Abstract
The sequential ring-opening polymerizations (ROP) of ε-caprolactone (ε-CL) and L-lactide (LLA) with benzo-12-crown-4-imidazole carbene (B-12-C-4imY) as the catalyst have been performed. Using either benzyl alcohol or ethylene glycol as an initiator, the corresponding poly(ε-caprolactone)-poly(L-lactide) (PCL-b-PLLA) diblock or poly(L-lactide)-poly(ε-caprolactone)-poly(L-lactide) (PLLA-PCL-PLLA) triblock copolymers were easily prepared. The results indicated that B-12-C-4imY was quite effective for the copolymerization. The diblock copolymerization of ε-CL with LLA could only be achieved when ε-CL was first polymerized followed by LLA. Feeding the two monomers simultaneously, however, only resulted in the formation of LLA homopolymers. Thermogravimetric analysis (TGA) measurements demonstrated that block copolymers exhibited the decomposition temperature lower than the PCL homopolymer. The copolymers were characterized by 1H NMR and 13C NMR, FT-IR, GPC, and DSC analyses. 20 × 10 mm2 rectangular specimens made of the triblock copolymer were allowed to degrade in a pH = 7.4 phosphate buffer at 37 °C. Degradation was monitored by various analytical techniques such as GPC, IR, and ESEM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past decades, aliphatic polyesters have attracted much attention, because they are biodegradable in the human body as well as in the Earth’s environment. Among all the aliphatic polyesters, poly(ε-caprolactone) (PCL), poly(L-Lactide) (PLLA), and their copolymers have been widely used in medical applications, for instance, in bone fracture fixation, biodegradable sutures, drug delivery systems, and tissue temporary scaffolds [1,2,3,4]. PCL exhibits remarkable drug permeability, good elasticity, and thermal properties, however, its degradation rate is very slow. On the contrary, PLLA has good mechanical properties but it is hardly permeable to most drugs, and its half-life time is much shorter than that of PCL [5,6,7]. Thus, random or block copolymerization of ε-CL with LLA may lead to a wide range of polyesters, exhibiting various degradation behavior, permeability, mechanical properties, etc. Random copolymerization is known to provide new materials with properties intermediate between those of the parent homopolymers [8]. In contrast to random copolymers, block copolymers are generally multiphase materials that provide for additivity of the phase properties [9]. The block copolymerization of ε-CL and LLA is an effective way of combining the permeability of the PCL segments and the rapid biodegradation of the PLLA components. Furthermore, the thermal and mechanical properties of PCL and PLLA also are adjusted and balanced by modification of monomer composition and molecular weight of the block copolymers.
Most polyesters are generally produced by the ROP of cyclic esters with metallic catalysts based on Al, Zn, Ti, Sn and Ca [10,11,12,13,14]. The cytotoxicity and difficulty in removal of the catalysts from the obtained polymers have limited their use in many cases [15]. Therefore, many efforts have been devoted to the development of new catalysts and/or initiators that would be well tolerated by the organism [16, 17]. Organocatalysts provide a metal-free approach to obtain well-defined polyesters, which can be carried out under mild conditions [18, 19]. As compared to metal-based catalysts, organocatalysts are usually insensitive to oxidation and resistant to hydrolysis but readily prepared. Among the successful organocatalysts for block ROP of ε-CL and LLA include 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)/4-(dimethylamino)pyridine (DMAP)/trifluoroacetic acid (TFA) system [20], diphenyl phosphate (DPP)/DMAP binary catalyst [21], methanesulfonic acid (MSA) or DPP/8-diazabicyclo[5.4.0]undec-7-ene (DBU) binary catalyst [22], and N-heterocyclic carbenes (NHCs). Recent studies have shown that organocatalysts NHCs have emerged enormous potential for the synthesis of block copolymers due to their structural diversity and high activity under relatively mild reaction conditions [23,24,25,26]. In 2003, Waymouth et al. [27] have successfully prepared the diblock copolymers of ε-CL and LLA catalyzed by 1,3-bis(phenyl)imidazolin-2-ylidene/BnOH system. In 2014, Zhang et al. [28] has reported the synthesis of 1-isopropyl-3-(4-methoxyphenyl)imidazol-2-ylidene which was an effective catalyst for diblock and triblock copolymerization of ε-CL and LLA. Nevertheless, the ROP of cyclic esters using NHCs substituted by the aliphatic and aromatic groups was usually accompanied by transesterification reactions at high conversion, thus resulting in less controlled ROP processes and broad distribution products [29]. In view of above deficiency, we introduced crown ethers into NHCs. Crown ethers are the most common host molecules in supramolecular chemistry and possess unique performance on the special recognition of small molecules and metal ions. Hence, the incorporation of macrocyclic host benzocrown ethers could vary their nucleophilic or steric hindrance properties of NHCs selectively and promoted the synthesis of polyesters with desirable properties.
In the present contribution, we report the ability of B-12-C-4imY to promote the sequential ring-opening copolymerization of ε-CL and LLA under mild conditions. The thermal properties and structures of PCL-b-PLLA and PLLA-PCL-PLLA copolymers were measured. A proposed monomer activation mechanism will also be discussed. The hydrolytic degradation of triblock copolymers was studied compared with that of PCL homopolymers.
Experimental
Materials
ε-CL which was provided by Alfa Aesar was purified by vacuum distillation over calcium hydride (CaH2) and stored over 4 Å molecular sieves under nitrogen. LLA was synthesized from L-lactic acid and purified by recrystallization in ethyl acetate three times,then dried for 48 h in vacuum at 40 °C. Tetrahydrofuran (THF) was freshly distilled from sodium-benzophenone. Benzyl alcohol and ethylene glycol were dried over CaH2 for 2 days, then distilled under reduced pressure and kept over activated 4 Å molecular sieves. B-12-C-4imY catalyst was prepared according to literature [30].
Copolymerization
PCL–b-PLLA copolymer was synthesized by ROP of successively added ε-CL and LLA using B-12-C-4imY as catalyst. In a typical procedure, ε-CL (0.70 g, 6.13 mmol) was dissolved in THF (3.38 mL) in previously flamed and argon-purged 20 mL ampoules using Schlenk techniques. The initiator, benzyl alcohol was added. After the injection of B-12-C-4imY catalyst, the mixture was reacted at 20 °C for 20 min. Upon completion of the reaction, a THF solution of LLA (0.88 g, 6.13 mmol) was introduced. After the desired reaction time, the polymer was precipitated by methanol and dried under vacuum to constant weight. PLLA-PCL-PLLA copolymer was prepared by a similar method. Ethylene glycol was used as the initiator in synthesis of PLLA-PCL-PLLA.
Measurements and characterization
1H and 13C NMR spectra were obtained from a Bruker ASCENDTM 600 MHz spectrometer instrument with CDCl3 as a solvent and chemical shifts were reported in ppm relative to tetramethylsilane (Me4Si) as an internal standard.
Thermal stability of the copolymer was examined using TGA (Metler Toledo TGA/DSC1). The temperature was ramped to 550 °C at 10 °C/min under a continuous nitrogen flow rate of 50 mL/min, using Al2O3 crucibles and 10 mg of sample for each analysis.
The thermal property of the polymer was measured on a NETZCH DSC 200F3 system under a nitrogen atmosphere. Sample was loaded into an aluminum DSC pans and initially heated from 20 °C to 150 °C to erase thermal history at a heating rate of 10 °C/min, followed by cooling to −60 at 40 °C/min after stopping at 150 °C for 1 min and finally heating to 150 °C at 10 °C/min.
The number-average molecular weight (Mn) and the polydispersity index (PDI) were measured on a PL-GPC220 gel permeation chromatography machine equipped with two columns (PL gel 10 μm MIXED-B 300 mm × 7.5 mm, PL gel 10 μm Guard 50 mm × 7.5 mm). The analysis was performed with THF as an eluent at a flow rate of 1.0 mL/min. The narrow polystyrene standards were used to calibrate the standard curve of molecular weight.
The surface morphology of films was examined by JSM-7500F Field Emission Scanning Electron Microscope (ESEM), micrographs being obtained under reduced pressure at room temperature.
Hydrolytic degradation
A 10 w/v % PLLA-PCL-PLLA or PCL solution in dichloromethane was used to cast the circular films of 85 mm diameter with 0.1 mm thickness, respectively. Films were dried in a vacuum oven at 40 °C for 48 h. Then the rectanglular specimens (20 × 10 mm2) were cut from the parent film. Each dried films were immersed in phosphate-buffered solution (PBS) (pH = 7.4) at a temperature of 37 °C. The hydrolyzed samples were subsequently weighted at several times.
Results and discussion
Synthesis of PCL-b-PLLA block copolymer
The sequential ring-opening copolymerization of ε-CL and LLA was investigated with B-12-C-4imY, combined to a benzyl alcohol as an initiator. The diblock copolymerization was successful only when ε-CL needed to be polymerized before LLA due to much lower reactivity and the fact that it could not be initiated by end group of PLLA in this case. (Table 1, NO.1). In contrast, attempt to prepare the diblock copolymer in the reverse order (Table 1, NO.2) or to synthesize random copolymer by simultaneously polymerizing a mixture of ε-CL and LLA failed, both yielding exclusively homopolymer of LLA. (Table 1, NO.3). Thus, it was essential to follow the order of CL-LLA. The IR spectra of NO.1, NO.2, NO.3 polymers are shown in Fig. 1. The main difference between PCL-b-PLLA and PLLA in the IR spectra was the carbonyl absorption band region. In the IR spectrum of NO.1, the carbonyl absorption band became wide and split into two peaks. The peak at 1759 cm−1 corresponded to the carbonyl absorption of PLLA units, while the peak at 1728 cm−1 was assigned to the carbonyl absorption of PCL units. It was important to prove that the PLLA chains existed in the copolymer as a block.
The block copolymerization was carried out with B-12-C-4imY in THF using different catalyst concentrations and the results are compiled in Table 2. The monomer conversion and molecular weight of copolymer increased with the increasing catalyst concentration from 5.00 × 10−3 to 7.50 × 10−3 mol/L. With higher catalyst concentration, the monomer conversion and molecular weight of PCL-b-PLLA decreased and the molecular weight distribution broadened. This is may be due to yielding more and shorter polymeric chains caused by the formation of more active species at the higher catalyst concentration.
The effects of the polymerization temperature and reaction time on the copolymerization are listed in Table 3. The results indicate that 25 °C and 40 min are the most suitable conditions for the copolymerization of ε-CL and LLA in THF. When the polymerization was conducted at 25 °C for a longer reaction time, the polymer with lower molecular weight and broader molecular weight distribution was obtained, which might be due to the transesterification reaction during the polymerization process. Increasing reaction temperature accelerated the polymerization, but the polymers produced were easily degradable.
Synthesis of PLLA-PCL-PLLA block copolymer
The influence of the monomer/catalyst ([M]/[C]) molar ratio on the triblock copolymerization is shown in Table 4. The yield and the molecular weight of copolymer changed from 83.9% to 89.7% and 38.7 kg/mol to 41.3 kg/mol, respectively, with increasing [M]/[C] molar ratio below 350 (Table 4, entries 1–3). At higher [M]/[C] molar ratio (>350, Table 4, entries 4–5), the molecular weight dropped owing to decreasing number of active species. Therefore, the favorable [M]/[C] molar ratio was 350.
Table 5 illustrates that the optimum temperature and time of the triblock copolymerization are about 25 °C and 40 min. It was also found that a further prolongation of the reaction time (Table 5, entries 4–5) resulted in lower molecular weight and broader molecular weight distribution because the usually slow transesterification was not negligible after reaching the reaction equilibrium. Higher temperature (>25 °C, Table 5, entries 8–9) accelerated the rate of intermolecular transesterification and thermal degradation reaction and brought about decreasing molecular weight of PLLA-PCL-PLLA, whereas below the optimum temperature (Table 5, entries 6–7) the polymerization reaction proceeded slowly.
Copolymers characterizations
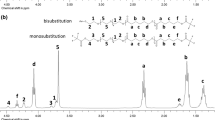
The 1H NMR spectrum of PCL-b-PLLA is shown in Fig. 2a. The peak assigned to the methylene protons (-CH2-OH) of the PCL block at 3.62 ppm disappeared, and a resonance at 4.36 ppm (i) corresponding to the formed PLLA end group was found, which indicated that the terminal hydroxyl group of the PCL macroinitiator successfully initiated the polymerization of LLA. In addition, typical proton signals of PLLA and PCL components in PLLA-PCL-PLLA spectrum (Fig. 2b) were observed. Signals at 1.40 (g) and 5.1 ppm (f) were assigned to PLLA blocks, 1.3, 1.6, 2.30 and 4.06 ppm (d, c, b, e) to the different methylene protons (-CH2-) of PCL blocks, respectively.
The 13C NMR spectra (Fig. 3) of PCL-b-PLLA and PLLA-PCL-PLLA revealed only two resonances between 168 and 175 ppm, one of the PCL carbonyl at 173.7 ppm and the other of the PLA carbonyl at 169.6 ppm. All these indicated that a transesterification reaction of PCL did not occur during the ROP of LLA to obtain the block copolymers PCL-b-PLLA and PLLA-PCL-PLLA.
Figure 4 exhibits the GPC traces of PCL-b-PLLA (a), PCL (b), PLLA-PCL-PLLA (c) and HO-PCL-OH (d). For the PCL-b-PLLA and PLLA-PCL-PLLA copolymers, the elution time decreased after the addition and polymerization of LLA, implying the successive growth of PLLA blocks. All tested samples showed single peak, no other small peaks. These observations further demonstrated that the purified copolymers were pure block copolymers.
The DSC curves of the PLLA-PCL-PLLA (A), PCL-b-PLLA (B), PLLA (C) and PCL (D) are presented in Fig. 5. The PCL-b-PLLA diblock copolymer showed a double melting peak at 55.8 and 156.1 °C, reflecting the presence of two crystalline domains. The melting peaks of PCL block and PLLA block were identified at 52.8 °C and 160.8 °C, respectively, corresponding to the melting peaks of PLLA-PCL-PLLA in the crystal phase. Moreover, the melting points of PCL-b-PLLA and PLLA-PCL-PLLA were different from those of their corresponding homopolymers. This suggested that the crystallization of PCL block was restricted by the crystallized PLLA block. Meanwhile, the crystallization of the PLLA block was also restricted by the crystallized PCL block. These results indicated that the crystallization of both the PCL and the PLLA blocks mutually interfered with each other.
The determination of the decomposition characteristics of the copolymers were performed by TGA. Also, the TGA analyses of the PCL homopolymer (A), PCL-b-PLLA (B) and PLLA-PCL-PLLA (C) were recorded for comparison, as seen in Fig. 6. Figure 6a shows the TGA curves for PCL, PCL-b-PLLA and PLLA-PCL-PLLA at 10 °C/min and Fig. 6b illustrates the first derivative TGA (DTG) curves for PCL, PCL-b-PLLA and PLLA-PCL-PLLA. Table 6 summarized the degradation temperatures at 5% weight loss (T5) and the temperatures at the maximum mass loss rate (Tmax) for PCL, PCL-b-PLLA and PLLA-PCL-PLLA from the TGA and DTG curves. It can be seen from Table 6 and Fig. 6 that T5 and Tmax of PCL were 370.9 and 410.4 °C, respectively. However, T5 and Tmax of PCL-b-PLLA were 278.6 and 358.1 °C, and T5 and Tmax of PLLA-PCL-PLLA were 298.1 and 347.5 °C, respectively. Obviously, T5 and Tmax of PCL-b-PLLA and PLLA-PCL-PLLA were lower than PCL. This can be attributed to the poorer thermal stability of the PLLA segments than that of the PCL segments in PCL-b-PLLA and PLLA-PCL-PLLA.
Mechanism
The mechanism of the diblock copolymerization of ε-CL with LLA is illustrated in Scheme 1. The NHC ring opens the ε-CL monomer to form a zwitterionic acylimidazole intermediate. According to the mechanism, after proton transfer from the alcohol initiator and subsequent displacement of the bound catalyst by the as-generated alkoxide (RO), a new chain-extended alcohol is generated, with concomitant release of the NHC. The α-chain end of PCL bears the ester from initiating BnOH and ω-chain end is a hydroxyl group. Propagation then proceeds in the same way, i.e. by reaction of the chain-extended alcohol with an activated monomer. The hydroxyl-terminated PCLs as macroinitiators for the ROP of LLA, finally generate high molecular weight polymers with narrow molecular weight distribution [28, 31].
Degradation behavior
The formed films of PCL-b-PLLA and PLLA polymers were not cut into rectangular dimensions because they easily cracked down. Thus, the PLLA-PCL-PLLA triblock copolymer and PCL homopolymer as objects were undertaken for hydrolysis studies. All the sample films were introduced into tubes filled with pH = 7.4 PBS. The tubes were placed in the thermostated water-bath at 37 °C.
Figure 7 presents the weight loss profiles of the PLLA-PCL-PLLA copolymers and PCL homopolymers in the course of PBS degradation. As could be seen, PLLA-PCL-PLLA copolymers showed the rapid weight loss rate. After 120 days, PLLA-PCL-PLLA lost 2.3% of its initial weight. Afterwards, weight loss increased progressively to reach 4.6% at the end of 180 days. In contrast, PCL presents lower degradation rate, 1.9% of weight loss being obtained after 180 days. It was found that PLLA-PCL-PLLA copolymers exhibited higher degradability than PCL homopolymers. This reason could be assigned to the lower overall crystallinity of PLLA-PCL-PLLA as compared to PCL.
The molecular weight change induced by hydrolysis can be more clearly seen in GPC spectra. Figure 8 shows GPC spectra of the PLLA-PCL-PLLA films hydrolyzed for different periods of time. There was no significant change in Mn of the copolymer as degradation proceeds up to 60 days. Apparently beyond 60 days, the position of the initial main peak did not change during hydrolysis, but the PDI gradually became broader. The PDI increased from initial 1.25 to 2.8 at day 240, which was ascribe to the presence of PLA-oligomers. As evident from GPC spectra of PCL, the molecular weight decrease rate of PCL was very slow. Meanwhile, Mn of PCL decreased from initial 4.31 kg/mol to 3.2 kg/mol at day 240, while the PDI remained almost unchanged.
IR analysis is a means to examine qualitatively compositional changes of the PLLA-PCL-PLLA copolymers. Figure 9 presents the IR spectra of PLLA-PCL-PLLA copolymers before and after degradation. The copolymers showed initially two the carbonyl stretching bands at 1757 cm−1 (PLLA) and 1725 cm−1 (PCL), and C-H stretching bands at 2995 cm−1 (PLLA) and at 2867 cm−1 (PCL). On the spectrum at day 60, the bands at 1757 cm−1 (PLLA) and at 1725 cm−1 significantly decreased, indicating the losses of PLLA component and PCL component. At the same time,it was observed that the intensity of band decreased rapidly at 2995, 2867 cm−1. At 150 days, the IR spectrum was in accord with 60 days. However, after hydrolysis 210 days, the intensity bands at 1757 and 1725 cm−1 remain at the same level.
The films of PLLA-PCL-PLLA and PCL were initially opaque due to crystallization. After immersion in a buffer of pH 7.4 at 37 °C, they became whitish and brittle. Morphology was followed by ESEM which provided a convenient method to monitor the changes of surface morphology during degradation. The surface of PLLA-PCL-PLLA film initially appeared rather smooth. No obvious spherulites were observed (Fig. 10a). After 60 days, spherulites with distinct boundaries were detected (Fig. 10b), which may be attributed to PCL block since PCL crystallizes more easily than PLLA as shown in Fig. 5. When the film was subjected to hydrolyze above 150 days, the ESEM image of copolymer appeared the larger size of spherulites (Fig. 10c, d). Appearance of spherulites probably resulted from internal erosion which removed the amorphous domains from the internal. However, the surface of PCL films had no distinct spherulites were observed within 60 days degradation (not shown). As discussed above, the spherulite should be attributed to PCL block, taking into account the amorphousness of PLLA block. The degradation of PLLA-PCL-PLLA copolymers followed a general diffusion-reaction mechanism. The amorphous PLLA region in copolymers was firstly hydrolyzed, and yielded PCL-rich crystalline polymer residues in the film that will require much longer time period to be resorbed in phosphate-buffered solution. These results are in agreement with weight losses of the films.
Conclusions
In summary, B-12-C-4imY is highly efficient organocatalyst for the block copolymerization of ε-CL and LLA under mild condition. More importantly, well-defined block copolymers (PCL-b-PLLA and PLLA-PCL-PLLA) exhibited predictable Mn and relatively narrow PDI, and their structures were proved by 1H, 13C NMR and IR spectral data. Furthermore, the TGA and DSC analyses showed that the thermal stability of block copolymers were poorer than PCL homopolymers. Obviously, this work provides an effective avenue to synthesize block copolymers with well-defined structures and potential applications as advanced materials. The hydrolytic degradation of the copolymers was performed in PBS at 37 °C. The introduction of PLLA component increased the rate of degradation compared to PCL film.
References
Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J (2005) Synthesis and evaluation of a star amphiphilic block copolymer from poly(ε-caprolactone) and poly(ethylene glycol) as a potential drug delivery carrier. Bioconjug Chem 16:397–405
Ho MH, Hou LT, Tu CY, Hsieh HJ, Lai JY, Chen WJ, Wang DM (2006) Promotion of cell affinity of porous PLLA scaffolds by immobilization of RGD peptides via plasma treatment. Macromol Biosci 6:90–98
Meng FL, Zheng SX, Zhang WA, Li HQ, Liang Q (2006) Nanostructured thermosetting blends of epoxy resin and amphiphilic poly(ε-caprolactone)-block-polybutadiene-block-poly(ε-caprolactone) triblock copolymer. Macromolecules 39:711–719
Wang CH, Hsiue GH (2005) Polymer-DNA hybrid nanoparticles based on folate-polyethylenimine-block-poly(L-lactide). Bioconjug Chem 16:391–396
Albertsson AC, Varma IK (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4:1466–1486
Arbaoui A, Redshaw C (2010) Metal catalysts for ε-caprolactone polymerization. Polym Chem 1:801–826
Dijkstra PJ, Du HZ, Feijen J (2011) Single site catalysts for stereoselective ring-opening polymerization of lactides. Polym Chem 2:520–527
Pitt CG, Marks TA, Schindler A (1980) Biodegradable drug delivery systems based on aliphatic polyesters: application to contraceptives and narcotic antagonists. In: Baker R (ed) Controlled release of bioactive materials. Academic Press, New York
Riess C, Hurtrez C, Bahadur P (1985) Block copolymers. Encyclopedia of polymer science and engineering 2nd edn. Wiley, New York
Wilson JA, Hopkins SA, Wright PM, Dove AP (2015) Synthesis of ω-pentadecalactone copolymers with independently tunable thermal and degradation behavior. Macromolecules 48:950–958
Dechy-Cabaret O, Martin-Vaca B, Bourissou D (2004) Controlled ring-opening polymerization of lactide and glycolide. Chem Rev 104:6147–6176
Bouyahyi M, Duchateau R (2014) Metal-based catalysts for controlled ring-opening polymerization of macrolactones: high molecular weight and well-defined copolymer architectures. Macromolecules 47:517–524
Pérez Y, del Hierro I, Zazo L, Fernández-Galán R, Fajardo M (2015) The catalytic performance of metal complexes immobilized on SBA-15 in the ring opening polymerization of ε-caprolactone with different metals (Ti, Al, Zn and mg) and immobilization procedures. Dalton Trans 44:4088–4101
Gilmour DJ, Webster RL, Perry MR, Schafer LL (2015) Titanium pyridonates for the homo- and copolymerization of rac-lactide and ε-caprolactone. Dalton Trans 44:12411–12419
Platel RH, Hodgson LM, Williams CK (2008) Biocompatible initiators for lactide polymerization. Polym Rev 48:11–63
Penczek S, Cypryk M, Duda A, Kubisa P, Slomkowski S (2007) Living ring-opening polymerizations of heterocyclic monomers. Prog Polym Sci 32:247–282
Gupta AP, Kumar V (2007) New emerging trends in synthetic biodegradable polymers-polylactide: a critique. Eur Polym J 43:4053–4074
Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL (2007) Organocatalytic ring-opening polymerization. Chem Rev 107:5813–5840
Ottou WN, Sardon H, Mecerreyes D, Vignolle J, Taton D (2016) Update and challenges in organo-mediated polymerization reactions. Prog Polym Sci 56:64–115
Guillerm B, Lemaur V, Ernould B, Cornil J, Lazzaroni R, Gohy J-F, Dubois P, Coulembier O (2014) A one-pot two-step efficient metal-free process for the generation of PEO-b-PCL-b-PLA amphiphilic triblock copolymers. RSC Adv 4:10028–10038
Makiguchi K, Kikuchi S, Yanai K, Ogasawara Y, Sato S, Satoh T, Kakuchi T (2014) Diphenyl phosphate/4-dimethylaminopyridine as an efficient binary organocatalyst system for controlled/living ring-opening polymerization of L-lacitde leading to diblock and end-functionalized poly(L-lactide)s. J Polym Sci, Part A: Polym Chem 52:1047–1054
Wang X, Liu JQ, Xu SQ, Xu JX, Pan XF, Liu JJ, Cui SD, Li ZJ, Guo K (2016) Tranceless switch organocatalysis enables multiblock ring-opening copolymerizations of lactones, carbonates, and lactides: by a one plus one approach in one pot. Polym Chem 7:6297–6308
Dove AP, Pratt RC, Lohmeijer BGG, Culkin DA, Hagberg EC, Nyce GW, Waymouth RM, Hedrick JL (2006) N-heterocyclic carbenes: effective organic catalysts for living polymerization. Polymer 47:4018–4025
Xiao XD, Bai YL, Liu JQ, Wang JW (2016) Synthesis of novel pillar[5]arene-based N-heterocyclic carbene ligands for Pd-catalysed heck reactions. Tetrahedron Lett 57:3385–3388
Coulembier O, Mespouille L, Hedrick JL, Waymouth RM, Dubois P (2006) Metal-free catalyzed ring-opening polymerization of β-lactones: synthesis of amphiphilic triblock copolymers based on poly(dimethylmalic acid). Macromolecules 39:4001–4008
Raynaud J, Absalon C, Gnanou Y, Taton D (2009) N-heterocyclic carbene-induced zwitterionic ring-opening polymerization of ethylene oxide and direct synthesis of α, ω-difunctionalized poly(ethylene oxide)s and poly(ethylene oxide)-b-poly(ε-caprolactone) block copolymers. J Am Soc 131:3201–3209
Nyce GW, Glauser T, Connor EF, Mock A, Waymouth RM, Hedrick JL (2003) In situ generation of carbenes: a general and versatile platform for organocatalytic living polymerization. J Am Chem Soc 125:3046–3056
Zhang LF, Li N, Wang Y, Guo JZ, Li JF (2014) Ring-opening block copolymerization of ε-caprolactone with L-lactide catalyzed by N-heterocyclic carbenes: synthesis, characteristics, mechanism. Macromol Res 22:600–605
Kamber NE, Jeong W, Gonzalez S, Hedrick JL, Waymouth RM (2009) N-heterocyclic carbene for the organocatalytic ring-opening polymerization of ε-caprolactone. Macromolecules 42:1634–1639
Bai JH, Wu N, Wang Y, Li QR, Wang XQ, Zhang LF (2016) Triblock and pentablock copolymerizations of ε-caprolactone with L-lactide catalyzed by N-heterocyclic carbene. RSC Adv 6:108045–108050
Coulembier O, Lohmeijer BGG, Dove AP, Pratt RC, Mespouille L, Culkin DA, Benight SJ, Dubois P, Waymouth RM, Hedrick JL (2006) Alcohol adducts of N-heterocyclic carbenes: latent catalysts for the thermally-controlled living polymerization of cyclic esters. Macromolecules 39:5617–5628
Acknowledgements
This work was supported by Basic Research Project of Shanxi Province of China (No.2015011029), Undergraduate Innovative Experiment Program of Shanxi Normal University (No.SD2014CXXM-36) and Shanxi Province Education Innovation Project for Postgraduate (No.2015BY38).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Wu, N., Bai, J. et al. Diblock and triblock copolymers catalyzed by benzo-12-crown-4 bridged N-heterocyclic carbene: synthesis, characterization and degradation behavior. J Polym Res 25, 254 (2018). https://doi.org/10.1007/s10965-018-1639-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1639-7