Abstract
Ibuprofen (IBP), a commonly recommended nonsteroidal, antipyretic, analgesic and anti-inflammatory drug, is a widespread ecological contaminant with harmful consequences. This issue has necessitated the development of a comprehensive scientific strategy for the removal of drugs as well as their by-products from the environment. Biodegradation is a method for breaking down complex chemicals into simpler forms that is both eco-friendly and effective. The current research studies the biodegradation of IBP by isolating a promising bacterial strain isolated from a pharmaceutical industrial wastewater. Microbacterium paraoxydans (Genbank accession numbers: Forward OL614700 and Reverse OL614701) was identified as the bacterium strain by 16S rRNA sequencing. Enhancement of IBP biodegradation efficiency was attained by optimization of parameters using central composite design (CCD). Due to their critical impact on IBP degradation, five parameters were selected for optimization: pH, yeast extract, temperature, inoculum dose, incubation time, and agitation speed. The other two parameters, concentration and time, were kept unchanged. At ideal conditions of pH 7, inoculum dose OD600, agitation speed of 150 rpm, 30 °C temperature, and 0.3% yeast extract, the target drug concentration was reduced by 92.01%. The results suggest that Microbacterium paraoxydans could be an efficient microbe for degrading pharmaceutical wastewater and reducing harmful environmental effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emerging pollutants have caused widespread concern in recent years due to their negative impacts on the marine and earthly environment. Pharmaceutical active chemicals, illicit drugs, surfactants, endocrine disruptors, and other toxins are examples of developing pollutants (Iovino et al. 2015). The annual intake of pharmaceutically active compounds (PhACs) is in the hundreds of tons. These compounds then build up in the environment, either as a result of human waste or as a result of the widespread disposal of expired medications. There are other manufacturing outlets that discard such unneeded medications in their immediate surroundings, damaging nearby water sources. The surface as well as groundwater become significantly contaminated as a result of the immersive consequences, as do other spheres of the climate, such as the surrounding soil and air. As a result of this condition, toxic water finds its way into numerous drinkable water sources, harming a wide range of living creatures (Zwiener 2007). Various investigations have confirmed PhACs' strong bioactivity, demonstrating their existence and posing a serious concern even at low concentrations.
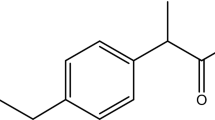
IBP is one of the most important PhACs, as well as the world's most widely used medicines. IBP, also known as 2-(4-isobutyl phenyl) propionic acid, is a nonsteroidal anti-inflammatory medicine (NSAID) that is used to reduce inflammation, pain, and fever. Because of its lipophilic feature, IBP can easily pass through the cellular membrane and induce significant bioaccumulation in aquatic flora and animals. In humans, IBP deposition can induce liver damage, renal failure, aseptic meningitis, gastrointestinal toxicity, and bone fracture healing can be hampered (Show et al. 2021). As a result, these compounds in wastewater have sparked an important field of research into their existence, effects, and, most crucially, their effective removal so that wastewater can be reused.
Volatilization (Matamoros et al. 2016), nano-filtration (Maryam et al. 2020), ion exchange (Jiang et al. 2015), membrane separation (Couto et al. 2018), and other traditional methods have all been used. However, they all require a lot of maintenance and investment, as well as the by-product that these processes produce cannot be clear completely. In the search for alternatives, the bioremediation process stood out since, in addition to having a high removal capacity, it did not produce poisonous by-products. Bioremediation techniques are important in treating wastewater and are generally eco-friendly approaches. Through microbial degradation, this technology accomplishes a significant reduction in harmful constituents by turning them into comparatively harmless compounds, viz., carbon dioxide and water. Other benefits of bioremediation over other procedures include cost-effectiveness, ease of operation, lower sludge generation, less energy usage, and so on. The interactions of three components determine the biodiversity of this technique: contaminants, microorganisms, and the environment. The connections between these parameters are significant in predicting the efficiency of bioremediation and have an impact on biodegradability, bioavailability, and physiological needs (Show et al. 2021). Different microorganisms, such as Bacillus thuringiensis (Almeida et al. 2013), Phanerochaete chrysosporium (Langenhoff et al. 2013), Phaeodactylum tricornutum (Santaeufemia et al. 2018), Micrococcus yunnanensis (Sharma et al. 2019), Nannochloropsis sp. (Encarnação et al. 2020a), and others, have been used by different researchers in various studies to aid in the remediation of IBP from waste products (Table 1).
The optimization of the physicochemical parameters is essential for effective degradation. The optimizing techniques include the conventional change of one variable per time (COVT) approach, which is time-consuming, inconvenient, and inefficient. As a result, response surface methodology (RSM)-guided methods like Box–Behnken design (BBD) and central composite design (CCD) can be adopted to solve the issue of manual optimization, because data fed to RSM-guided technique will estimate the optimal condition based on the mathematical model with an analysis of variance (ANOVA) verifying the authenticity of the mathematical model selected to predict the optimal response. CCD is a 2n full-factorial method; here n specifies the number of optimization parameters (Show et al. 2020). Presently, biological procedures are now intensively studied using statistical methods and experimental designs owing to their robustness and reproducibility (Sarkar and Dey 2020).
The main objective of this study was to isolate a potential bacterial strain capable of degrading IBP under optimal cultural conditions. The strain was isolated from pharmaceutical wastewater of East India Pharmaceutical Private Limited, Durgapur, West Bengal, India (Fig. 1), and its degradation of IBP was studied. The most acceptable parametric conditions for maximal IBP elimination by the isolated bacteria were determined using the central composite design process. To the best of our knowledge, the literature lacks studies on IBP removal using Microbacterium paraoxydans. This study elucidates details regarding its isolation from wastewater and optimization, which are prerequisites for enhanced IBP biomitigation.
Materials and methods
Chemicals
IBP and methanol used in this experiment was purchased from Sigma-Aldrich (St. Louis, MO). For screening and biodegradation studies, mineral salt medium (MSM) was utilized which contained (g/L): 1.0 g NaNO3, 0.1 g MgSO4.7H2O, 0.5 g K2HPO4, and 1.0 g NaCl.
Enrichment and isolation of the IBP degrading bacteria
Isolation of a potent IBP degrader was carried out according to reported literature (Sarkar and Dey, 2020) (Sarkar and Dey 2020), with slight modifications. Pharmaceutical waste water collected from an industrial outlet (East India Company, Durgapur, India) served as the inoculum source of the IBP degrader. 5 ml of the waste water sample was inoculated in 45 mL of MSM medium supplemented with 1 mg/L of IBP as the main carbon source as well as energy in a 250-mL Erlenmeyer flask and incubated at 30 °C at 120 rpm. The removal of IBP was monitored every 24 h at 220 nm (Show et al. 2020; Dai et al. 2019) using UV–vis spectrophotometer (REMI UV-2310, Kolkata, India). After the complete removal of IBP, 5 ml of suspension has been used as an inoculum for the following transfer with a higher concentration and incubated under the same conditions. For proper acclimatization of the enriched culture, two to three transfers were made at the same concentration of IBP. The concentration range used during the enrichment process was 1– 20 mg/L, with a step-wise increment of 5 mg/L, beyond which the suspension failed to utilize IBP. After acclimatization for about three months, the enriched culture was plated on MSM agar plate supplemented with 15 mg/L IBP and incubated at 30 °C till the appearance of colonies. After 5 days of incubation, three morphologically different colonies were observed. Each of these isolates was streaked as pure cultures on MSM agar plates and was then separately tested for their IBP degrading potential. The isolate with the maximum IBP degrading efficiency was chosen for further studies. The isolated bacterial culture was maintained as pure culture on MSM agar plates containing 15 mg/L IBP and in glycerol stocks at 4 °C.
Biochemical, morphological, and phylogenetic characterization of the isolated bacteria
The morphology of the isolated strain has been observed by scanning electron microscopy (SEM) in ZEISS instrument. As the biomass (raw and spent) was non-conductive in nature, it was covered with platinum before SEM analysis to improve the surface of the material conductivity.
Biochemical characterization of the isolate was performed by a biochemical test kit (KB-002 5KT, Himedia) according to the manual instructions. The biochemical reactions were concluded to be positive or negative depending upon any color changes in the corresponding wells.
Phylogenetic analysis of the bacterial strain has been done based on the 16S rRNA homology with the related species. After the extraction of bacterial DNA, 16S RNA amplicon was created utilizing the 8F and 1492 R primers, followed by its sequencing utilizing the Big Dye Termination technique. The generated consensus sequences of the 16S rRNA are then aligned with the non-redundant database of the NCBI Genbank using BLAST alignment tool. The first eleven sequences are chosen based on the maximal identity score which are then aligned by multiple alignment software, CLUSTAL W. The evolutionary history was inferred by using the maximum likelihood method and Tamura-Nei model (Kimura 1980). Neighbor-Join and BioNJ algorithms were used estimate the pairwise distance between the sequences. Evolutionary analyses were conducted in MEGA X (Kumar et al. 2018). The sample was sent to Barcode Biosciences, Bangalore, for the analysis of the bacterial culture and construction of the phylogenetic tree.
Optimization of the physicochemical parameters by CCD
Environmental conditions are known to alter the process performance of a bioprocess. In this study, the influence of crucial factors that affect the growth of Microbacterium paraoxydans and the biodegradation of IBP were investigated to assess their impact on the bioprocess. The variables studied were pH, temperature, agitation speed, yeast extract, inoculum dose, concentration, and duration. Based on previously conducted experiments, the maximum IBP concentration to be degraded by the isolate was 15 mg/l within 120 h of incubation. As a result, A = pH, B = temperature, C = speed, D = yeast extract, and E = inoculum dose are the five factors that were changed during the course of the experiment while maintaining the substrate concentration and incubation time constant. The number of runs N is defined by nc number of central runs, 2n factorial runs, and 2n axial runs in central composite design optimization. Equation (1), which is presented below, can be used to correlate them:
For each process parameter, CCD uses five alternative levels in coded forms for the formation of the experimental matrix: low (-α), − 1, 0, + 1, and high (+ α). After that, the model equation may be generated for regression analysis utilizing ANOVA, and the model's performance aspects can be checked and validated by statistical parameters in analysis of variance study. The pH (6,7,8), temperature (28 °C, 30 °C, 32 °C), agitation speed (120, 150, 180) rpm, yeast extract (0.2, 0.3, 0.4) %, and inoculum dose (0.1, 0.5, 0.9) OD600 ranges for each parameter in the five-factor five-level CCD shown in Table 2. Experiments were carried out for each parametric combination in order to obtain the response yield.
Assessment of growth rate of the isolated strain
The growth kinetics analysis of Microbacterium paraoxydans was carried out to ascertain if the particular strain of bacteria isolated from wastewater is capable of adapting to a new environment and metabolizing the substrate into which it was introduced.
The optical density (OD) at 600 nm was obtained using a UV-spectrophotometer to investigate the growth of the isolated strain of bacteria Microbacterium paraoxydans. Figure 2 represents the growth pattern of the bacteria. Dry cell weight (DCW) in g/L was calculated gravimetrically after drying the bacteria cells at 80 °C, according to Zakaria's methodology. Linear regression was used to determine the association between DCW and OD. The variation in concentration of biomass (g/L) throughout a culture time in hours (h) was used to determine the specific growth rate, μ (h−1) [Eq. (2)],
where a1 and a0 signify the initial and final biomass concentration (g/L) at the times t0 and t1 hours correspondingly (Samanta et al. 2020). The specific growth rate was found to be 0.00034 h−1 for 15 mg/L concentration.
Analytical methods
The biodegradation of IBP was investigated under a range of settings, such as pH (6,7,8), temperature (28 °C, 30 °C, 32 °C), agitation speed (120, 150, 180) rpm, yeast extract (0.2, 0.3, 0.4)%, inoculum dose (0.1, 0.5, 0.9) OD600, concentration (1, 5, 10, 15, 20) mg/L, and time (24, 48, 72, 96, 120) h. The studies were carried out in a 250-mL conical flask with 50 mL of working solution with varied IBP concentrations. After a predetermined time period, 5 ml of the culture was collected and centrifuged at 8000 rpm at 4 °C for 20 min to determine the residual IBP. The supernatant has been spectrophotometrically examined at 440 nm. The cell pellets, thus obtained, were then thoroughly cleaned with deionized water, and dried for 48 h at 80 °C in an air oven at a fixed weight. The biomass was calculated as the dry weight of the culture (g/L). All experiments were carried out in triplicate, with the average results being presented.
Results and discussion
Characterization of the IBP degrading bacteria
The surface morphology of the microbial biomass was studied using SEM in Fig. 3a and b. Before and after IBP uptake, considerable surface topography alterations in the biomass were found. In raw biomass, the surface texture was dense rather than porous. The attachment of IBP, i.e., blackish lump-like deposition onto the biomass surface, was detected as shown in Fig. 3a. However, after IBP uptake (Fig. 3b), there was no longer any blackish lump deposition on the biomass surface. Gram staining revealed that the isolate was gram-positive rods with yellow pigmentation (Chorost et al. 2018).
Table 3 shows the biochemical screening of the isolated bacterial strain.
Phylogenetic analysis of the bacterial strain was done according to the 16S rRNA homology with the related species shown in Fig. 4 According to the phylogenetic analysis, the isolate was identified to be Microbacterium paraoxydans (Genbank accession numbers: Forward OL614700 and Reverse OL614701).
Optimization by CCD approach and effect of the operative parameters on the process output
ANOVA study
When using the CCD to optimize the process, the response must be recorded and evaluated for each parametric combination; this relates to the percentage of IBP removed. ANOVA may be used to create a mathematical model equation for optimized process prediction by analyzing the impact of each parametric value on the response. Statistical parameters like F value (Fischer's exact test), SS values (sum of squares) and p value (probability of error) for every parameter can be evaluated using ANOVA. The high F value for a model is usually desired for significance, and a p value lesser than 0.05 (5% deviation owing to chance) ensures validity of the model. Table 4 shows that the model has an F value of 56.90 and a p value of 0.0001 according to ANOVA results. The SS value for each parameter in the method also assists in comprehending that every operational variable impacts the expected response. These can be converted to percentages as indicated in Eq. (4) and used to calculate a contribution to optimum response, as given in Table 5.
SSf stands for the sum of squares for a specific parameter, whereas SSM stands for the sum of squares for the entire model. The clearance % was found to be highly impacted by yeast extract and agitation speed, also with squared value of yeast extract having the utmost effect on the response.
Model equation, fit statistics and validation
ANOVA must be used to build a regression model equation in order to mathematically evaluate the process under investigation. This permits to see how each individual process variable affects the process response and influences the output in either a positive or negative way. The elimination percentage is influenced by the individual, combined, and squared coefficients at various levels of each operating parameter, as given in Eq. (5). A positive coefficient is indicated by a ' + ' sign, whereas a negative coefficient is shown by a '-ve' sign. The coefficients indicate whether the process reaction is constructive or destructive.
The obtained values could be used to generate a linearized plot, often known as a 'predicted vs actual graph,' as presented in Fig. 5, which is essential to determine how well the actual values suit the desired model equation parameters, as indicated via R2 values. The fit as well as model comparison statistics of the generated regression model are summarized in Table 6. In order for the model to be accurate, the difference between the predicted as well as adjusted R2 values should be lesser than 0.2. As shown in the table, the adequate precision (AP) value is 25.2995 that are substantially greater than 4, indicating that the signal-to-noise ratio (SNR) is high enough for unexpected mistakes to have any influence on the design area.
Effects of parameters
Effect of pH
The effect of pH on IBP biodegradation by the isolated strain was studied at pH of 6, 7, 8, whereas other parameters were kept constant, i.e., 15 mg/L initial IBP concentration, 0.1% yeast extract, 5 mL inoculum dosage, 160 rpm mixing intensity, 30 °C temperature, and 24 h daily readings were recorded for 5 days. The maximum removal was found to be 85.54% for pH 7 shown in Fig. 6a. IBP has a pKa value of 4.9, and more than 99 percent of the drug is available in anionic form at pH greater than 6.5. As a result, variations in breakdown efficiency at pH 7.0 may not be linked to various ionic forms of IBP. Another reason seems to be that the observed variation could reflect a functional state of a protein in the process of degradation. IBP is accessible in an uncharged form at low pH (4.0–5.0) and interacts with the surface of bacteria cells. However, in the uncharged form, this could indicate that IBP degrades more easily and quickly. These findings appear to validate this finding. In numerous studies, researchers have found that pH 7 is the optimal pH for bacteria growth. For example, Sharma et al. (2019) isolated the most viable bacterial strains from a pharmaceutical industry site and investigated IBP biodegradation. Out of the six isolated strains, Micrococcus yunnanensis (GenBank: MG 493,238) was identified as the best potential bacteria to degrade IBP. According to high-performance liquid chromatography, concentration of drug was decreased by 90.37% at optimal condition (carbon 0.15%, nitrogen 0.1%, pH 7, inoculum size 2% (w/v), 150 rpm agitation speed, time 12 h and temperature 25 °C). Similarly in another study, Bacillus thuringiensis B1(2015b) degrades IBP at a concentration of 25 mg/L in the presence of glucose as an extra carbon source done by Marchlewicz et al. (2017). The bacteria have been extracted by centrifugation (4500 g) at 4 °C for 15 min. The pellets were rinsed and resuspended in 50 mM phosphate buffer at pH 7.0. The whole-cell suspensions were then sonicated (six times for 15 s) and centrifuged at 9000 rpm for 30 min at 4 °C. The enzyme assays employed clear supernatants as crude cell extracts. qmax = 0.24 0.02 mg/mL/h and Ks = 2.12 0.56 mg/L, correspondingly, were the maximum specific IBP elimination rate and the half-saturation constant value.
Effect of temperature
The second most crucial factor affecting both biomass growth as well as IBP degradation was determined to be temperature. Microbial growth and IBP biodegradation improved when the temperature was raised from 28 to 30 °C, which could be attributed to the bacteria's increased metabolic activity. Increased temperatures from 30 to 32 °C (Fig. 6b), on the other hand, resulted in lesser biomass growth and, as a result, lower IBP degradation. This could be due to the denaturation of proteins and enzymes caused by microbe growth and substrate metabolism. Furthermore, a rise in temperature has been shown to accelerate the inhibitory impact of substrates and alter membrane mobility (Sarkar et al. 2022).
Effect of agitation speed
Agitation was found to be a significant factor. The experiment was done at agitation speed of 120, 150, and 180 rpm, whereas other parameters were kept constant. The maximal removal was found to be 93.77% at 150 rpm (Fig. 6c). This is due to the fact that agitation increases mass transfer of oxygen and is one of the most important elements in a biologically mediated procedure to ensure homogeneous O2 circulation within a reaction mechanism. IBP biodegradation is influenced by the amount of dissolved oxygen in the media. Bacterial development is influenced by O2, which in turn affects the biodegradation procedure (Sarkar et al. 2022).
Effect of yeast extract
The yeast extract was determined to be an important factor in growth of microbe. The concentration of yeast extract was increased from 0.2 to 0.3%, which had a huge effect on biomass growth as well as IBP biodegradation and removal % was 80.29% shown in Fig. 6d. This is because yeast extract is a source of nitrogen which assists in the growth of biomass and the synthesis of cellular components. Since the bacterial culture has larger nutritional necessities when grown in a media supplemented with raised IBP concentration, the rate of IBP breakdown at higher substrate concentrations has been shown to rise with the increase in concentration of yeast extract. IBP is hazardous to microbial cells at high concentrations, with different bacteria having varied IBP toxicity tolerance limits (Sarkar and Dey 2020). Because of its adverse effect on the growth of most microbes, the bulk of IBP biodegradation investigations have been undertaken in the range of concentrations of 1–20 mg/L.
Effect of inoculum dose
The inoculum dose is another significant component that influences biomass growth and IBP breakdown. The inoculum dose of 0.5 OD600 had the greatest impact on the process output in this investigation and the percentage removal was 67.41% (Fig. 6e). When the inoculum size is increased, the lag phase is reduced, which speeds up the degradation process. Furthermore, a large inoculum size assists in overcoming IBP toxicity, providing tolerance, and increasing in its degradation (Sarkar and Dey 2020).
Effects of interactive parameters
Figure 7a shows a three dimensional plot of combined impacts of pH (A) and temperature (B) while controlling for other variables such as agitation speed (150 rpm), yeast extract (0.3%), and inoculum dose (0.5 OD600). The removal % appears to vary more with temperature in this graph. Figure 7b shows the effect of pH (A) and agitation speed (C) on the percentage of IBP removed at a fixed temperature (30 °C), inoculum dose (10 mL), and yeast extract (0.3%) in 3D plot. This graph demonstrates that removal percentage increases more with agitation speed since agitation enhances mass transfer of oxygen and is one of the most significant factors in a biologically intermediated technique to ensure uniform O2 circulation inside a reaction mechanism. The 3D surface plot at stable temperature (30 °C), agitation speed (150 rpm), and inoculum dose (0.5 OD600) as shown in Fig. 7c describes the interactive effect of pH (A) and yeast extract (D) on IBP removal, and it can be seen from this plot that IBP elimination percentage tends to vary more with yeast extract. Similarly, Fig. 6d depicts the combined effect of pH (A) and inoculum dose (E) on IBP elimination percentage while all other parameters remain constant. The percentage of IBP removed changed significantly with inoculum dose in this graph. Figure 7e illustrates the influence of temperature (B) and agitation speed (C) on the percentage of IBP eliminated while keeping the pH (7), yeast extract (0.3%), and inoculum dose (0.5 OD600) constant. The removal percentage varies considerably with temperature in this graph. The 3D surface graph in Fig. 7f demonstrates the percentage of IBP removed by the interactive influence of temperature (B) and yeast extract (D) while maintaining other parameters constant, including pH (7), agitation speed (150 rpm), and inoculum dose constant (0.5 OD600). The percentage of IBP eliminated varied considerably with temperature.
The elimination percent of IBP by the interactive influence of temperature (B) and inoculum dose (E) is shown in Fig. 7g, with all other parameters held constant and the elimination percent shown to vary more with temperature in this graph. The 3D surface plot in Fig. 6h illustrates the interactive effect of agitation speed (C) and yeast extract (D) on IBP removal at a constant pH (7), temperature (30 °C), and inoculum dose (0.5 OD600), and it can be shown that the amount of IBP removed varies more with yeast extract. Figure 7i shows the combined effect of agitation speed (C) and inoculum dose (E) on IBP removal percentage, with all other parameters kept constant. In this graph, the percentage of IBP eliminated increased substantially with agitation speed. Lastly, Fig. 7j indicates the interactions between yeast extract (D) and inoculum dose (E) while controlling the other parameters, i.e., pH (7), agitation speed (150 rpm), and temperature (30 °C). The removal percent appears to vary more with yeast extract in this plot because yeast extract is a source of nitrogen, which helps in the growth of biomass as well as the synthesis of cellular components.
Optimum conditions for IBP biodegradation
Initially the percentage degradation predicted by CCD was 91.56%. Then the methodology was validated by conducting confirmatory biodegradation experiments at the optimal conditions, i.e., pH 7, temperature 30 °C, agitation speed 150 rpm, yeast extract 0.3% and 0.5 OD600 inoculum dose predicted by RSM-CCD was 92.01% of IBP biodegradation. The overall degradation enhancement was found to be 0.49%. The model is validated since the values are in close agreement with each other.
Conclusion
Microbes have a broad and enhanced catabolic potential, making them a possible means for environmental remediation. A highly potent bacterium Microbacterium paraoxydans was able to utilize 15 mg/L of IBP as the main source of carbon, isolated from pharmaceutical wastewater in the current investigation. The CCD approach was used to optimize process parameters, which decreases the cost and time limits associated with standard optimization processes. The results of the CCD technique demonstrated the optimal conditions for IBP biodegradation. The maximum IBP elimination of 92.01% was obtained by bacterial biomass. These findings suggest that the isolated bacterium could be utilized to treat contaminated effluents containing IBP, and RSM-CCD approach can be utilized to develop realistic bioremediation methods.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Almeida B, Kjeldal H, Lolas I, Knudsen AD, Carvalho G, Nielsen KL, Barreto Crespo MT, Stensballe A, Nielsen JL (2013) Quantitative proteomic analysis of ibuprofen-degrading Patulibacter sp. Strain I11. Biodegradation 24:615–630
Chorost MS, Smith NC, Hutter JN, Ong AC, Stam JA, McGann PT, Kamau E (2018) Bacteraemia due to Microbacterium paraoxydans in a patient with chronic kidney disease refractory hypertension and sarcoidosis. JMM Case Rep. https://doi.org/10.1099/jmmcr.0.005169
Couto FC, Lange LC, Amaral MCS (2018) A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J Water Process Eng 26:156–175
Dai L, Wu TQ, Xiong YS, Ni HB, Ding Y, Zhang WC, Yu J (2019) Ibuprofen-mediated potential inhibition of biofilm development and quorum sensing in Pseudomonas aeruginosa. Life Sci 237:116947. https://doi.org/10.1101/576447
Delgado-Moreno L, Bazhari S, Nogales R, Romero E (2019) Innovative application of biobed bioremediation systems to remove emerging contaminants: adsorption, degradation and bioaccesibility. Sci Total Environ 651:990–997
Encarnação T, Aguiar A, Palito C, Pais AA, Campos MG, Sobral AJ, Burrows HD (2020a) Development and validation of a RP-HPLC method for the simultaneous analysis of paracetamol, ibuprofen, olanzapine, and simvastatin during microalgae bioremediation. MethodsX 7:101083
Encarnação T, Palito C, Pais AACC, Valente AJM, Burrows HD (2020b) Removal of pharmaceuticals from water by free and Imobilised microalgae. Molecules 25:1–13
Fortunato MS, Fuentes Abril NP, Martinefski M, Trípodi V, Papalia M, Rádice M, Korol SE (2016) Aerobic degradation of ibuprofen in batch and continuous reactors by an indigenous bacterial community. Environ Technol 37(20):2617–2626
Girijan S, Kumar M (2019) Immobilized biomass systems: an approach for trace organics removal from wastewater and environmental remediation. Curr Opin Environ Sci Health 12:18–29
Iovino P, Canzano S, Capasso S, Erto A, Musmarra D (2015) A modeling analysis for the assessment of ibuprofen adsorption mechanism onto activated carbons. Chem Eng J 277:360–367
Jiang M, Yang W, Zhang Z, Yang Z, Wang Y (2015) Adsorption of three pharmaceuticals on two magnetic ion-exchange resins. Res J Environ Sci 31:226–234
Jiménez-Silva VA, Santoyo-Tepole F, Ruiz-Ordaz N, Galíndez-Mayer J (2019) Study of the ibuprofen impact on wastewater treatment mini-plants with bioaugmented sludge. Process Saf Environ 123:140–149
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Struct 16:111–120
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
Langenhoff A, Inderfurth N, Veuskens T, Schraa G, Blokland M, Roeleveld KK, Rijnaarts H (2013) Microbial removal of the pharmaceutical compounds ibuprofen and diclofenac from wastewater. Biomed Res Int, 1–9
Marchlewicz A, Domaradzka D, Guzik U, Wojcieszyńska D (2016) Bacillus thuringiensis B1(2015b) is a Gram-positive bacteria able to degrade naproxen and ibuprofen. Water Air Soil Pollut 227:197
Marchlewicz A, Guzik U, Katarzyna Hupert-Kocurek K, Nowak A, Wilczyńska S, Wojcieszyńska D, D, (2017) Toxicity and biodegradation of ibuprofen by Bacillus thuringiensis B1(2015b). Environ Sci Pollut Res 24:7572–7584. https://doi.org/10.1007/s11356-017-8372-3
Marco-Urrea E, Pérez-Trujillo M, Vicent T, Caminal G (2009) Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 74:765–772
Maryam B, Buscio V, Odabasi SU, Buyukgungor H (2020) A study on behavior, interaction and rejection of Paracetamol, Diclofenac and Ibuprofen (PhACs) from wastewater by nanofiltration membranes. Environ Technol Innov 18:100641
Matamoros V, Uggetti E, García J, Bayona JM (2016) Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: a laboratory scale study. J Hazard Mater 301:197–205
Navrozidou E, Melidis P, Ntougias S (2019) Biodegradation aspects of ibuprofen and identification of ibuprofen-degrading microbiota in an immobilized cell bioreactor. Environ Sci Pollut Res 26:14238–14249
Samanta S, Datta D, Halder G (2020) Biodegradation efficacy of soil inherent novel sp. Bacillus tropicus (MK318648) onto low density polyethylene matrix. J Poly Res 27(10):1–16
Santaeufemia S, Torres E, Abalde J (2018) Biosorption of ibuprofen from aqueous solution using living and dead biomass of the microalga Phaeodactylum tricornutum. J Appl Phycol 30:471–482
Sarkar P, Dey A (2020) 4-Nitrophenol biodegradation by an isolated and characterized microbial consortium and statistical optimization of physicochemical parameters by Taguchi Methodology. J Environ Chem Eng 8(5):104347
Sarkar P, Show S, Tiwari H, Dey A (2022) Strategic utilization of groundnut shell (Arachis hypogaea) immobilized bacterial consortium for enhanced 4-nitrophenol remediation: statistical optimization, kinetic modeling, and reusability. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02458-5
Sharma K, Kaushik G, Thotakura N, Raza K, Sharma N, Nimesh S (2019) Fate of ibuprofen under optimized batch biodegradation experiments using Micrococcus yunnanensis isolated from pharmaceutical sludge. Int J Environ Sci Technol 16(12):8315–8328
Show S, Karmakar B, Halder G (2020) Sorptive uptake of anti-inflammatory drug ibuprofen by waste biomass–derived biochar: experimental and statistical analysis. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00922-8
Show S, Chakraborty P, Karmakar B, Halder G (2021) Sorptive and microbial riddance of micro-pollutant ibuprofen from contaminated water: a state of the art review. Sci Total Environ 786:147327
Sutherland DL, Ralph PJ (2019) Microalgal bioremediation of emerging contaminants-Opportunities and challenges. Water Res 164:114921
Zwiener C (2007) Occurrence and analysis of pharmaceuticals and their transformation products in drinking water treatment. Anal Bioanal Chem 387(4):1159–1162
Acknowledgements
Sincere thanks to Dr. Soumya Banerjee for his assistance in preparation of the manuscript. The authors would like to extend their gratitude to the Department of Chemical Engineering as well as Biotechnology at NIT-Durgapur, India. Prof. Apurba Dey provided laboratory facilities, while Mr. Harshit Tiwari and Mr. Pankaj Garkoti, both M.Tech students at the institute, provided important support in completing this experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Show, S., Sarkar, P., Barman, S. et al. Microbial remediation of ibuprofen contaminated water using novel isolate Microbacterium paraoxydans. Chem. Pap. 77, 517–531 (2023). https://doi.org/10.1007/s11696-022-02499-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02499-0