Abstract
In this study, (S)-SEGPHOS was adopted as chiral extractant to recognize amino acid enantiomers. Phenylalanine (Pha), homophenylalanine (Hpha), 4-nitro-phenylalanine (Npha), phenylglycine (Phg), 3-chloro-phenylglycine (Cphg), and tyrosine (Tys) were selected as substrates to estimate the enantioselectivities of (S)-SEGPHOS-metal complexes. (S)-SEGPHOS-Pd was an excellent chiral extractant to recognize Npha with operational enantioselectivity (α) was 4.25. And (S)-SEGPHOS-Cu was a good chiral extractant to recognize Pha, Hpha, Phg, Tys, and Cphg with α were 2.87, 1.93, 2.28, 4.07, and 2.94, respectively. After optimization by response surface methodology, the highest performance factors (pf) for Pha, Hpha, Phg, Npha, Tys, and Cphg were 0.22893, 0.11085, 0.14003, 0.25476, 0.21414, and 0.23142, respectively. Based on experimental results, the possible recognize mechanisms were discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The enantiomers of some chiral drugs have different pharmacology activities in the treatment of human diseases. Thus, preparation of enantiomerically pure drugs is of great concern in pharmaceutical industry (Ager 2005; Sheldon 1993; Herráez-Hernández and Campins-Falcó 2000). Nowadays, the common methods for preparation of enantiomerically pure compounds are natural sources, asymmetric catalysis, and raceme separation. Separation of racemic mixture is a useful technique. For instance, crystallization (Tulashie et al. 2010), chromatography (Schulte and Strube 2001), capillary electrophoresis (Zhou et al. 1995), and chiral extraction (Schweitzer et al. 1968; Bowman et al. 1968) are widely used in the separation of enantiomers. Among them, chiral extraction is a potentially attractive method which is cheaper and easier to scale up to a commercial scale and has a large application range. Since the chiral extraction is a highly useful resolution method, it is not surprising that considerable efforts have been undertaken for finding new and efficient chiral extractants.
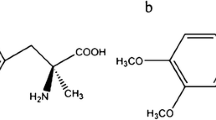
(S)-SEGPHOS is a famous chiral diphosphine ligand that has exhibited high stereoselectivity in asymmetric synthesis. For instance, (S)-SEGPHOS-metal complex is an effective chiral catalyst in asymmetric carbonyl-ene reaction (Mikami et al. 2004), hydroamination (Hu et al. 2010), and hydrogenation (Wang et al. 2010). The chemical structure of (S)-SEGPHOS is shown in Fig. 1. Recently, some chiral diphosphine ligands, such as (S)-BINAP and (S)-MeO-BIPHEP, have exhibited promising application in enantioseparation of amino acid enantiomers. For instance, (S)-BINAP-Cu was an excellent chiral extractant to separate phenylalanine with α of 5.203 (Tang et al. 2012a, b). (S)-MeO-BIPHEP-Cu showed considerable ability to separate tyrosine with α of 4.22 (Liu et al. 2019a). (S)-SEGPHOS also belongs to chiral diphosphine ligand and it has been proved to be effective chiral catalyst in asymmetric reaction. It might be another ligand that has potential application in enantioseparation of amino acid enantiomers.
In this work, (S)-SEGPHOS was introduced to separate dl-amino acids. Phenylalanine (Pha), homophenylalanine (Hpha), 4-nitro-phenylalanine (Npha), phenylglycine (Phg), 3-chloro-phenylglycine (Cphg), and tyrosine (Tys) were employed as substrates to evaluate the enantioselectivity of (S)-SEGPHOS. The chemical structures of these amino acids are shown in Fig. 2. The optimum conditions of resolution of dl-amino acids were determined. Moreover, the possible chiral recognition mechanisms were discussed.
Experimental
Materials
Amino acid enantiomers, methanol (HPLC), (S)-SEGPHOS, (CH3CN)2PdCl2, [(CH3CN)4Cu]PF6, and [(C6H5)3P]2NiCl2 were purchased from J & K Chemical Technology. Other reagents (analytic grade unless stated otherwise) were purchased from Sinopharm Chemical Reagent Co., Ltd (China). All reagents were used without further treatment.
Preparation of organic phase and aqueous phase
The (S)-SEGPHOS-metal complexes in organic phase were prepared as follows: Briefly, the mixtures of (S)-SEGPHOS (0.1 mmol) and metal precursors (0.1 mmol, (CH3CN)2PdCl2, [(CH3CN)4Cu]PF6 or [(C6H5)3P]2NiCl2) were dissolved and stirred in 100 mL organic solvent. After 12 h, the organic phase was obtained with (S)-SEGPHOS-metal molarity of 1.0 mmol/L. The amino acid enantiomers in aqueous phase were prepared as follows: dl-amino acid (0.1 mmol) was dissolved in 50 mL 0.1 mol/L PBS (pH 6–10) to obtain aqueous phase with molarity of 2.0 mmol/L.
Chiral extraction of amino acids
The typical chiral extraction procedure was: 2.0 mL (S)-SEGPHOS-metal complexes solution and 2.0 mL amino acid enantiomer solution were added into a 10 mL tube. Then, the tube was shaken in a water-bathing constant temperature vibrator for 12 h at temperature of 5–25 °C. Let stand for 12 h, the water solution was filtered by filter membrane (0.45 μm). The concentrations of amino acid enantiomers in samples were detected by HPLC method that described in our precious work (Liu et al. 2019a). The contents of dl-amino acids were obtained considering the mean value of triplicate assays.
Calculation of operation parameters
The distribution ratios (kL and kD), operational enantioselectivity (α), enantiomeric excess (ee), fraction of enantiomer (f), and performance factor (pf) were calculated according to Eqs. (1), (2), (3), (4), (5), and (6), respectively:
where CL,w and CD,w represent the concentrations of l- and d-amino acid in aqueous phase at equilibrium, respectively. CL,org and CD,org represent the concentrations of l- and d-amino acid in organic phase at equilibrium, respectively. Ci,org represents the concentration of enantiomer in organic phase at equilibrium. Ci represents the initial total concentration of enantiomer in aqueous phase (Ma et al. 2019).
Optimization methods
The chiral extraction processes were optimized by response surface methodology. Box–Behnken design, a well-known second-order symmetrical design, was employed to optimize the chiral extraction processes (Bezerra et al. 2008). Numerous variables may affect the response of the system studied. In this work, the screened variables were selected according to the results of single-factor experiments. The response in extraction was pf. To determine a maximum response, quadratic polynomial model is selected as the polynomial function to fit the experimental data and it is presented below:
where b0 is constant, and bi, bii, and bij are the linear, quadratic, and interactive regression coefficients of the model, respectively. Xi are the screened variables (Bezerra et al. 2008).
Results and discussion
Effects of metal precursors on extractions
Table 1 shows the effects of metal precursors on extractions. (S)-SEGPHOS-Pd exhibited good ability to recognize Npha with α was 3.31. (S)-SEGPHOS-Cu exhibited good abilities to recognize Pha, Hpha, Phg, Tys, and Cphg with α were 2.87, 1.93, 2.28, 4.07, and 2.94, respectively. (S)-SEGPHOS-Ni complex showed poor abilities with α were all below 1.4. In addition, when (S)-SEGPHOS-Pd complex was used as extractant, kD were bigger than kL. This result indicated that d-amino acids were preferentially recognized by (S)-SEGPHOS-Pd complexes. In contrast, the values of kL were all bigger than kD when (S)-SEGPHOS-Cu complex was used as extractant. l-Amino acids were preferentially recognized by (S)-SEGPHOS-Cu complex. This phenomenon suggested that the preferred enantiomers for (S)-SEGPHOS could be changed using different metal precursors. As a whole, (S)-SEGPHOS-Pd complex was selected to recognize Npha, and (S)-SEGPHOS-Cu complex was selected to recognize Pha, Hpha, Phg, Tys, and Cphg.
Effects of solvents on extractions
The noncovalent interactions and the conformation of the chiral partners in enantioselective host–guest complexation can be influenced by organic solvent. Thus, the influences of solvents on extractions were investigated. The results in Table 2 showed that the highest α for Pha, Hpha, Phg, Tys, and Cphg were obtained when 1,2-dichloroethane was used as organic solvent. And the highest α for Npha was obtained when dichloromethane was used as organic solvent. In addition, the lowest α were obtained when chlorobenzene was used as solvent. This is probably because that π–π interaction between extractants and substrates is an important factor in chiral recognition (Liu et al. 2019b, c). The π–π interaction between (S)-SEGPHOS-metal complexes and amino acids would be weakened by chlorobenzene. Therefore, 1,2-dichloroethane was chosen as the suitable solvent for extraction of Pha, Hpha, Phg, Tys, and Cphg. Dichloromethane was adopted as the suitable solvent for extraction of Npha.
Effects of pH on extractions
Basic group (amino) and acidic group (carboxyl) in amino acids could be affected by pH. In this study, the stereoselectivities of (S)-SEGPHOS with different pH were investigated and the results are shown in Fig. 3. The change trends of kL and kD revealed that distribution ratios (k) increased substantially with the rising of pH ranging from 6 to 10. This result indicated that (S)-SEGPHOS-metal complexes preferred to combine with anionic amino acids. The α values for Pha, Hpha, Phg, Npha, Tys, and Cphg were around 2.8, 2.0, 2.2, 3.5, 3.9, and 2.7, respectively, indicating that (S)-SEGPHOS-metal complexes retained high stereoselectivities at high pH. Figure 3c reveals that the change trends of ee were inversely correlated to pH. The results of Fig. 3a, c revealed that high distribution ratios were obtained at high pH while high ee were obtained at low pH. Therefore, it was hard to select the optimum pH. Performance factor (pf) could be adopted to comprehensively estimate the efficiency of chiral extractant. High pf means that the enantiomer is purified with high purity and high yield simultaneously (Koska and Haynes 2001). As shown in Fig. 3d, high pf for Pha, Hpha, Phg, Npha, Tys, and Cphg were obtained with pH in range of 8–10, 7–9, 7–9, 7–9, 6.5–8.5, and 9–11, respectively.
Effects of extractant concentration on extractions
Figure 4 shows the effects of extractant concentration on extractions. With the increasing of extractant concentration, there were more ligand sites that could combine with substrates. Thus, concentration had positive effect on k values. The curves of α indicated that (S)-SEGPHOS-metal complexes retained good abilities to recognize Pha, Hpha, Phg, Npha, Tys, and Cphg with α were about 3.2, 2.0, 2.1, 3.0, 3.3, and 3.4, respectively. The curves of ee indicated that high ee were obtained at low concentration. When extractant concentration was low, competition between d- and l-enantiomers was high. Thus, high ee was obtained. (S)-SEGPHOS-metal concentrations exhibited positive effects on k but negative effects on ee. The influences of concentration on pf were also tested. The optimal concentrations of (S)-SEGPHOS-Cu complex for Pha, Hpha, Phg, Tys, and Cphg were 1.0–3.0 mmol/L. And the optimal concentration of (S)-SEGPHOS-Pd complex for Npha was 0.5–1.5 mmol/L.
Effects of temperature on extractions
Figure 5 shows the change trends of k, α, ee, and pf with extraction temperature in range of 5–25 °C. The curves of kL and kD in Fig. 5a revealed that there were more enantiomers distributed from aqueous phase to organic phase with the rising of temperature. The possible reason for the increase of distribution ratios is that the physical distribution coefficient of enantiomers in organic phase is higher at high temperature. The increase in temperature weakened the discrimination ability of the (S)-SEGPHOS-metal complexes toward amino acid enantiomers because of the intensified thermal motion of the extractants and the enantiomers. Thus, an increasing temperature led to the reduction of α and ee. The change trends of pf indicated that the higher pf were obtained at temperature of 5–15 °C.
Optimization of the extractions
Based on the results discussed above, temperature (X1), (S)-SEGPHOS-metal concentration (X2), and pH (X3) were selected as variables. Three levels were applied to each variable as tabulated in Table 3.
Table 4 shows the experimental data obtained in Box–Behnken design. The experimental data were fitted to a second-order polynomial model and regression coefficients were obtained. The fitness of model to the responses was evaluated by analysis of variance (Chew et al. 2017; Liu et al. 2014; Long et al. 2013; Park et al. 2007). The models for Pha, Hpha, Phg, Npha, Tys, and Cphg were given as Eqs. (8)–(13), respectively:
Table 5 shows the P values of regression coefficients for above models. The P values of models were all below 0.05, indicating that models (Eqs. 8–13) showed good approximation. In addition, lack-of-fit analysis (P > 0.05) revealed that equations were adequately fitted to experimental data. The factors which affect pf significantly could also be determined by P values. For instance, pf of Pha was affected significantly by concentration (X2), quadratic levels of temperature (X21), concentration (X22), and pH (X23), interaction among quadratic levels of temperature and concentration (X21X2). Taking into account the significant factors, Eqs. (8)–(13) can be simplified to Eqs. (14)–(19), respectively:
Figure 6 shows the response surface plots of Pha (I), Hpha (II), Phg (III), Npha (IV), Tys (V), and Cphg (VI). For instance, Fig. 6(III) shows the response surface plots of Phg. Figure 6a3, b3 reveals that the change trends of pf increased rapidly with the rising of temperature (5–10 °C), and then decreased with further increasing of temperature (10–15 °C). Figure 6a3, c3 indicates that pf slightly increased and then sharply decreased with the increase in concentration. Figure 6b3, c3 shows that pf slightly increased with pH increasing from 7.0 to 7.5, and then decreased with pH above 7.5. Higher pf were obtained at pH around 7.5. For Pha, Hpha, Npha, Tys, and Cphg, the effects of temperature/concentration, temperature/pH, and concentration/pH on pf have also been fully displayed in Fig. 6(I), (II), (IV)–(VI), respectively.
According to Eqs. (14)–(19), the optimal parameters in extractions were determined by numerical optimization. As shown in Table 6, the optimal extraction condition for Pha was temperature 5.0 °C, extractant concentration 2.1 mmol/L, and pH 10.0 with predicted pf of 0.22484. The experimental pf were also determined at their optimum extraction conditions. The experimental pf for Pha, Hpha, Phg, Npha, Tys, and Cphg were 0.22893, 0.11085, 0.14003, 0.25476, 0.21414, and 0.23142, respectively. The relative errors of Pha, Hpha, Phg, Npha, Tys, and Cphg were 1.78%, 2.38%, 2.42%, 3.07%, 0.17%, and 3.29%, respectively, indicating that the models were sufficient to predict the values of pf.
Comparison of enantioselectivities between (S)-BINAP, (S)-MeO-BIPHEP, and (S)-SEGPHOS
A comparison of the enantioselectivities of (S)-SEGPHOS obtained in this work with those of (S)-BINAP and (S)-MeO-BIPHEP reported by literature was analyzed. (S)-BINAP showed excellent ability to recognize Pha with α of 5.203 (Tang et al. 2012a, b). (S)-MeO-BIPHEP had good ability to recognize Tys with α of 4.22 (Liu et al., 2019a). (S)-SEGPHOS showed good ability to recognize Npha and Cphg with α of 4.25 and 2.94, respectively. For Hpha and Phg, there were no significant differences on enantioselectivities between these three chiral diphosphine ligands. The above results indicated that the steric and/or electronic properties have significant influence on enantioselectivities of chiral extractants. The highest enantioselectivity is obtained when extractant and substrate have matching stereochemical structure and electronic property.
The possible recognize mechanisms
The possible chiral recognition mechanisms between (S)-SEGPHOS-metal complexes and dl-amino acids are shown in Fig. 7. The results in Table 1 revealed that π–π interaction between phenyl groups in (S)-SEGPHOS and substrate was an important acting force in extraction. The k values in Fig. 3a revealed that (S)-SEGPHOS-metal complexes preferred to combine with anionic substrates. Therefore, the coordination between carboxyl and metal ion was another important acting force in extraction (Liu et al. 2019b, c). The stereoscopic structures of tetradentate copper and palladium have big difference. Tetradentate copper has a tetrahedral configuration and tetradentate palladium has a quadrilateral configuration (Otter et al. 1998; Aslanidis et al. 1993). In chiral recognition, L-amino acid could coordinate with (S)-SEGPHOS-Cu to form a tetrahedral configuration. The combining capacity between l-amino acid and (S)-SEGPHOS-Cu was higher in extraction. Thus, l-amino acids were preferentially recognized by (S)-SEGPHOS-Cu. When (S)-SEGPHOS-Pd was used as extractant, amino acid could not coordinate with (S)-SEGPHOS-Pd to form a quadrilateral configuration. The steric hindrance between –NH2 and CH3CN would also weaken the combining capacity between l-amino acid and (S)-SEGPHOS-Pd. In addition, –NH2 in d-amino acid could form hydrogen bond with H2O. Thus, kD were higher than kL when (S)-SEGPHOS-Pd was used as extractant.
Conclusions
(S)-SEGPHOS-metal complexes, similar to other chiral diphosphine ligands, also showed good abilities to recognize amino acid enantiomers. (S)-SEGPHOS-Pd exhibited excellent ability to recognize Npha with α of 4.25. And (S)-SEGPHOS-Cu exhibited good abilities to recognize Pha, Hpha, Phg, Tys, and Cphg with α were 2.87, 1.93, 2.28, 4.07, and 2.94, respectively. After optimization by response surface methodology, the optimal extraction conditions were obtained and the maximum experimental pf for Pha, Hpha, Phg, Npha, Tys, and Cphg were 0.22893, 0.11085, 0.14003, 0.25476, 0.21414, and 0.23142, respectively. The tetrahedral configuration of Cu(I) and quadrilateral configuration of Pd(II) lead to the preferred enantiomers for (S)-SEGPHOS-Cu and (S)-SEGPHOS-Pd are l-amino acids and d-amino acids, respectively. This work provided a new chiral extractant that had good application in enantioseparation of amino acid enantiomers. And it has potential application in recognition of chiral drugs which has structural unit of amino acid.
References
Ager DJ (2005) Handbook of chiral chemicals, 2nd edn. CRC Press, Boca Raton
Aslanidis P, Hadjikakou SK, Karagiannidis P (1993) Four-coordinate copper(I) iodide complexes with triphenylphosphine and heterocyclic thione ligands. The crystal structure of [Cu(PPh3)2(pymtH)I]. Polyhedron 12(18):2221–2226. https://doi.org/10.1016/S0277-5387(00)88260-1
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5):965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Bowman NS, McCloud GT, Schweitzer GK (1968) Partial resolution of some organic racemates by solvent extraction. J Am Chem Soc 90(14):3848–3852. https://doi.org/10.1021/ja01016a046
Chew S-C, Tan C-P, Nyam K-L (2017) Application of response surface methodology for optimizing the deodorization parameters in chemical refining of kenaf seed oil. Sep Purif Technol 184:144–151. https://doi.org/10.1016/j.seppur.2017.04.044
Herráez-Hernández R, Campı́ns-Falcó P (2000) Chromatographic separation of chlorthalidone enantiomers using β-cyclodextrins as chiral additives. J Chromatogr B 740(2):169–177. https://doi.org/10.1016/S0378-4347(00)00103-1
Hu A, Ogasawara M, Sakamoto T, Okada A, Nakajima K, Takahashi T, Lin W (2010) Palladium-catalyzed intermolecular asymmetric hydroamination with 4,4′-disubstituted BINAP and SEGPHOS. Adv Synth Catal 348(15):2051–2056. https://doi.org/10.1002/adsc.200606208
Koska J, Haynes CA (2001) Modelling multiple chemical equilbria in chiral partition systems. Chem Eng Sci 56(20):5853–5864. https://doi.org/10.1016/S0009-2509(00)00419-X
Liu X, Yang D-L, Liu J-J, Xu K, Wu G-H (2014) Modeling of supercritical fluid extraction of flavonoids from Calycopteris floribunda leaves. Chem Pap 68(3):316–323. https://doi.org/10.2478/s11696-013-0451-4
Liu X, Ma Y, Cao T, Tan D, Wei X, Yang J, Yu L (2019a) Enantioselective liquid–liquid extraction of amino acid enantiomers using (S)-MeO-BIPHEP–metal complexes as chiral extractants. Sep Purifi Technol 211:189–197. https://doi.org/10.1016/j.seppur.2018.09.068
Liu X, Ma Y, Liu Q, Wei X, Yang J, Yu L (2019b) Chiral extraction of amino acid and mandelic acid enantiomers using chiral diphosphine ligands with tunable dihedral angles. Sep Purif Technol 221:159–165. https://doi.org/10.1016/j.seppur.2019.03.085
Liu X, Ma Y, Xu L, Liu Q (2019c) Enantioselective liquid–liquid extraction of 3-chloro-phenylglycine enantiomers using (S,S)-DIOP as extractant. Chirality 31(9):750–758. https://doi.org/10.1002/chir.23111
Long A, Zhang H, Lei Y (2013) Surfactant flushing remediation of toluene contaminated soil: optimization with response surface methodology and surfactant recovery by selective oxidation with sulfate radicals. Sep Purif Technol 118:612–619. https://doi.org/10.1016/j.seppur.2013.08.001
Ma Y, Liu X, Zhou W, Cao T (2019) Enantioselective liquid-liquid extraction of dl-mandelic acid using chiral diphosphine ligands as extractants. Chirality 31(3):248–255. https://doi.org/10.1002/chir.23054
Mikami K, Aikawa K, Kainuma S, Kawakami Y, Saito T, Sayo N, Kumobayashi H (2004) Enantioselective catalysis of carbonyl-ene and Friedel-Crafts reactions with trifluoropyruvate by ‘naked’ palladium(II) complexes with SEGPHOS ligands. Tetrahedron Asymmetry 15(24):3885–3889. https://doi.org/10.1016/j.tetasy.2004.10.022
Otter CA, Bardwell DA, Couchman SM, Jeffery JC, Maher JP, Ward MD (1998) Coordination chemistry of mixed pyridine-phenol ligands; mononuclear palladium (II) and dinuclear copper (II) complexes of derivatives of bidentate N, O-chelating ligands based on 2-(2-hydroxyphenyl) pyridine. Polyhedron 17(2–3):211–220. https://doi.org/10.1016/S0378-4347(00)00103-1
Park PK, Kim EY, Chu KH (2007) Chemical disruption of yeast cells for the isolation of carotenoid pigments. Sep Purif Technol 53(2):148–152. https://doi.org/10.1016/j.seppur.2006.06.026
Schulte M, Strube J (2001) Preparative enantioseparation by simulated moving bed chromatography. J Chromatogr A 906:5824–5828. https://doi.org/10.1016/S0021-9673(00)00956-0
Schweitzer GK, Supernaw IR, Bowman NS (1968) The solvent extraction resolution of some optically-active neutral chelates. J Inorg Nucl Chem 30(7):1885–1890. https://doi.org/10.1016/0022-1902(68)80364-1
Sheldon RA (1993) Chirotechnology: industrial synthesis of optically active compounds. Marcel Dekker Press, New York
Tang K, Fu T, Zhang P (2012a) Equilibrium studies on enantioselective liquid–liquid extraction of homophenylalanine enantiomers with metal-BINAP complexes. Pro Biochem 47(12):2275–2283. https://doi.org/10.1016/j.procbio.2012.09.004
Tang K, Fu T, Zhang P (2012b) Equilibrium studies on enantioselective liquid–liquid extraction of phenylalanine enantiomers using BINAP–metal complexes. J Chem Eng Data 57(12):3628–3635. https://doi.org/10.1021/je300846m
Tulashie SK, Lorenz H, Seidel-Morgenstern A (2010) Solubility of mandelic acid enantiomers and their mixtures in three chiral solvents. J Chem Eng Data 55:5196–5200. https://doi.org/10.1021/je1006955
Wang DW, Wang DS, Chen QA, Zhou YG (2010) Asymmetric hydrogenation with water/silane as the hydrogen source. Chemistry 16(4):1133–1136. https://doi.org/10.1002/chem.200902790
Zhou SY, Zuo H, Stobaugh JF, Lunte CE, Lunte SM (1995) Continuous in vivo monitoring of amino acid neurotransmitters by microdialysis sampling with online derivatization and capillary electrophoresis separation. Anal Chem 67:594–599. https://doi.org/10.1021/ac00099a017
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant no. 51703060) and Graduate Student Research Innovation Fund of Hunan Province (Grant no. CX2018B673).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, Xiong Liu states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Ma, Y., Cao, T. et al. Chiral extraction of amino acid enantiomers using (S)-SEGPHOS-metal complexes as extractants. Chem. Pap. 74, 1229–1239 (2020). https://doi.org/10.1007/s11696-019-00970-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00970-z