Abstract
Lipid oxidation is the major chemical phenomenon leading to the deterioration of edible oils by the diminution of their organoleptic and nutritional qualities. This study was aimed at profiling a phenolic extract from sweet Basil cultivated in Morocco and to investigate its possible protective effect against the oxidative process in sunflower edible oil as a natural alternative to synthetic antioxidants. The extract provides significant prevention of sunflower oil peroxidation after storage at 37 °C for 60 days. However, the effect was relatively less than that exerted by the BHA. Against, the phenolic extract significantly decreased the peroxidation value of oil after heating at 370 °C/5 h; the effect was comparable to that of BHA. Besides, the extract protects sunflower oil against oxidation induced by copper ion, but this activity remains statistically lower than that exerted by the BHA. Moreover, the DPPH radical scavenging activity of the phenolic extract is less than that recorded by the BHA. This extract inhibits also the oxidative bleaching of β-carotene relatively less than BHA. The extract contains 153.19 ± 1.05 mg/g total phenol. The HPLC analysis showed that the extract contains eight phenolic acids (caftaric acid, gallic acid, chlorogenic acid, caffeic acid, chicoric acid, rosmarinic acid, carnosic acid and p-hydroxybenzoic acid) and two flavonoids (rutin and luteolin-7 glucoside). In conclusion, we suggest that the phenolic Basil extract is a good source of natural antioxidants that might be exploited in edible oil preservation.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipid oxidation is the primary factor leading to the chemical deterioration of edible oil and fat-rich foods (Choe and Min 2006). This phenomenon is triggered by the reaction of oxygen with unsaturated fatty acids and progressed by the intervention of free radicals in stored oils (Taghvaei and Jafari 2015). Indeed, among the direct factors that induce oil oxidation is the process of frying, which is one of the most commonly used procedures for the preparation and production of foods around the world. The oil is continuously heated at high temperatures in the presence of moisture and atmospheric oxygen, which promotes lipid oxidation; this is a problem that mainly affects organoleptic and nutritional qualities of edible oils (Li et al. 2008).
Hence, the prevention of foodstuffs oxidation during their technological transformations, storage, and distribution is highly needed. Thus, among the effective technological treatments, the addition of antioxidants to oils and fat-rich foods (Taghvaei and Jafari 2015). However, synthetic antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are now recognized as having adverse effects on human health and they are readily volatile at high temperatures (Kahl and Kappus 1993). So, the stabilization of vegetable oils has been the subject of much research to replace synthetic antioxidants with natural ones with remarkable antioxidant power (Mira-Sánchez et al. 2020).
In this context, the extracts of several aromatic and condimental plants such as rosemary, marjoram, thyme, and oregano showed an interesting antioxidant activity in edible oil (Wang et al. 2018; Kozłowska and Gruczyńska 2018). The observed beneficial effect was generally exerted by phenolic compounds, which can be exploited industrially in the prevention of the oxidative deterioration of dietary lipids (Wang et al. 2018; Mira-Sánchez et al. 2020).
Sweet Basil plant (Ocimum basilicum L.), a member of the Lamiaceae family, is a popular food seasoning ingredient in the Mediterranean diet. This plant grows in mountain regions, including Africa, Asia, and South America. It is an aromatic annual herb known as an important economic crop having wide applications in the cosmetic and pharmaceutical industries (Purushothaman et al. 2018). Thus, except for the flavor-enhancing characteristic, basil leaves may be an edible plant with existing ideal antioxidant properties to extend the food shelf life and reduce the risks of lipid oxidation-related diseases in the human body (Sestili et al. 2018). In Morocco, sweet basil is cultivated as a medicinal plant, and it is widely used in cooking for its culinary attributes. Previous studies have shown that phenolic compounds and especially phenolic acids are the major phytochemicals found in the basil. However, the nature and amount of these phenolics widely depend on the origin of the plant (Kwee and Niemeyer 2011; Omoba et al. 2019).
Given the considerable interest gained by these phytochemicals in recent years, several techniques have been exploited to identify and measure the polyphenols in plant extracts. However, the RP-HPLC coupled with diode array detector (DAD) or mass spectrometry (MS) has been the major technique widely used for polyphenol analysis (Giusti et al. 2017; Figueroa et al. 2018; Fu et al. 2020).
In fact, classic methods such as thin-layer chromatography and paper chromatography have been previously used to analyze phenolic compounds. However, the resolution and time of analysis given by such methods have been generally inadequate (Molnár-Perl and Füzfai, 2005). The gas chromatography combined with mass spectrometry (GC–MS) provides an excellent resolution but the volatilization increase the analysis time (Soleas and Goldberg, 2001). Other developed methods such as capillary zone electrophoresis (CZE), HPLC–MS and HPLC–DAD have been shown to be more reproducible, efficient and satisfactory for the polyphenol analysis (Rodrigues Sá et al. 2017).
Rodrigues Sá et al. (2017) developed and validated a method using HPLC–DAD to quantify bioactive phenolics in herbal medicines containing Cynara scolymus, Maytenus ilicifolia Mart ex Reiss and Ptychopetalum uncinatum. The author and collaborators recently used the same technique to determination phenolic acids and flavonoids in dried fruits and capsules containing Goji berries (Lycium barbarum L.) (Rodrigues Sá et al. 2019). On the other hand, the HPLC–DAD/ESI-MSn method was also used to characterize and quantify phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells (Weisz et al. 2009). Recently, Oliveira et al. (2018) developed and validated an UHPLC-DAD method which allows quantifying simultaneously 19 phenolic compounds in aromatic plants from Lamiaceae and Asteraceae families.
To our knowledge, it is difficult to choose simple and efficient analytical methods that allow analyzing both phenolics acids and flavonoids of sweet basil. The HPLC–DAD method used in this study present the advantage to be simple, rapid and less expensive. However, a number of phenolics were easily assessed in different plant materials using other more expensive and complex chromatography techniques with MS/MS or Q-TOF–MS detectors (Figueroa et al. 2018). Furthermore, the UHPLC methods have been widely used in the last decade due to their advantages compared to the classic HPLC. The method makes it possible to reduce the analysis time, the solvent consumption and the limit of detection (Oliveira et al. 2018).
The aim of the present study is highlighting the antioxidant properties and the phenolic profile of sweet basil cultivated in Morocco using a HPLC–DAD method. The identification of the major phenolic compounds of the Moroccan sweet basil and their antioxidant activity could help academic researchers and food industrials to select for novel natural phytochemicals as oil stabilizers.
Experimental
Materials
The edible sunflower oil was bought from a local market (Society Lesieur-Cristal, Morocco). Only one lot of oil was purchased in 1/2 L translucent polyethylene terephthalate (PET) bottles and stored at 4 °C before use. It is indicated in the product label that the oil contains 14.54 ± 0.4% saturated fatty acids (SFA), 24.30 ± 0.6% monounsaturated fatty acids (MUFA) and 61.3 ± 1.2% polyunsaturated fatty acids (PUFA) including 2.5 ± 0.1% omega-3.
All the solvents and reagents are of analytical grade and purchased from Sigma Chemical Co (Germany): Caffeic acid (purity ≥ 99%), rosmarinic acid (purity ≥ 98%), rutin (purity ≥ 94%), luteolin-7 glucoside (purity ≥ 99%), malondialdehyde (purity ≥ 96%), thiobarbituric acid (purity ≥ 96%), trichloroacetic acid (purity ≥ 99%), Folin-Ciocalteu reagent, sodium carbonate (Na2CO3, purity ≥ 99.5%), copper sulfate (CuSO4, 5 H2O, purity ≥ 98%), linoleic acid (purity ≥ 99%), β-carotene (purity ≥ 95%), tween 80, sodium thiosulfate (Na2S2O3, purity ≥ 99%), potassium iodide (KI, purity ≥ 99%) and butylated hydroxyanisole (purity ≥ 98.5%).
Methods
Preparation of the Basil phenolic extract
Sweet Basil (O. basilicum L.) was purchased from a herbalist in Oujda city (Eastern Morocco). The plant was authenticated by a botanist (Prof. B. Haloui, Faculty of Sciences, Oujda, Morocco), and a voucher specimen was deposited at the Department of Biology, Faculty of Sciences, Oujda (collection LO15). The basil phenolic extract was prepared according to the method previously described by Amrani et al. (2006) slightly modified. The dried powder from aerial parts of the plant was extracted by infusion in boiled distilled water for 30 min. The extract obtained was concentrated in the rotatory evaporator under reduced pressure at 60 °C and then placed in a drying oven (40 °C) to obtain the crude material in the form of brownish fine powder. The powder was stored at – 20 °C until use. The yield of extraction, in terms of the starting dried plant material, was 27%.
Dosage of total polyphenols
Total polyphenol amount of basil extract was determined according to the Folin–Ciocalteu procedure as described by Bekkouch et al. (2019) with some modifications. A volume of 0.5 mL of plant extract properly diluted (0.25 mg/mL in distilled water) was mixed with 0.25 mL of Folin–Ciocalteu reagent and 0.5 mL of a saturated solution of sodium carbonate 20%. The coloration was allowed to develop for 30 min in the dark. Then, the blue color was measured spectrophotometrically at 725 nm against a blank where the sample was replaced by the 0.5 mL of distilled water. The amount of total phenols was calculated from a calibration curve of rosmarinic acid standard solutions (5, 10, 15, 20 and 25 µg/mL in methanol, R2 > 0.99) and expressed as mg rosmarinic acid/g dry extract. All measurements were done in triplicate.
HPLC analysis of the basil phenolic extract
The HPLC analysis of the phenolic Basil extract was carried out according to the method described by Tsumbu et al. (2012), slightly modified to ameliorate the resolution of peaks. The separation was performed on a Shimadzu LC-10AS apparatus with a Diode Array Detector (SPD-10A. Shimadzu) using a Spherisorb ODS II reverse phase (RP18) analytical column (250 × 4.6 mm, particle size 5 mm). 20 µL samples of the basil extract (1 mg/mL in methanol) were filtered prior to analysis through a 0.45 µm syringe filter and injected three times into the HPLC. The separation was undertaken at 40 °C and a flow rate of 1 mL/min using the following gradient of aqueous orthophosphoric acid (0.3%) (A) and acetonitrile (B): 0–20 min: 7–17% B, 20–30 min: 17% B, 30–45 min: 17–25% B, 45–60 min: 25–40% B, 60–65 min: 40–10% B, 65–70 min: 10% B. UV spectra were collected over the range of 220–400 nm and the chromatogram was recorded at 280 nm. The phenolic compounds were identified by their retention times and UV spectra using a database of analytical standards. Individual phenolic acids quantification was performed with calibration curves of external standards cafeic and rosmarinic acids dissolved in methanol separately at concentrations of 0.1, 0.5, 1, 5, 10, 20 and 40 µg/mL generated by plotting HPLC peak areas against the concentrations (R2 > 0.99). Flavonoids were quantified using a calibration curves prepared with standards rutin and luteolin-7 glucoside at concentrations of 0.1, 0.5, 1, 5, 10, 20 and 40 µg/mL (R2 > 0.99). The compounds identified that no commercial standard is available were quantified using the calibration curves belonging to the available standard with the most similar UV absorption spectrum and from the same phenolic class.
Method validation
The HPLC–DAD method was validated for linearity, specificity, recovery, accuracy, limit of detection (LOD), limit of quantification (LOQ), and system suitability. Calibration plots of peak area against concentration were constructed after triplicate analysis of solutions at seven different concentrations in the range 0.1–40 μg/mL. The phenolic compounds used in the study were caffeic, gallic, chlorogenic and rosmarinic acids, rutin and luteolin-7 glucoside. The LOD of the method was defined as the amount in a sample for which the signal was three times the baseline noise (S/N > 3). The LOQ of the method was defined as the amount in a sample for which the signal was ten times the baseline noise (S/N > 10). At this level accuracy and precision were acceptable. Percentage recovery and precision were determined after six replicate analyses. To assess intra-day variation, calibration plots were prepared twice on the same day, and on three consecutive days to assess the inter-day variation.
Study of the preservative effect of Basil extract and BHA against peroxidation of sunflower oil during storage
The study of the stabilizing effect of the phenolic extract and BHA on the sunflower oil during storage was conducted, in triplicate, according to the method described by Baştürk et al. 2018, with some minor modifications. The concentration of the phenolic extract was chosen according to previously reported works (Taghvaei and Jafari 2015) and after preliminary study showing that the 200 mg/kg gives the maximum stabilizing effect.
-
Control: 5 g of sunflower oil stored at 37 °C for 60 days in a ventilated oven.
-
Phenolic extract-treated oil: 5 g of sunflower oil were added with 200 mg/kg of the phenolic Basil extract and stored at 37 °C for 60 days.
-
BHA-treated oil: 5 g of sunflower oil were supplemented with the BHA at a dose of 200 mg/kg and stored at 37 °C for 60 days.
At the end of the experiment, the peroxide value was determined in the samples as follow: each sample was dissolved in15 mL of acetic acid-chloroform mixture (3:2 V/V) under stirring. Then, 1 mL of a saturated potassium iodide (KI) solution was added and the reaction was allowed to do for 30 min. In acid medium, the peroxide reacts with KI to produce the iodine which was assayed with a sodium thiosulfate (Na2S2O3) solution (0.01 N). The peroxide value was expressed as milliequivalent active oxygen per Kg of oil (meqO2/Kg oil).
Study of the preservative effect of Basil extract and BHA against peroxidation of sunflower oil under frying
The investigation of the antioxidant effect of basil phenolic extract and BHA on sunflower heated oil was carried out according to the following experimental design as previously described.
-
Control: 5 g of non treated and non-heated oil;
-
Heated control oil: 5 g of sunflower oil heated at 370 °C for 5 h;
-
Phenolic extract-treated oil: 5 g of sunflower oil, treated with the phenolic extract at a dose of 200 mg/kg and heated at 370 °C for 5 h;
-
BHA-treated oil: 5 g of sunflower oil treated with the BHA at a dose of 200 mg/kg and heated at 370 °C for 5 h.
At the end of the experiment, the samples were cooled and the peroxide values were determined as described above. All treatments were done in triplicate.
Study of the effect of phenolic Basil extract and BHA on inhibition of copper-induced oxidation of sunflower oil
This study was carried out using copper accelerating the oil oxidation as an experimental model. Thus, to determine the oil peroxidation, the produced malondialdehydes (MDA) as secondary products of the oxidative process were quantified as thiobarbituric acid reactive substances (TBARS) according to the procedure described by Ramchoun et al. (2015) slightly modified. The untreated control contained 40 μL of sunflower oil only. In the oxidized control, 40 μL of sunflower oil were incubated with 10 μL of copper sulfate (CuSO4) solution (0.33 mg/mL). In the tested samples, the oil (40 μL) was incubated with copper sulfate and phenolic extract or BHA dissolved in methanol at different concentrations (100, 200, 400, 600, 800, and 1000 μg/mL). The preparations were stirred and incubated 24 h at 37 °C in a ventilated oven. Then, all the samples were added with 0.5 mL of thiobarbituric acid (0.8%) and 0.5 mL of trichloroacetic acid (20%) and heated at 90 °C in a water bath for 30 min. After cooling, 2 mL of n-butanol were added and the solutions were centrifuged at 4500 rpm for 15 min. The absorbance of the colored layer was recorded at 532 nm. The amounts of TBARS were calculated from a calibration curve of MDA made of increasing concentrations at 50, 100, 150, 200, and 250 nM (R2 = 0.997). All the assays were done in triplicate.
Free radical scavenging activity of the phenolic Basil extract and BHA
The free radical scavenging activities of the basil phenolic extract and BHA were determined using the DPPH (2.2-diphenyl-1-picryl-hydrazyl) assay as previously described by Bekkouch et al. (2019). Thus, 5 μL of the phenolic extract or BHA, dissolved in methanol, were completed at 2.5 mL by a methanol DPPH solution (0.1 mM) to have final concentrations of 0.5, 10, 25, 50, 100, 200 and 400 μg/mL.
The samples were incubated 30 min in the dark and then the absorbance of the mixtures was measured at 517 nm. The blank solution was prepared by replacing the samples with the same volume of distilled water. The free radical scavenging activity (RSA) was calculated according to the following formula: \({\text{RSA }}\left( \% \right)\, = \,\left( {{\text{A}}_{{{\text{blank}}}} {-}{\text{A}}_{{{\text{sample}}}} /{\text{A}}_{{{\text{blank}}}} } \right) \, \times {1}00.\) The IC50 (concentration providing 50% radical swiping) values were calculated from the plotted graph of scavenging activity against concentrations of the samples. All tests were done in triplicate.
Study of the effect of phenolic Basil extract and BHA on inhibition of β-Carotene Oxidative Bleaching induced by linoleic acid degradation
The oxidative bleaching of β-carotene was induced by lipoperoxyl radicals produced by the autooxydation of linoleic acid. This experimental model was used to evaluate the effect of Basil phenolic extract and BHA on the neutralization of lipoperoxyl radicals and the prevention of lipid oxidation according to the method described by Leouifoudi et al. (2015) slightly modified. Thus, a mixture of β-carotene–linoleic acid was prepared as follows: 2 mg of β-carotene dissolved in 1 mL of chloroform were mixed with 20 mg of linoleic acid and 200 mg of Tween 80. After evaporation of chloroform, 100 mL of distilled water were added to form the emulsion linoleate-β-carotene. 5 μL of Basil phenolic extract or BHA dissolved in methanol at different concentrations (10, 25, 50, 100, 200, 400 μg/mL) were completed to 2.5 mL with the emulsion linoleate-β-carotene. Absorbance values were read at 492 nm before (blank) and after 24 h incubation.
The inhibition of β-carotene oxidative bleaching was calculated according to the following formula: \(\% {\text{ inhibition}}\, = \,{1}00{-}\left[ {\left( {\left( {{\text{A}}_{{{\text{blank}}}} {-\!\!-}{\text{A}}_{{{\text{sample}}}} } \right)/{\text{ A}}_{{{\text{blank}}}} } \right) \times {1}00} \right]\) and IC50 values were calculated from the plotted graph of antioxidant activity against concentrations of the samples. The measurements were done in triplicate.
Statistical analysis
The data were analyzed using student t-test and one way ANOVA test. P values less than 0.05 were considered as statistically significant. The results are expressed as mean ± SD (n = 3).
Results
Total polyphenol content and HPLC analysis of the Basil extract
The dosage of total polyphenol showed that the Basil extract contained 153.19 ± 1.05 mg rosmarinic acid equivalent/g dry extract. Fig. 1 depicts the HPLC chromatogram of the extract. We identified 10 major phenolic compounds (Table 1) of which 8 are phenolic acids namely caftaric acid 27.3 ± 0.93 mg/g (38.3%), gallic acid 3.4 ± 0.12 mg/g (4.7%), chlorogenic acid 1.55 ± 0.08 mg/g (2.17%), caffeic acid 4.5 ± 0.15 mg/g (6.31%), chicoric acid 9.1 ± 0.46 mg/g (12.7%), rosmarinic acid 18.2 ± 0.65 mg/g (25.5%), carnosic acid 3.2 ± 0.14 mg/g (4.49%) and p-hydroxybenzoic acid 1.58 ± 0.06 mg/g (2.21%), and 2 are flavonoids namely rutin 0.7 ± 0.01 mg/g (0.98%) and luteolin-7 glucoside 1.7 ± 0.09 mg/g (2.38%).
Method validation
The data of method validation were summarized in Table 2. We showed that retention time was highly repeatable, both for single standards and for mixture of the phenolic compounds. Moreover, the R2 values for the compounds were ≥ 0.99, confirming the linearity of the method. The LOD values ranged from 0.24 to 0.92 μg/mL and LOQ from 0.95 to 1.25 μg/mL. This result could confirm the capacity for quantification of the analyzed phenolic compound. The high recovery values (> 86%) and the good repeatability indicated that the method was satisfactory accurate (Table 2).
Protective effect of the phenolic Basil extract and BHA against oxidation of sunflower oil during storage
Our finding shows that the storage of sunflower oil at 37 °C during 60 days exerted a significant increase in peroxide value compared to control (the same oil before storage) (+ 1600%, P < 0.001). However, the pre-treatment of the oil with the phenolic Basil extract at 200 mg/kg provides a significant oxidative stabilization. The phenolic extract decreases the peroxide value by 56.8% compared to untreated oil (P < 0.001) (Fig. 2). Moreover, the BHA reduces the oxidation process of the stored oil by 62.2% comparatively to untreated oil (P < 0.001). So, we can conclude that the phenolic Basil extract was relatively less efficient than the synthetic antioxidant BHA (P < 0.05) (Fig. 2).
Protective effect of the phenolic Basil extract and BHA against oxidation of sunflower oil after heating
In this study, we demonstrated that the heating of sunflower oil at 370 °C for 5 h induces a significant increase in peroxide value compared to unheated oil (+ 277%, P < 0.001) (Fig. 3). However, the addition of basil phenolic extract or BHA at a concentration of 200 mg/kg significantly prevents oxidative degradation of the oil during frying. The phenolic extract decreased the oil peroxide value by 57.8% (P < 0.001) comparatively to control (oil fried without any additive). In the same conditions, BHA reduced the peroxide value with a score comparable to that exerted by the extract (57%, P < 0.001). In terms of comparison, there was no statistical difference between the effects exerted by the phenolic extract and BHA (P > 0.05) (Fig. 3).
Effect of phenolic Basil extract and BHA on inhibition of copper-induced oxidation of sunflower oil
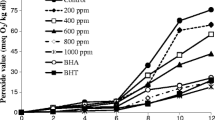
Using the accelerating sunflower oil oxidation model, we demonstrated that the copper induced a significant increase in oil oxidative process (+ 486%, P < 0.001) compared to the control (oil incubated without copper ion). However, the pre-treatment with phenolic Basil extract decreased the oil oxidation in a dose-dependent manner. Indeed, the phenolic extract reduced the amount of TBARS by 10%, 23%, 35%, 66%, 77% and 83% at doses of 100, 200, 400, 600, 800 and 1000 µg/mL, respectively (Fig. 4). The synthetic antioxidant BHA decreased the oil oxidation by 34%, 40%, 47%, 52%, 55%, and 63%, respectively, at the same concentrations listed above (Fig. 4). After calculation of IC50, we concluded that the protective effect exerted by the phenolic Basil extract (IC50 = 403.42 ± 5.2 µg/mL) was statistically less than that exerted by the BHA (IC50 = 445.85 ± 8.15 µg/mL) (P < 0.01).
Free radical scavenging activity of the phenolic Basil extract and BHA
The free radical scavenging is the most recognized mechanism by which antioxidants inhibit lipid peroxidation. In this work, we observed a positive correlation between the concentrations of phenolic Basil extract or BHA and the scavenging of DPPH radical (Fig. 5). The percentages of inhibition were 24%, 32%, 48%, 61%, 86%, 91.6% and 92% at doses of 0.5, 10, 25, 50, 100, 200 and 400 μg/mL of phenolic Basil extract, respectively. The BHA acts by 38%, 44%, 56%, 61%, 88%, 91% and 92% at the same concentrations described above. When comparing IC50 of the tested compounds, we concluded that the phenolic extract exerted a good anti-radical scavenging activity against the DPPH radical with an IC50 = 11.59 ± 1.26 μg/mL which was, however, significantly less than that recorded by BHA; IC50 = 5.16 ± 0.56 μg/mL (P = 0.001).
Effect of phenolic Basil extract and BHA on β-carotene oxidative bleaching
The β-carotene bleaching model indirectly measured the potential of tested samples to neutralize the lipoperoxyl radicals produced by the oxidation of linoleic acid. Our results showed that the phenolic Basil extract and BHA inhibit oxidation of linoleic acid and, consequently, the discoloration of β-carotene in a dose-dependent manner (Fig. 6). As can be seen, the extract inhibits oxidation process of linoleic acid by 16%, 38%, 48%, 57%, 69% 76% and 82% at doses of 0.5, 10, 25, 50, 100, 200 and 400 μg/mL, respectively. However, the BHA inhibits oxidation by 20%, 55%, 69%, 71%, 76%, 77 and 82% at the same concentrations. Comparing the effects of the tested compounds in inhibition of linoleic acid β-carotene-system oxidation, we note that the phenolic Basil extract presents a considerable activity with an IC50 = 20.45 ± 5.87 μg/mL. However, this activity is statistically lower than that of BHA, having an IC50 = 7.04 ± 1.24 μg/mL (P < 0.05).
Discussion
The Sunflower oil (Helianthus annuus L.) is rich in unsaturation represented especially by linoleic acid (48–74%) and oleic acid (14–39%), this property makes the oil more sensible to auto-oxidation and less stable at frying process (Madhujith and Sivakanthan 2019).
The auto-oxidation of edible oils during storage resulted from the reaction of unsaturated fatty acids and molecular oxygen involving a free-radical chain mechanism (Madhujith and Sivakanthan 2019). The unstable primary oxidative products, hydroperoxides, are rapidly decomposed to secondary products especially aldehydes, ketones, alcohols, and acids, which cause the rancidity of stored oil (Madhujith and Sivakanthan 2019). Furthermore, during the frying process, a number of undesirable reactions take place such as thermal oxidation, polymerization and hydrolysis of triglycerides. These reactions products especially ketones and polymer can significantly change the quality of frying oil (Gertz 2000).
Synthetic antioxidants were widely used by the food industry to prevent lipid oxidation; however, their use as food additives is strictly regulated today due to their harmful effects on human health (Madhujith and Sivakanthan 2019).
In this connection, spices are a lavish source of polyphenolic compounds that possess the strong antioxidant capacity and can be potentially used to substitute the synthetic antioxidants and confer some beneficial health effects (Horuz and Maskan 2015). In this context, we assayed the effect of phenolic Basil extract on sunflower oil peroxidation in comparison to the synthetic antioxidant BHA.
Thus, we demonstrated that the Basil extract significantly stabilizes the sunflower oil stored at 37 °C for 60 days or heated at frying temperature. Our results are in accordance with several previous works studying the beneficial effect of natural plant extracts in the stabilization of edible oils during storage. In fact, Mikołajczak et al. (2020) recently highlighted the effect of 23 edible flowers on the stabilization of flaxseed oil and chia seed oil. Furthermore, Asnaashari et al. (2015) demonstrated that the leaves extract from raspberry (Rubus fruticosus) showed a higher effect on the oxidative stability of sunflower oil. Another study data revealed the rosemary (Rosmarinus officinalis L.) extracts to be a potent antioxidant for stabilization of sunflower oil (Mezza et al. 2018). Furthermore, it was noticed by Başturk and coworkers (2018) that natural antioxidants (Salvia officinalis L., Mentha arvensis L., Rhus coriaria L., Thymus vulgaris) showed a positive effect on corn oil stability. After analyzing these works, we concluded that the preservative effect of natural plant extracts against oil oxidation was mainly due to their high content of phenolic compounds. These observations led us to suggest that the prevention of sunflower oil oxidation by the sweet basil extract might be due to its phenolic compounds, which are considered as major candidates acting as antioxidant agents on oil after long period storage or heating. In fact, we identified 10 phenolic compounds in the basil extract representing 71.23 mg/g. The most quantitatively represented are caftaric acid (38.3%), chicoric acid (12.7%), and rosmarinic acid (25.5%). According to this result, we suggest that the protective effect of the extract against oil deterioration could be especially due to these three major phenolic acids. Besides, this effect could also be defined as the result of additive activities from each compound acting alone or in synergy with the other phenolic compounds present in the extract and other antioxidants probably present in the oil. In this context, many previous reports suggested the implication of phenolic compounds such as carnosic acid, rosmarinic acid, quercetin, kaemferol, and myricetin in the oxidative stability of edible oils (Yanishlieva and Marinova 2001).
When we compared the stabilizing effect of the phenolic Basil extract and BHA, it was noticed that the plant extract was relatively less efficient than the BHA on stored oil at 37 °C for two months. We noted that the percentages of inhibition of the oxidative process exerted by BHA and the extract are very close while the difference is statistically significant. This difference could be explained by the fact that the phenolic Basil extract is not pure and can contain other compounds devoid of antioxidant activity. This hypothesis could be partially confirmed by the quantitative HPLC analysis showing that the extract contained only 71.23 mg polyphenol/g dry extract. This is in accordance with the results recently reported by Baştürk et al. (2018) when compared the effects of some herbal extracts on oxidative stability of corn oil under accelerated oxidation conditions in comparison with some commonly used antioxidants. However, when compared the exerted effects on oil stabilization under heating, we concluded that there was any statistical difference between the basil extract and BHA. This result is not contradictory to the first, but the heating conditions make BHA instable and very volatile; therefore its initial concentration decreases in the heated oil samples, and consequently, its antioxidant activity decreases. This instability of synthetic antioxidants is an inconvenient for their use in heated oil comparatively to natural compounds, which are more thermostables (Kahl and Kappus 1993).
To understand the mechanisms by which the phenolic Basil extract can prevent oil oxidation, we studied the copper ion-induced sunflower oil oxidation in the presence or absence of the plant extract, and we showed that this extract significantly inhibits copper-accelerating oil oxidation. The result led us to suggest that the phenolic compounds of Basil extract might be acting as potent copper chelator agents, the mechanism by which natural polyphenols can inhibit lipid oxidation. Indeed, the chelation of Cu2+ is a potent mechanism involved in the inhibition of free radical propagation and lipid peroxidation (Losada-Barreiro and Bravo-Diaz 2017). Our result agrees with previous reports demonstrating that polyphenols can act as a metal chelator and prevent lipid peroxidation. Gulcin et al. (2010) demonstrated that polyphenol-rich extract from propolis inhibited lipid peroxidation of a linoleic acid emulsion and possessed cupric ions (Cu2+) and ferric ions (Fe3+) reducing ability. Besides, sumac and thyme extracts significantly inhibited TBARS and conjugated dienes formation in corn oil under accelerated oxidation at 60 °C for 6 weeks (Baştürk et al. 2018). Recently, Mikołajczak et al. (2020) demonstrated that phenolic extracts from nasturtium, marigold scattered, dog rose, and daylilies flowers were characterized by a high DPPH radical inhibition value and improved oxidative stability of the cold-pressed flax and chia seed oils.
On the other hand, as demonstrated in this work, the preservation of sunflower oil against oxidation can also be explained by the anti-radical effect of the phenolic compounds present in the Basil extract. In fact, these compounds are able to exert a free radical neutralization resulting in the inhibition of polyunsaturated fatty acid peroxidation (Köksal et al. 2017). In general, the number and position of hydrogens donated by the aromatic ring of polyphenols directly determine their antioxidant capacities (Köksal et al. 2017). The free radical scavenging activities of the Basil extract might be due to the presence of phenolic acids with high ability to donate protons and stabilize the radicals. Furthermore, the property of the Basil extract to slow down the β-carotene bleaching indicated its antioxidant activity involving lipoperoxyl radicals scavenging and its ability to inhibit lipid peroxidation. This activity might be attributed mainly to the phenolic compounds, as demonstrated by Djenidi et al. (2020). The phenolic compounds can scavenge the alkyl peroxyl radical generated by auto-oxidation of linoleic acid, and then the β-carotene bleaching was stopped. The mechanism of the anti-β-carotene bleaching effect of phenolic compounds was based on hydrogen atom transfer (HAT). The HAT mechanism involves the abstraction of a hydrogen atom from a phenolic hydroxyl group by peroxyl radicals, which is followed by the rapid recombination of peroxyl and the resulting aryloxyl radicals to yield non-radical products (Losada-Barreiro and Bravo-Diaz 2017).
This study provided new and valuable data on the phenolic composition of sweet basil cultivated in Morocco as well as its antioxidant and preservative effect against sunflower oil oxidative degradation. Comparatively to BHA, the extract presents the advantage that it’s natural, safer and healthier that is why its use in oil stabilization can bring added value, unlike synthetic antioxidants. Besides, the extract contains natural polyphenols that are less volatile and more stable at high temperatures, which mean that they better support food production processes such as frying, cooking or baking. However, in the form of crud material, the extract remains relatively less efficient than the BHA.
Conclusion
This work revealed original data on the phenolic composition of Moroccan sweet basil and its protective effect against sunflower oil oxidation during storage and frying. We highlighted that the extract, rich in phenolic compounds, incorporated into edible sunflower oil improves its oxidative stability and extends its frying life. Moreover, the extract possesses potent scavenging activity against DPPH and lipoperoxyl radicals and inhibits copper-induced oil oxidation.
The HPLC–DAD method used in the characterization of the basil extract is more accurate, sensitive, easy to operate, reliable and reproducible compared with other analytical methods. Furthermore, all the methods used to study the anti-radical and the protective effects of the extract against oil oxidation are widely documented in the domain of food sciences for their sensibility and reproducibility. Based on this data, the Basil phenolic extract can be considered as a promising source of antioxidants. However, further investigations in the subject matter are needed to develop new and useful preservatives of natural origin and to enhance their practical applicability in vegetable oils and other food products. So, among the research necessary to improve the oil stabilizing effect of Basil extract is the purification of the most abundant phenolic compounds and their application alone or in combination as well as the research of possible toxic effects of such products.
References
Amrani S, Harnafi H, Bouanani N, Aziz M, Serghini Caid H, Manfredini S, Besco E, Napolitano M, Bravo E (2006) Hypolipidaemic activity of aqueous Ocimum basilicum extract in acute hyperlipidaemia induced by Triton WR-1339 in rats and its antioxidant property. Phytother Res 20:1040–1045. https://doi.org/10.1002/ptr.1961
Asnaashari M, Tajik R, Haddad Khodaparast MH (2015) Antioxidant activity of raspberry (Rubus fruticosus) leaves extract and its effect on oxidative stability of sunflower oil. J Food Sci Technol 52:5180–5187. https://doi.org/10.1007/s13197-014-1564-7
Baştürk A, Ceylan M, Çavuş M, Boran G, Javidipour I (2018) Effects of some herbal extracts on oxidative stability of corn oil under accelerated oxidation conditions in comparison with some commonly used antioxidants. LWT Food Sc Tech 89:358–364. https://doi.org/10.1016/j.lwt.2017.11.005
Bekkouch O, Harnafi M, Touiss I, Khatib S, Harnafi H, Alem C, Amrani S (2019) In vitro antioxidant and in vivo lipid-lowering properties of zingiber officinale crude aqueous extract and methanolic fraction: a follow-up study. Evidence-Based Compl Alt Med 2019:1–13. https://doi.org/10.1155/2019/9734390
Choe E, Min DB (2006) Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Saf 5:169–186. https://doi.org/10.1111/j.1541-4337.2006.00009.x
Djenidi H, Khennouf S, Bouaziz A (2020) Antioxidant activity and phenolic content of commonly consumed fruits and vegetables in Algeria. Progress Nutr 22: 224–235. https://doi.org/10.23751/pn.v22i1.7701
Figueroa JG, Borrás-Linares I, Lozano-Sánchez J, Segura-Carretero A (2018) Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res Int 105:752–763. https://doi.org/10.1016/j.foodres.2017.11.082
Fu Q, Tong C, Guo Y, Xu J, Shi F, Shi S, Xiao Y (2020) Flavonoid aglycone–oriented data-mining in high-performance liquid chromatography–quadrupole time-of-flight tandem mass spectrometry: efficient and targeted profiling of flavonoids in Scutellaria barbata. Anal Bioanal Chem 412:321–333. https://doi.org/10.1007/s00216-019-02238-72020
Gertz C (2000) Chemical and physical parameters as quality indicators of used frying fats. Eur J Lipid Sci Technol 102:566–657. https://doi.org/10.1002/1438-9312(200009)102:8/9%3C566:AID-EJLT566%3E3.0.CO;2-B
Giusti F, Caprioli G, Ricciutelli M, Vittori S, Sagratini G (2017) Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem 221:689–697. https://doi.org/10.1016/j.foodchem.2016.11.118
Gulcin I, Bursal E, Sehitoglu MH, Bilsel M, Goren AC (2010) Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem Toxicol 48:2227–2238. https://doi.org/10.1016/j.fct.2010.05.053
Horuz Tİ, Maskan M (2015) Effect of the phytochemicals curcumin, cinnamaldehyde, thymol and carvacrol on the oxidative stability of corn and palm oils at frying temperatures. J Food Sci Tech 52:8041–8049. https://doi.org/10.1007/s13197-015-1913-1
Kahl R, Kappus H (1993) Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Lebensm Unters Forsch 196:329–338. https://doi.org/10.1007/bf01197931
Köksal E, Bursal E, Gülçin İ, Korkmaz M, Çağlayan C, Gören AC, Alwasel SH (2017) Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int J Food Prop 20:514–525. https://doi.org/10.1080/10942912.2016.1168438
Kozłowska M, Gruczyńska E (2018) Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem Papers 72:2607–2615. https://doi.org/10.1007/s11696-018-0516-5
Kwee EM, Niemeyer ED (2011) Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem 128:1044–1050. https://doi.org/10.1016/j.foodchem.2011.04.011
Leouifoudi I, Harnafi H, Zyad A (2015) Olive mill waste extracts: polyphenols content, antioxidant, and antimicrobial activities. Adv Pharmacol Sci 2015:1–11. https://doi.org/10.1155/2015/714138
Li Y, Ngadi M, Oluka S (2008) Quality changes in mixtures of hydrogenated and non-hydrogenated oils during frying. J Sci Food Agr 88:1518–1523. https://doi.org/10.1002/jsfa.3239
Losada-Barreiro S, Bravo-Diaz C (2017) Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur J Med Chem 133:379–402. https://doi.org/10.1016/j.ejmech.2017.03.061
Madhujith T, Sivakanthan S (2019) Oxidative stability of edible plant oils. Ref Series Phytochem. https://doi.org/10.1007/978-3-319-78030-6_94
Mezza GN, Borgarello AV, Grosso NR, Fernandez H, Pramparo MC, Gayol MF (2018) Antioxidant activity of rosemary essential oil fractions obtained by molecular distillation and their effect on oxidative stability of sunflower oil. Food Chem 242:9–15. https://doi.org/10.1016/j.foodchem.2017.09.042
Mikołajczak N, Anna Sobiechowska D, Tańska M (2020) Edible flowers as a new source of natural antioxidants for oxidative protection of cold-pressed oils rich in omega-3 fatty acids. Food Res Int. https://doi.org/10.1016/j.foodres.2020.109216
Mira-Sánchez MD, Castillo-Sánchez J, Morillas-Ruiz JM (2020) Comparative study of rosemary extracts and several synthetic and natural food antioxidants. Relevance of carnosic acid/carnosol ratio. Food Chem. https://doi.org/10.1016/j.foodchem.2019.125688
Molnár-Perl L, Füzfai Z (2005) Chromatographic, capillary electrophoretic and capillary electrochromatographic techniques in the analysis of flavonoids. J Chromatogr A 1073:201–227. https://doi.org/10.1016/j.chroma.2004.10.068
Oliveira AS, Ribeiro-Santos R, Ramos F, Conceição Castilho M, Sanches-Silva A (2018) UHPLC-DAD multi-method for determination of phenolics in aromatic plants. Food Anal Methods 11:440–450. https://doi.org/10.1007/s12161-017-1015-y
Omoba OS, Olagunju AI, Salawu SO, Boligon AA (2019) HPLC-DAD phenolic profiling and in vitro antioxidant activities of three prominent nigerian spices. Prev Nutr Food Sci 24:179–186. https://doi.org/10.3746/pnf.2019.24.2.179
Purushothaman B, Prasanna Srinivasan R, Suganthi P, Ranganathan B, Gimbun J, Shanmugam KA (2018) Comprehensive review on Ocimum basilicum. J Nat Remedies 18: 71–85. https://doi.org/10.18311/jnr/2018/21324
Ramchoun M, Sellam K, Harnafi H, Alem C, Benlyas M, Khallouki F, Amrani S (2015) Investigation of antioxidant and antihemolytic properties of Thymus satureioides collected from Tafilalet Region, south-east of Morocco. Asian Pac J Trop Biomed 5:93–100. https://doi.org/10.1016/S2221-1691(15)30151-9
Rodregez Sá R, Matos RA, Silva VC, da Cruz CJ, da Silva SC, dos Santos WNL, de Freitas Santos Júnior A, (2017) Determination of bioactive phenolics in herbal medicines containing Cynara scolymus, Maytenus ilicifolia Mart ex Reiss and Ptychopetalum uncinatum by HPLC-DAD. Microchem J 135:10–15. https://doi.org/10.1016/j.microc.2017.07.009
Rodrigues Sá R, da Cruz Caldas J, de Andrade Santana D, Vieira Lopes M, dos Santos WNL, Graças Andrade Korn M, de Freitas Santos Júnior A (2019). Multielementar/centesimal composition and determination of bioactive phenolics in dried fruits and capsules containing Goji berries (Lycium barbarum L). Food Chem 273:15-23. https://doi.org/10.1016/j.foodchem.2018.05.124
Sestili P, Ismail T, Calcabrini C, Guescini M, Catanzaro E, Turrini E, Layla A, Akhtar S, Fimognari C (2018) The potential effects of Ocimum basilicum on health: a review of pharmacological and toxicological studies. Expert Opin Drug Metab Toxicol 14:679–692. https://doi.org/10.1080/17425255.2018.1484450
Soleas GJ, Yan J, Goldberg DM (2001) Ultrasensitive assay for three polyphenols (catechin, quercetin and resveratrol) and their conjugates in biological fluids utilizing gas chromatography with mass selective detection. J Chromatogr B 757:161–172. https://doi.org/10.1016/S0378-4347(01)00142-6
Taghvaei M, Jafari SM (2015) Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol 52:1272–1282. https://doi.org/10.1007/s13197-013-1080-1
Tsumbu CN, Deby-Dupont G, Tits M, Angenot L, Frederich M, Kohnen S, Mouithys-Mickalad A, Serteyn D, Franck T (2012) Polyphenol Content and Modulatory Activities of Some Tropical Dietary Plant Extracts on the Oxidant Activities of Neutrophils and Myeloperoxidase. Int J Mol Sci 13:628–650. https://doi.org/10.3390/ijms13010628
Wang Y-Z, Fu S-G, Wang S-Y, Yang D-J, Chen, Y-C (2018) Effects of a natural antioxidant, polyphenol-rich rosemary (Rosmarinus officinalis L.) extract, on lipid stability of plant derived omega-3 fatty-acid rich oil. LWT Food Sci Tech 89: 210–216. https://doi.org/10.1016/j.lwt.2017.10.055
Weisz GM, Kammerer DR, Carle R (2009) Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSn. Food Chem 115:758–765. https://doi.org/10.1016/j.foodchem.2008.12.074
Yanishlieva NV, Marinova EM (2001) Stabilisation of edible oils with natural antioxidants. Eur J Lip Sc Tech 103: 752–767. https://doi.org/10.1002/1438-9312(200111)103:11<752::aid-ejlt752>3.0.co;2-0
Acknowledgements
This work is part of a project funded by the CNRST (Centre National pour la Recherche Scientifique et Technique, Maroc) the UMP (Université Mohamed Premier, Oujda, Maroc) and the ANPMA (Agence Nationale des Plantes Medicinales et Aromatiques, Maroc).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khatib, S., Harnafi, M., Touiss, I. et al. HPLC–DAD profiling of a phenolic extract from Moroccan sweet Basil and its application as oxidative stabilizer of sunflower oil. Chem. Pap. 75, 1907–1917 (2021). https://doi.org/10.1007/s11696-020-01472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01472-z