.org/10.1073/pnas.2423744
Krafft MP, Goldmann M (2003) Monolayers made from fluorinated amphiphiles. Curr Opin Colloid Interf Sci 8:243–250. https://doi.org/10.1016/S1359-0294(03)00046-3
Krafft MP, Riess JG (2009) Chemistry, physical chemistry, and uses of molecular fluorocarbon−hydrocarbon diblocks, triblocks, and related compounds unique “apolar” components for self-assembled colloid and interface engineering. Chem Rev 109(5):1714–1792
Li GL, Zheng LQ, Xiao JX (2009) Synthesis and surface activities of organic solvent-soluble fluorinated surfactants. J Fluorine Chem 130:674–681. https://doi.org/10.1016/j.jfluchem.2009.05.006
Li GL, Gao YA, Li XW, Liu J, Zheng LQ, Xing H, Xiao JX (2010) Aggregation behavior of N-alkyl perfluorooctanesulfonamides in dimethyl sulfoxide solution. J Colloid Interf Sci 342:372–381. https://doi.org/10.1016/j.jcis.2009.10.058
Mustan F, Politova-Brinkova N, Rossetti D, Rayment P, Tcholakova S (2022) Oil soluble surfactants as efficient foam stabilizers. Colloids Surf A 633:127874. https://doi.org/10.1016/j.colsurfa.2021.127874
Padoan G, Darmanin T, Zaggia A, Amigoni S, Conte L, Guittard F (2015) Characterization of air/water interface adsorption of a series of partially fluorinated/hydrogenated quaternary ammonium salts. J Fluorine Chem 178:241–248. https://doi.org/10.1016/j.jfluchem.2015.07.023
Pan LY, Feng GQ, Liu JW, Mao JC, Wang HB (2017) Oil soluble polymers as a viscosity-reducing agent for super heavy oil. Pet Sci Technol 35:747–753. https://doi.org/10.1080/10916466.2016.1273240
Parent ME, Yang J, Jeon Y, Toney MF, Zhou ZL, Henze D (2011) Influence of surfactant structure on reverse micelle size and charge for nonpolar electrophoretic inks. Langmuir 27:11845–11851. https://doi.org/10.1021/la202660d
Peshoria S, Nandini D, Tanwar RK, Narang R (2020) Short-chain and long-chain fluorosurfactants in firefighting foam: a review. Environ Chem Lett 18:1277–1300. https://doi.org/10.1007/s10311-020-01015-8
Read RW, Wang X (2012) A structure–function study of the surface tension changes of m-xylene in the presence of fluorous 1H–1,2,3-triazoles and tetrazoles. J Fluorine Chem 135:25–32. https://doi.org/10.1016/j.jfluchem.2011.07.030
Shen Y, Duhamel J (2008) Micellization and adsorption of a series of succinimide dispersants. Langmuir 24:10665–10673. https://doi.org/10.1021/la801416a
Wu WH, Wang JL, Zhou YQ, Sun Y, Zhou X, Zhang AD (2021) Design, synthesis and application of short-chained perfluorinated nitrogenous heterocyclic surfactants for hydrocarbon subphases. J Fluorine Chem 252:109919. https://doi.org/10.1016/j.jfluchem.2021.109919
Wu WH, Wang JL, Zhou YQ, Sun Y, Duan J, Zhang A (2023) Formulation and performance of aqueous film-forming foam fire extinguishing agent composed of a short-chain perfluorinated heterocyclic surfactant as the key component. Chem Paper 77(11):6763–6771. https://doi.org/10.1007/s11696-023-02975-1
Zhang D, Sha M, Xing P, Pan R, Lin X, Jiang B (2019) Synthesis of novel oil-soluble fluorinated surfactants via Wittig-Horner reaction. Tetrahedron 75:1652–1657. https://doi.org/10.1016/j.tet.2019.01.053
Zhou R, Jin Y, Shen Y, Zhao P, Zhou Y (2021) Synthesis and application of non-bioaccumulable fluorinated surfactants: a review. J Leather Sci Eng 3:1–5
Zhu YW, Yi WB, Cai C (2011) A recyclable fluoroalkylated 1,4-disubstituted [1,2,3]-triazole organocatalyst for aldol condensation of aldehydes and ketones. J Fluorine Chem 132:71–74. https://doi.org/10.1016/j.jfluchem.2010.11.004
Zhu DY, Cheng F, Chen Y, Jiang SC (2012) Preparation, characterization and properties of anionic gemini surfactants with long rigid or semi-rigid spacers. Colloids Surf a: Physicochem Eng Asp 397:1–7. https://doi.org/10.1016/j.colsurfa.2012.01.006
Acknowledgements
The authors gratefully acknowledge the financial supports from the Introductory Research Project of Shiyan (21Y13), the Natural Science Foundation of Hubei Provincial (2022CFB854) and the Key Research and Development Program of Hubei Province (2023BAB183).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, WH., Zhou, YQ., Sun, Y. et al. Design, synthesis, and structure–activity relationships of oil-soluble fluorinated surfactants with fluorocarbon/hydrocarbon hybrid chain. Chem. Pap. (2024). https://doi.org/10.1007/s11696-024-03656-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11696-024-03656-3