Abstract
Ibuprofen has become an emerging pollutant in aquatic ecosystems, and therefore, it is necessary to develop efficient methods for its elimination. Bioremediation based on the use of a biological material as sorbent is a good alternative. For this reason, the sorption characteristics of ibuprofen using living or dead biomass of the microalga Phaeodactylum tricornutum have been tested in this study. Kinetics, isotherms, and maximum sorption capacity were investigated and discussed. Both living and dead biomass showed a similar efficiency; around 99.9% of ibuprofen was removed even when the initial concentration of ibuprofen tested reached 2 mg L−1 and 0.8 g L−1 of biomass used. Based on the Langmuir isotherm, the maximum sorption capacity was 3.97 mg g−1 at 18 °C, agitation speed 200 rpm, and pH 8.2 after 6 h of contact time. Results indicated that the removal efficiency increased as pH decreased and was higher at pH 2. Six consecutive sorption-regeneration cycles were assayed, and after 3 cycles, there was a loss of only 10.7% in efficiency, which remained stable thereafter. Therefore, the results indicate that the biomass of this microalga is a good and eco-friendly alternative for applications that require the removal of ibuprofen from aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals are substances with biological activity that are used worldwide to treat diseases in humans and animals. In recent years, there is an increasing concern about the growing consumption and use of pharmaceutically active compounds (PhACs) and their subsequent continuous release to the aquatic environment, forming part of the so-called emerging pollutants. The non-steroidal anti-inflammatory drugs (NSAIDs) are one of the major classes of PhACs. There are many ways through which NSAIDs enter into aquatic environments, like effluents of wastewater treatment plants (Gros et al. 2010), hospital wastewater effluents (Hartmann et al. 1998), or even groundwater (Sacher et al. 2001). This uncontrolled discharge cause adverse effects in these environments (Mezzelani et al. 2016).
Ibuprofen is one of the most highly utilized NSAIDs worldwide. It is used for the treatment of fever and to relieve pain in general. This compound has been recognized, in recent years, as an emerging pollutant since it causes toxic effects in the environment (Hernando et al. 2006). This involves a clear need to create and develop effective treatments to remove this compound from aquatic media.
There are a number of treatments used to eliminate ibuprofen from aqueous solutions, such as electrochemical degradation (Ciríaco et al. 2009), UV degradation (Iovino et al. 2016), photo-Fenton reaction (Klamerth et al. 2010), ultrasonic degradation (Méndez-Arriaga et al. 2008), membrane bioreactor technology (Sipma et al. 2010), membrane filtration (nanofiltration and reverse osmosis) (Westerhoff et al. 2005), or degradation using Fe2+/Oxone/UV processes (Gong et al. 2017). However, these kinds of treatments have different drawbacks and limitations; for example, they require high-tech operations, skilled personnel, or high cost equipment. In addition, these methods can generate toxic waste and even an incomplete removal. These disadvantages imply the need to develop new and effective methods for the removal of ibuprofen. In this sense, a simple alternative is the use of a material as sorbent of contaminants (Kyzas et al. 2015; Nanaki et al. 2015). Many sorbents have been tested for the removal of ibuprofen; for instance, mesoporous silica SBA-15 60 (Bui and Choi 2009), activated carbon (Mestre et al. 2007), or carbon from municipal waste (Mestre et al. 2009). Within these methods, the technology of biosorption has gained greater interest in recent years because the use of this methodology has more advantages (it does not produce by-products and is environmentally friendly) and less constraints (Gadd 2009). This process uses a biological material as sorbent. Biosorbents such as potato peel-modified carbon (Kyzas and Deliyanni 2015), pine chip bark-modified carbon (Junga et al. 2013), Kigelia pinnata (Lawal and Moodley 2016), and mung bean (Vigna radiata) (Mondal et al. 2016b) have been proven to remove ibuprofen. However, the use of biomass from microorganisms has been demonstrated to be more effective (Priyadarshani et al. 2011). In particular, the use of microalgal biomass is showing good possibilities in the field of biosorption. There are two ways to use the biomass from microalgae, either as dead biomass or living biomass. The use of dead biomass as biosorbent of contaminants has some advantages; for example, it is independent of growth and is not subject to limitations of toxicity. However, the use of living biomass has gained great attractiveness, due to the possibility to store large amounts of contaminant (bioaccumulation) or to transform it in less active forms (biotransformation), improving the elimination process.
The aim of this work is to investigate the kinetics, isotherms, and capacity of ibuprofen removal by biomass obtained from a microalgal species. The selected microalga was the diatom Phaeodactylum tricornutum; this species is easy to culture, with multiple applications, and the production of this biomass can be considered cheap (Molina Grima et al. 2003; Borowitzka 2013). In addition, the living biomass of this microalga has proved its effectiveness in the removal of other pollutants (Santaeufemia et al. 2016; Torres et al. 2014). For this purpose, living and dead biomass of this microalga were used and compared. Three kinetic models (pseudo-first-order, pseudo-second-order, and intraparticle diffusions) and four sorption isotherm models (Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich) were used to characterize the process.
Materials and methods
Microorganism
The used biomass came from the marine microalga Phaeodactylum tricornutum Bohlin (strain CCAP 1055/1). This microalga was cultured in laboratory under appropriate conditions in natural seawater enriched with Algal-1 medium (Herrero et al. 1991) at 18 ± 2 °C, under a light intensity of 68 μmol photons m−2 s−1 using cool fluorescent light (Osram L36 W/765, Germany) and with a light/dark cycle of 12:12 h. Natural sterile air was constantly bubbled at a flow rate of 10 L min−1.
Both living and dead biomass of this microalga were used in the experiments. The dead biomass was obtained by freeze-drying. A volume of the stock culture of P. tricornutum was centrifuged at 4500×g and 4 °C for 15 min. The pellet was resuspended in a solution of ammonium formate (1%) to remove salts and was centrifuged again. Finally, the pellet was lyophilized. After lyophilization, the dried biomass was stored in a desiccator to avoid moisture absorption. The living biomass was obtained from an appropriate volume of the stock culture of the microalga (at the middle of the logarithmic phase) and in order to obtain a number of cells equivalent to the amount of lyophilized biomass used in the experiments with dead biomass. This volume was calculated taking into account the culture cell density (obtained by counting in the Neubauer chamber) and the cell dry weight.
Reagents
Ibuprofen sodium salt, phosphoric acid and NaOH (reagent grade), acetonitrile, and methanol (HPLC grade) were purchased from Sigma (USA). Double-deionized water with 18.2 MΩ cm−1 of resistivity was obtained from a Milli-Q system (Millipore, USA). The seawater used for the experiments was natural organic-free seawater with a salinity of 35‰ and pH = 8.2. The natural seawater was passed through a Millipore filter of nitrocellulose (Millipore Iberica, Madrid) with a pore size of 0.22 μm and through a charcoal column to remove organic substances. Finally, this seawater was sterilized at 121 °C for 20 min.
A stock solution of ibuprofen was freshly prepared by dissolving ibuprofen in methanol to obtain a final concentration of 1 mg mL−1. Phosphoric acid was prepared in Milli-Q water to obtain a final concentration of 10 mM and adjusted to pH 7 by adding NaOH. All solvents were filtered through a 0.22-μm Millipore filter.
Biosorption and regeneration experiments
The biosorption experiments were carried out in Kimax glass tubes using sterile seawater as aqueous solution (necessary to keep the biomass of this microalga alive) for 8 h, at a constant temperature of 18 ± 2 °C and under an illumination of 68 μmol photons m−2 s−1. The Kimax tubes were gently shaken to ensure homogeneity on an orbital shaker (Skyline S-3.08 M) at 200 rpm. To carry out the experiments, appropriate volumes of the seawater and of the ibuprofen stock solution were added to each Kimax tube to obtain final concentrations of 0.1, 0.5, 0.75, 1, 2, 2.5, 5, 7.5, 10, or 15 mg L−1. Then, an appropriate amount of the lyophilized biomass or an appropriate volume of the stock culture of the living microalga was placed in the tubes (this volume was previously taken into consideration to not vary the final concentration of ibuprofen). All procedures were performed under axenic conditions. The biomass concentration was equivalent to 0.4 and 0.8 g L−1 of dry biomass. Two control experiments were included, one control with ibuprofen but without biomass and another with ibuprofen and without biomass but in darkness. All the biosorption experiments were carried out in triplicate. Samples were collected at times 0, 0.083, 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, and 8 h. The collected samples were centrifuged for 10 min at 14000×g, and the supernatant was always kept in darkness and under congelation (− 20 °C) for further analysis by high-performance liquid chromatography (HPLC).
Additional biosorption experiments were also carried out to investigate the effect of pH on the ibuprofen removal by biomass of P. tricornutum. For these experiments, dead biomass of this microalga was used. The experiments were performed during 8 h at various pH values (2, 4, 6, 8, and 10) with a biomass concentration of 0.4 g L−1 and an ibuprofen concentration of 2.5 mg L−1. Hydrochloric acid or sodium hydroxide was added to the seawater solution to adjust the desired pH. Control tubes with ibuprofen in seawater at the same pH but without biomass were included.
In addition, regeneration of the P. tricornutum biomass for ibuprofen removal was investigated. Dead biomass was used for these experiments. The microalgal biomass was regenerated six times, and the efficiency of the process was measured for each cycle. Each cycle consisted in the following procedure: After a removal process, the biomass was centrifuged and a sample of the supernatant was obtained to measure the residual concentration of ibuprofen. The obtained biomass was resuspended in an appropriate volume of methanol to eliminate the sorbed ibuprofen. Then, the solution was centrifuged and the biomass was resuspended in sterile seawater. After the elimination of the seawater by another centrifugation, a new ibuprofen solution in seawater was added to the clean biomass to initiate a new removal cycle. A biomass concentration of 0.4 g L−1 and an ibuprofen concentration of 2.5 mg L−1 were used for these experiments.
Analytical methods
The concentration of ibuprofen remaining in the supernatants was measured by HPLC using a Hewlett-Packard 1050 equipped with an UV detector and a reverse-phase Zorbax Eclipse XDB-C18 column (4.6 mm × 250 mm × 5 μm). The mobile phase consisted of a mixture of phosphoric acid 10 mM/acetonitrile (65:35, v/v) pH = 7. Isocratic elution with a constant flow rate of 1 mL min−1 at room temperature was used. The injection volume was 20 μL. The detector wavelength was set at 220 nm. The estimated limit of detection (LOD) was 0.02 mg L−1.
The amount of ibuprofen removed per gram of the biosorbent at each sampling time q t (mg g−1) was calculated as follows:
where C t (mg L−1) is the ibuprofen concentration in the solution at time t, C c (mg L−1) is the ibuprofen concentration in solution at that same time in the control tubes exposed to the light but without biomass, V (L) is the volume used in the experiments, and m (g) is the mass of the biosorbent.
The percentage of ibuprofen removed (P t ) at time t from the solution was calculated as follows:
where C i (mg L−1) is the initial ibuprofen concentration in the solution.
Determination of sorption kinetics
The kinetic parameters are useful for the prediction of sorption rate, which gives important information for designing and modeling the process. Sorption kinetics is commonly described with pseudo-first-order and pseudo-second-order kinetic models; for this reason, these models were used in the present study. In addition, the intraparticle diffusion model was also included.
Pseudo-first-order kinetic model
The pseudo-first-order kinetic model (Lagergren 1898) has been extensively used to interpret the adsorption rate of organic compounds on different adsorbents. It can be represented by the following equation:
where q (mg g−1) is the amount of ibuprofen sorbed per unit of mass at time t, k 1 (h−1) is the rate constant of the first-order kinetic model, and q e is the amount of ibuprofen sorbed per unit of mass at equilibrium.
Pseudo-second-order kinetic model
The pseudo-second-order kinetic model (Blanchard et al. 1984) is represented by the following equation:
where k 2 (g mg−1 h−1) is the rate constant of the second-order kinetic model.
Intraparticle diffusion model
This model (Weber and Morris 1963) assumes that the adsorption mechanism occurs through the diffusion of adsorbate molecules into the pores of adsorbent material. It is a functional relationship found empirically, common to most adsorption processes, where uptake varies almost proportionally with t 0.5 rather than with the contact time t:
where k i (mg g−1 h-0.5) is the intraparticle diffusion rate constant.
Determination of biosorption isotherms
The Langmuir (Langmuir 1918), Freundlich (Freundlich 1906), Temkin (Temkin and Pyzhev 1940), and Dubinin-Radushkevich (Dubinin and Radushkevich 1947) isotherm models were considered to study the characteristics of the dead and living biomass of P. tricornutum in the removal of ibuprofen.
Langmuir isotherm
This model is represented by the following equation:
where q e (mg g−1) is the amount of ibuprofen sorbed at equilibrium per unit of mass, C e (mg L−1) is the ibuprofen concentration in solution at equilibrium, q max (mg g−1) is the maximum sorption capacity or theoretical isotherm saturation capacity, and K L (L mg−1) is the constant related to the affinity for the biomaterial. According to Hall et al. (1966), the essential features of the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor, R L , which is defined by the following equation:
where C i (mg L−1) is the initial ibuprofen concentration and K L (L mg−1) is the Langmuir constant.
Freundlich isotherm
The Freundlich isotherm has the following equation:
where K F (mg1−(1/n) L1/n g−1) is the Freundlich constant, an indicator of the sorption capacity, and n is of the intensity.
Temkin isotherm
The Temkin isotherm model is described by the following equation:
where A T (L mg−1) is the Temkin isotherm equilibrium binding constant, corresponding to the maximum binding energy; b T (g J mg−1 mol−1) is a constant related to the heat of sorption; R is the gas constant (8.314 J mol−1 K−1), and T is the absolute temperature.
Dubinin-Radushkevich isotherm
The Dubinin-Radushkevich isotherm model is represented by the following equation:
where B D is related to the free energy sorption per mole of the sorbate and ε is the Polanyi potential which is related to the equilibrium concentration as follows:
The apparent energy (E D , kJ mol−1) of sorption from the Dubinin-Radushkevich isotherm model can be computed using Eq. (12):
Determination of the bioconcentration factor
The bioconcentration factor (BCF) is defined as the ratio of the concentration of a chemical in the biomass to the concentration in the surrounding medium. For this reason, it is used to relate the pollutant in the biomass to the pollutant concentration in the solution. It can be represented by the following equation:
where C b is the concentration of ibuprofen in the algal biomass (mg ibuprofen removed kg−1 of biomass) and C i is the initial ibuprofen concentration (mg L−1).
Statistical analysis
Biosorption data were fitted to the kinetic and isotherm equations (Eqs. (3)–(6) and (8)–(10)) using non-linear regression analysis. All data represent the mean of three independent experiments, and the statistical analysis and plots were performed using SigmaPlot for Windows version 12.5 (Systat Software, Inc.).
In order to evaluate the goodness of the kinetic and isotherm models to the experimental data, different error functions were used (Table S1). If data derived from a model are similar to the experimental data, the value of the error functions will be low; otherwise, if they differ, the value will be high. Only in the case of r 2, a higher value indicates the best fit of the model. In all cases, the error function initially selected to minimize the non-linear regression was SSE. The values for all the other error functions were calculated with the obtained parameters.
The amount of ibuprofen removed by both biomass was compared by Student’s t test at the 95% confidence level (α = 0.05). One-way ANOVA and Tukey’s post hoc test were used to statistically assess the effect of pH and biomass regeneration (α = 0.05). These analyses were done using SPSS version 21 (SPSS Ibérica, Spain).
Results and discussion
Effect of culture conditions: photodegradation, contact time, and type of biomass
Photodegradation is a methodology used in some processes of ibuprofen elimination (Candido et al. 2016; Iovino et al. 2016). Since our experiments were carried out in the presence of light, the amount of photodegraded ibuprofen was quantified. For this purpose, experiments without biomass but exposed to the same conditions were carried out and their results were compared with those obtained in absence of light. No significant differences were observed between both experiments, and therefore, photodegradation of ibuprofen was negligible throughout the experiments with the conditions used.
The contact time is one of the most important parameters in a sorption process because it is necessary for the determination of the equilibrium state. Observing Fig. 1, the removal of ibuprofen by both biomasses increased with the increase in the contact time. The ibuprofen removal efficiency increased considerably during the initial stage of the process, and then, an equilibrium was reached. This equilibrium point was attained in 2–6 h depending on the initial ibuprofen concentration, reflecting a rapid removal of this compound. For this reason, a contact time of 8 h was suitable for studying the sorption of ibuprofen using living or dead biomass of P. tricornutum.
Evolution of the total amount of ibuprofen eliminated per unit of biomass throughout the contact time, considering living (a) and dead (b) biomass and using 0.4 g L−1 of biomass. c, d The same but using 0.8 g L−1 of biomass (pH = 8.2; agitation speed, 200 rpm; temperature, 18 °C). Data represent the means of three replicates, and bars indicate the standard deviation
The obtained times to reach the equilibrium point using this biomass were lower than in other studies with different organisms. For instance, the biomass of Eichhornia crassipe and Pistia stratiotes showed a higher adsorption equilibrium time, reaching a plateau at 12 days (Lin and KunLi 2016). Even in reactors planted with the submerged aquatic plant, Elodea canadensis was required 38 days of incubation to reach the equilibrium, using only an ibuprofen concentration of 0.01 mg L−1 and 27 g of fresh biomass (Matamoros et al. 2012). Less time was needed in the case of active duckweed reactors, where concentrations of ibuprofen (10 μM) were constant for 20–24 h and then decreased linearly, resulting in a depletion in 9 days (Reinhold et al. 2010). These results show that the biomass of P. tricornutum was more efficient, using less time and even with much higher concentrations of ibuprofen.
The statistical test comparing the amount of ibuprofen eliminated by both types of P. tricornutum biomass showed that there were no significant differences (paired Student’s t test, p = 0.05) between living and dead cells. Unlike other compounds such as oxytetracycline (Santaeufemia et al. 2016), ibuprofen does not seem to be able to enter easily in the microalgal cell and this compound only remains retained in the surface of the cell. This means that both types of biomass were equally effective. Although taking into account Fig. 1 and Table 2, a slightly higher efficiency of living biomass can be observed (not significant). Considering this result, it would be more appropriate to use dead microalgal biomass for the removal of ibuprofen since in this case the advantage of a bioaccumulation or a possible biodegradation disappears. However, it is not ruled out that if the duration of the experiments were higher, the difference between both biomasses could also increase because ibuprofen is considered as a biodegradable compound (Matamoros et al. 2012; Kruglova et al. 2014; Ding et al. 2017).
Effect of sorbent dose
In this study, two amounts of biomass were tested, equivalent to 0.4 and 0.8 g L−1 of dry biomass. As shown in Fig. 1 and in Table 1, the amount and the percentage of removed ibuprofen increased with the increase of sorbent dose but only from the initial ibuprofen concentration of 2 mg L−1. At concentrations below this value, both doses of sorbent had the same effectiveness. In this case, the difference was in the time to reach the equilibrium; a shorter time was observed in the highest sorbent dose. However, when the concentration of ibuprofen was higher than 2 mg L−1, as expected, the percentage of removed ibuprofen was doubled by doubling the amount of biomass. This was because an increase in the biomass concentration exposed more available surface area, which means more active adsorption sites.
Effect of the initial ibuprofen concentration
The total amount of ibuprofen removed per unit of biomass increased with the initial ibuprofen concentration in the solution (Fig. 1). However, if the removed amount is expressed as percentage in relation to the initial amount, the removal capacity decreased with the increase of the initial ibuprofen concentration. This indicates the lack of active sites when the concentration of ibuprofen was very high. The number of active sites becomes saturated at a certain concentration. In the case of P. tricornutum, this happened from the ibuprofen concentration of 1 with 0.4 mg L−1 of biomass. Instead, with 0.8 mg L−1 of biomass, this saturation was observed from 2.5 mg L−1 of ibuprofen. Thus, as shown in Table 1, for both biomasses, the percentage of total ibuprofen removed from the solution was around 99.9% when the initial ibuprofen concentration was 0.1–0.5 mg L−1. This high percentage was maintained even up to an ibuprofen concentration of 2.5 mg L−1 when the biomass dose was 0.8 g L−1. However, when the initial concentration was 15 mg L−1, the percentage removed was 20.8–21.2% (dead-living biomass) and 9.9–10.1% with 0.8 and 0.4 g L−1 of biomass, respectively.
Effect of the pH on ibuprofen biosorption
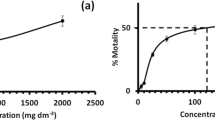
The effect of the different tested pH on the sorption characteristics of ibuprofen by the biomass of P. tricornutum is shown in Fig. 2. It was observed that as the pH decreased, the sorption of ibuprofen was higher, and this effect was more perceptible when the pH became acid. The highest sorption was obtained at pH 2. The ANOVA test showed that this effect was significant (F 4,10 = 328.77, p < 0.001), and the Tukey’s test allowed to demonstrate that all tested pH values were significantly different, influencing the removal of ibuprofen. In fact, an increase in the percentage of removed ibuprofen close to 20% was obtained at pH 2 in relation to the working solution (pH = 8.2). This behavior was also observed in other works of ibuprofen sorption. For instance, Cho et al. (2011) showed that the sorption of ibuprofen onto carbon nanotubes decreased as the pH increased from 4 to 10, and Guedidi et al. (2014) observed a similar decrease in ibuprofen adsorption on activated carbon cloths as the solution pH increased.
Amount of ibuprofen sorbed per unit of mass at equilibrium (red solid line) and the percent of ibuprofen removed (blue dotted line) at different pH. Dead biomass of P. tricornutum was used as sorbent (conditions: sorbent concentration, 0.4 g L−1; ibuprofen concentration, 2.5 mg L−1; contact time, 8 h; agitation speed, 200 rpm; temperature, 18 °C). Data represent the means of three replicates, and bars indicate the standard deviation
Bioconcentration factor
The bioconcentration factors in the steady-state equilibrium for the different ibuprofen concentrations and both biomasses are also listed in Table 1, and they were calculated by means of Eq. (13). This factor provides an index of the ability of the microalga to concentrate ibuprofen with respect to the concentration of this compound in the surrounding medium. As can be observed in Table 1, as the ibuprofen concentration increased in the solution, the bioconcentration factor decreased. The bioconcentration factors were always higher than 1 in all concentrations which means that ibuprofen was bioconcentrate.
Kinetics of sorption
The kinetics for ibuprofen sorption using living and dead biomass of P. tricornutum was studied at different sorbate concentrations. For that, different kinetic models were fitted to experimental data. The kinetic parameters obtained by nonlinear regression are listed in Table 2, and the measures of the goodness of fit with the statistical error deviation functions are listed in Tables S2 and S3. The pseudo-second-order kinetic model was the most suitable for the description of the ibuprofen sorption using this microalga. As shown in Table 3, the removal of ibuprofen considering both biomasses showed similar results. Considering the pseudo-second-order kinetic, an initial phase (0–2 h) of fast removal of ibuprofen is followed by a slow removal until an equilibrium was reached. In the experiments with an initial ibuprofen concentration of 0.1 mg L−1, the maximum amounts of removed ibuprofen (taking into account the pseudo-second-order model) were 0.27 and 0.24 mg g−1 after 6 h, for living and dead biomass, respectively, and 0.4 g L−1 of biomass (Table 2). However, these values decreased when the concentration of biomass was double (0.13 and 0.11 mg g−1 for living and dead biomass, respectively) since in this case, the concentration of ibuprofen was lower than that required to saturate the biomass. But the equality between both biomasses increased progressively as the initial concentration of ibuprofen increased since the biomass would be saturated when the concentrations were high. At higher concentrations of biomass, there are more vacant sites and therefore a higher gradient which would lead to more ibuprofen being sorbed faster. This was observed from the concentration of 2.5 mg L−1 of ibuprofen.
Biosorption isotherms
The sorption isotherms help to understand the mechanism of sorption because they indicate the amount of sorbate removed at a constant temperature and how the sorbate is distributed between the liquid and the solid phases when the sorption process reaches an equilibrium state. Four isotherm models were used to obtain the sorption data. The parameters obtained with the models are listed in Table 3. The validity of the models was assessed by the error functions r 2 and Δq t (Table S1). The plots of the non-linear adjustments to these models are shown in Fig. 3. The obtained results indicated that the order of the isotherm that best fits the four sets of experimental data in this study was Langmuir > Dubinin-Radushkevich > Freundlich > Temkin.
The Langmuir isotherm model showed the lowest values of Δq t and the highest r 2 for both biomasses, sorbent doses, and throughout the range of ibuprofen concentrations tested. This process assumes monolayer adsorption onto a surface containing a finite number of adsorption sites of uniform energies of adsorption and with no transmigration of sorbate in the plane of surface; all the adsorption sites are equivalent, and adsorbed molecules do not interact with each other (Langmuir 1918). With this model, the maximum monolayer sorption capacities at equilibrium were 3.68 and 3.97 mg of ibuprofen per gram with 0.4 and 0.8 g L−1 of biomass, respectively. A biomass concentration of 0.8 g L−1 was slightly more effective than 0.4 g L−1, but there were no significant differences between both types of biomass.
The essential features of the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor (R L ). The R L values were calculated for both biomasses and for each concentration assayed by means of Eq. (7). These values were plotted against the initial ibuprofen concentrations (Fig. 4). This parameter indicates the type of isotherm: unfavorable (R L > 1), favorable (0 < R L < 1), linear (R L = 1), or irreversible (R L = 0). All the obtained R L values for both biomasses were between 0 and 1, indicating that the sorption of ibuprofen by this microalgal biomass was favorable for all concentrations tested. As the initial ibuprofen concentration increased, R L decreased. This indicated that sorption was more favorable at higher concentrations.
As shown in Table 3, the Freundlich isotherm showed higher values than Langmuir and Dubinin-Radushkevich isotherms in the error function Δq t and a lower value in the r 2. This implies that the Freundlich isotherm was less suitable for the description of ibuprofen sorption using living or dead biomass of P. tricornutum. Despite this, its values were sufficiently representative. The K F value signifies the sorption capacity of the sorbent for ibuprofen, which reflects the affinity of this compound towards the biomass. Despite the conditions under which the experiments were performed, the values obtained were not low. Like other pharmaceuticals, the sorption of ibuprofen is strongly dependent on pH, decreasing the capacity as the pH increases (Bui and Choi 2009). In this case, the experiments were performed at pH 8.2 in order to keep the biomass alive. Ibuprofen pK a is 4.91; for this reason, ibuprofen is negatively charged at this pH like the microalgal surfaces. Since the obtained results indicated that both types of biomasses had the same capacity to remove ibuprofen, there would be no problem in using this biomass at much lower pH values, which would increase the capacity of this biomass. With a pH of 2, an increase of about 20% was obtained (Fig. 2). On the other hand, the 1/n constant serves to describe the linearity of sorption or the degree of curvature of the isotherms described throughout the range of concentrations tested. Typically, 1/n values range from 1 downwards. A value of 1 signifies that the sorption of the chemical was the same across the whole range tested. The 1/n parameter presented values far from 1 which describe highly curved isotherms. This means that when the concentration of ibuprofen increases, the sorption decreases, obtaining a L-type isotherm. This is indicative of saturation of adsorption sites available. In the case of this biomass, the saturation was produced from the ibuprofen concentration of 1–2 mg L−1, depending on the amount of biomass used (this is in agreement with the percentage of removal).
The Temkin isotherm presented the worst values for the error functions. The constant, b T , is related to heat of sorption which indicates if the sorption reaction is exothermic (b T > 1) or endothermic (b T < 1). As shown in Table 3, both living and dead biomass presented high values of this constant, indicating an exothermic process in this sorption.
The Dubinin-Radushkevich isotherm is useful to calculate the mean free energy of sorption. It predicts the nature of the sorbate sorption onto the sorbent and the magnitude of the apparent energy of sorption (E D ) which is useful for estimating the type of sorption. If this value is between 8 and 16 kJ mol−1, the sorption type can be explained by chemisorptions, and if this value is lower than 8 kJ mol−1, it indicates that the adsorption process might be dominated by physical mechanism. As can be observed in Table 3, all values were lower than 8 kJ mol−1, suggesting that the ibuprofen sorption was a physical process for both biomass.
Regeneration of the microalgal biomass
An evaluation of the behavior of the P. tricornutum biomass regarding its reusability for the removal of ibuprofen was carried out by conducting six sorption-desorption cycles. The ANOVA test indicated that there were significant differences in the efficiency of removal of ibuprofen (F 6,14 = 7.51, p < 0.001) during the cycles. The Tukey test showed a significant loss of effectiveness of 10.7% after 3 cycles, but then remained constant in the rest of the cycles without significant loss. As a result of this, the degree of recovery from and reusability of P. tricornutum biomass after 6 cycles is an added advantage. This indicates that this biomass was resistant to repeated different wash cycles and can be reused for further treatments.
Table 4 shows a comparison of the P. tricornutum biomass with other sorbents tested to remove ibuprofen. Although most of these sorbents are considered of high performance (activated carbon, biochar), the untransformed P. tricornutum biomass showed a good effectiveness in a short period of time; therefore, this biomass can compete with some of them and can be useful in biotechnology applications; in any case, this microalgal biomass could be a good alternative to remove ibuprofen because it is cheaper and easier to obtain (minimum processing) and this biomass can be used in different cycles without an excessive loss of effectiveness (Fig. 5). In addition, this type of biomass is considered eco-friendly, preventing the risk of production of by-products and other adverse effects on the aquatic environment. In fact, previous studies already have shown that algal treatment systems have a potential to remove micropollutants while simultaneously closing the cycle of nutrients in a safer way (de Wilt et al. 2016).
Amount of ibuprofen sorbed per unit of mass at equilibrium (red solid line) and the percent of ibuprofen removal (blue dotted line) through six sequential cycles of biomass reuse. P. tricornutum biomass concentration of 4 g L−1 and ibuprofen concentration of 2.5 mg L−1. Conditions are the same as indicated in Fig. 2. Data represent the means of three replicates, and bars indicate the standard deviation
Conclusions
This work showed that the microalgal biomass (in this case P. tricornutum) is a good alternative for ibuprofen biosorption from aqueous solutions. Both living and dead biomass of this microalga had almost the same results on the sorption of this emerging pollutant. The maximum sorption capacity obtained with the conditions assayed was 3.97 mg g−1 after 6 h of contact time and pH of 8.2. But the dead biomass of this microalga can be used at lower pH, increasing the efficiency. In addition, this biomass can be regenerated and reused in application cycles. Thus, this study demonstrates that the biomass of this microalga is a suitable and promising tool for ibuprofen removal from aqueous solutions.
References
Baccar R, Sarrà M, Bouzid P, Feki M, Blánquez J (2012) Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem Eng J 211-212:310–317
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18:1501–1507
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Bui TX, Choi H (2009) Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J Hazard Mater 168:602–608
Candido JP, Andrade SJ, Fonseca AL, Silva FS, Silva MRA, Kondo MM (2016) Ibuprofen removal by heterogeneous photocatalysis and ecotoxicological evaluation of the treated solutions. Environ Sci Pollut Res 23:19911–19920
Ciríaco L, Anjo C, Correia J, Pacheco MJ, Lopes A (2009) Electrochemical degradation of ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Electrochim Acta 54:1464–1472
Cho HH, Huang H, Schwab K (2011) Effects of solution chemistry on the adsorption of ibuprofen and triclosan onto carbon nanotubes. Langmuir 27:12960–12967
de Wilt A, Butkovskyi A, Tuantet K, Leal LH, Fernandes TV, Langenhoff A, Zeeman G (2016) Micropollutant removal in an algal treatment system fed with source separated wastewater streams. J Hazard Mater 304:84–92
Ding T, Yang M, Zhang J, Yang B, Lin K, Li J, Gan J (2017) Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J Hazard Mater 330:127–134
Dubey S, Dwivedi AD, Sillanpää M, Gopal K (2010) Artemisia vulgaris-derived mesoporous honeycomb-shaped activated carbon for ibuprofen adsorption. Chem Eng J 165:537–544
Dubinin MM, Radushkevich LV (1947) Equation of characteristic curve of activated charcoal. Proc Acad Sci Phys Chem Sect USSR 55:331–333
Essandoh M, Kunwar B, Pittman CU Jr, Mohan D, Mlsna T (2015) Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem Eng J 265:219–227
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28
Gong H, Chu W, Lam SH, Lin AY-C (2017) Ibuprofen degradation and toxicity evolution during Fe2+/Oxone/UV process. Chemosphere 167:415–421
Gros M, Petrović M, Ginebreda A, Barceló D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ Int 36:15–26
Guedidi H, Reinert L, Soneda Y, Bellakhal N, Duclaux L (2014) Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab J Chem 10:S3584–S3594
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Indust Eng Chem Fundamentals 5:212–223
Hartmann A, Alder AC, Koller T, Widmer RM (1998) Identification of fluoroquinolone antibiotics as the main source of umuC genotoxivity in native hospital wastewater. Environ Toxicol Chem 17:377–382
Hernando MD, Mezcuaa M, Fernández-Alba AR, Barceló D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69:334–342
Herrero C, Cid A, Fábregas J, Abalde J (1991) Yields in biomass and chemical constituents of four commercially important marine microalgae with different culture media. Aquac Eng 10:99–110
Iovino P, Chianese S, Canzano S, Prisciandaro M, Musmarra D (2016) Ibuprofen photodegradation in aqueous solutions. Environ Sci Pollut Res 23:22993–23004
Junga C, Park J, Lim KH, Park S, Heo J, Her N, Oh J, Yun S, Yoon Y (2013) Adsorption of selected endocrine disrupting compounds and pharmaceuticals on activated biochars. J Hazard Mater 263:702–710
Khalaf S, Al-Rimawi F, Khamis M, Zimmerman D, Shuali U, Nir S, Scrano L, Bufo SA, Karaman R (2013) Efficiency of advanced wastewater treatment plant system and laboratory-scale micelle-clay filtration for the removal of ibuprofen residues. J Environ Sci Heal 48:814–821
Klamerth N, Rizzo L, Malato S, Maldonado MI, Aguera A, Fernández-Alba AR (2010) Degradation of fifteen emerging contaminants at μg L−1 initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res 44:545–554
Kruglova A, Ahlgren P, Korhonen N, Rantanen P, Mikola A, Vahala R (2014) Biodegradation of ibuprofen, diclofenac and carbamazepine in nitrifying activated sludge under 12 °C temperature conditions. Sci Total Environ 499:394–401
Kyzas GZ, Deliyanni EA (2015) Modified activated carbons from potato peels asgreen environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem Eng Res Des 97:135–144
Kyzas GZ, Fuc J, Lazaridis NK, Bikiaris DN, Matis KA (2015) New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J Mol Liq 209:87–93
Lagergren S (1898) About the theory of so-called adsorption of soluble substance. Hand 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lawal IA, Moodley B (2016) Sorption mechanism of pharmaceuticals from aqueous medium on ionic liquid modified biomass. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.5063
Lin Y-L, KunLi B (2016) Removal of pharmaceuticals and personal care products by Eichhornia crassipe and Pistia stratiotes. J Taiwan Inst Chem Eng 58:318–323
Matamoros V, Nguyen L, Arias CA, Salvadó V, Brix H (2012) Evaluation of aquatic plants for removing polar microcontaminants: a microcosm experiment. Chemosphere 88:1257–1264
Méndez-Arriaga F, Torres-Palmaa RA, Pétriera C, Esplugas S, Gimenez J, Pulgarinc C (2008) Ultrasonic treatment of water contaminated with ibuprofen. Water Res 42:4243–4248
Mestre AS, Pires J, Nogueira JMF, Carvalho AP (2007) Activated carbons for the adsorption of ibuprofen. Carbon 45:1979–1988
Mestre AS, Pires J, Nogueira JMF, Parra JB, Carvalho AP, Ania CO (2009) Waste-derived activated carbons for removal of ibuprofen from solution: role of surface chemistry and pore structure. Bioresour Technol 100:1720–1726
Mezzelani M, Gorbi S, Da Ros Z, Fattorini D, d'Errico G, Milan M, Bargelloni L, Regoli F (2016) Ecotoxicological potential of non-steroidal anti-inflammatory drugs (NSAIDs) in marine organisms: bioavailability, biomarkers and natural occurrence in Mytilus galloprovincialis. Mar Environ Res 121:31–39
Molina Grima E, Belarbi EH, Acién Fernández FG, Robles Medina A, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotech Adv 20:491–515
Mondal S, Aikat K, Halder G (2016a) Biosorptive uptake of ibuprofen by chemically modified Parthenium hysterophorus derived biochar: equilibrium, kinetics, thermodynamics and modeling. Ecol Eng 92:158–172
Mondal S, Bobde K, Aikat K, Halder G (2016b) Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J Environ Manag 182:581–594
Nanaki SG, Kyzas GZ, Tzereme A, Papageorgiou M, Kostoglou M, Bikiaris DN, Lambropoulouc DA (2015) Synthesis and characterization of modified carrageenanmicroparticles for the removal of pharmaceuticals from aqueous solutions. Colloids Surf B Biointerfaces 127:256–265
Priyadarshani I, Sahu D, Rath B (2011) Microalgal bioremediation: current practices and perspectives. J Biochem Tech 3:299–304
Reinhold D, Vishwanathan S, Park JJ, Oha D, Saunders FM (2010) Assessment of plant-driven removal of emerging organic pollutants by duckweed. Chemosphere 80:687–692
Sacher F, Langea FT, Braucha H, Blankenhorn I (2001) Pharmaceuticals in groundwaters. Analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J Chromatogr A 938:199–210
Santaeufemia S, Torres E, Mera R, Abalde J (2016) Bioremediation of oxytetracycline in seawater by living and dead biomass of the microalga Phaeodactylum tricornutum. J Hazard Mater 320:315–325
Sipma J, Osuna B, Collado N, Monclús H, Ferrero G, Comas J, Rodriguez-Roda I (2010) Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination 250:653–659
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physiochim URSS 12:217–222
Torres E, Mera R, Herrero C, Abalde J (2014) Isotherm studies for the determination of Cd (II) ions removal capacity in living biomass of a microalga with high tolerance to cadmium toxicity. Env Sci Pollut Res Internat 21:12616–12628
Weber WJ, Morris JC (1963) Kinetics of adsorption carbon from solutions. J Sanitary Eng Div Proc 89:31–60
Westerhoff P, Yoon Y, Snyder S, Wert E (2005) Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ Sci Technol 39:6649–6663
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 722 kb)
Rights and permissions
About this article
Cite this article
Santaeufemia, S., Torres, E. & Abalde, J. Biosorption of ibuprofen from aqueous solution using living and dead biomass of the microalga Phaeodactylum tricornutum . J Appl Phycol 30, 471–482 (2018). https://doi.org/10.1007/s10811-017-1273-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1273-5