Abstract
The three-dimensional structure of hydrogels plays a leading role in several areas of applications. The hydrogels are more and more used as systems of immobilized and controlled release of biomolecules in biotechnology and bio-pharmacy industries. To improve protein adsorption capacity in poly(acrylamide) hydrogels, maleic acid co-monomer was included into the reaction mixture during hydrogel synthesis. So, hydrogels of poly(acrylamide) and its copolymers with diprotic maleic acid were prepared by copolymerization and chemical crosslinking with N,N′-methylene bis-acrylamide. Swelling behavior in distilled water, in physiological saline and in bovine serum albumin (BSA) solutions was studied. Influence of initial BSA concentration on hydrogel swelling and BSA adsorption was investigated. The high amount of maleic acid present in the hydrogels has a significant effect on the swelling behavior and BSA adsorption. Results showed that the pH sensitivity of hydrogels resulted in the high amount of adsorbed BSA. The adsorption isotherms were described by Langmuir and Freundlich models. The thermodynamic parameter (ΔG 0ads ) was determined for all obtained hydrogels. We demonstrated the favorable character and reversibility of the BSA adsorption process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Immobilization of labile bioactive substances, cells and molecules on porous support is a valuable technique used in different domains (Sassolas et al. 2012). Certain molecules like biomolecules and food ingredients are sensitive to their environment (temperature, pH and/or other parameters) and can be easily denatured with time. To preserve their natural activity, these biomolecules are protected by immobilization in a specific material (Pothakamury and Barbosa-Cánovas 1995). Immobilization technique ensures compatibility and stability of biomolecules and allows tailored a specific application. It can be used for control the release profile of bioactive compound and can cover an unpleasant odor or taste of biomolecules. Immobilized biomolecules are used in food technology (protein protection), in biotechnology (enzymes protection) and bio-pharmacy (protection and controlled release of drugs) (Chen et al. 2006; Murty et al. 2002). The improvement of novel systems able to immobilize large amounts of biomolecules represented an intensive and popular research field in the last years.
Hydrogels, especially poly(acrylamide), have found broad applications in immobilized enzyme systems and in biomolecule separation processes (Pollak et al. 1980; Kan et al. 2014; Zhao et al. 2014, 2015). Hydrogel is a crosslinked hydrophilic macromolecule which can be able to absorb huge amounts of fluids without changing their form. To improve biomolecules uptake capacity in poly(acrylamide) hydrogels, a co-monomer carrying some specific functional groups can be included into the reaction mixture during polymer synthesis. As an example, hydrogels of acrylamide and acidic comonomers can be prepared by co-polymerizing acrylamide (AAm) with a pH-sensitive comonomer, such as acrylic (AAc) or diprotic acids from maleic (MA) or itaconic (IA) acids (Solpan et al. 2003; Rintoul and Wandrey 2005; Ling and Lu 2008). The biocompatibility and non-toxicity of maleic acid have promoted its use for the preparation of pH-sensitive copolymers with acrylamide. The advantage of maleic acid is the great hydrophilicity; it has two ionizable groups with different pKa, showing a more pronounced pH sensitivity of the system. The addition of maleic acid in the poly(acrylamide) hydrogel can produce physically interactions between the hydrogel and the immobilized biomolecule, which can significantly increase the biomolecule uptake in hydrogel (Mellott et al. 2001).
The biomolecule can be immobilized onto hydrogels over several of methods and approaches. Among these, the swelling of hydrogels at equilibrium state in a biomolecule-containing solution is the most generally used approach. This procedure depends on water absorption capacity and changes in drug diffusivity within the matrix. Swelling increases polymer flexibility and makes pores bigger, resulting in higher drug mobility (Censi et al. 2012). Biomolecule of bovine serum albumin (BSA) was chosen as a model protein because it is well characterized and commonly used in protein adsorption studies (Ekici 2011).
In this work, we focus on how the anionic co-monomer acidity affects the sensitive protein adsorption, their uptake and the swelling behavior in BSA solution of the acrylamide-based hydrogels. For this, copolymers based on acrylamide (AAm) and maleic acid (MA) co-monomers were prepared by free radical crosslinking copolymerization at higher ratios of diprotic acid. The copolymers synthesized were used for BSA adsorption. The kinetic of swelling in BSA solution has been studied.
Experimental
Materials
Acrylamide (AAm) and maleic acid (MA) were supplied by Panreac Chemicals and were used as monomers. Reagents N,N′-methylene-bis-acrylamide (BisAAm), potassium persulfate (KPS) and N,N,N′,N′-tetramethyl ethylene diamine (TEMED) were supplied by Aldrich Chemicals and used as received.
Albumin from bovine serum (BSA), supplied by Aldrich Chemicals, was used as model protein for swelling and adsorption experiments. NaCl (Panreac Chemicals) used for salt solutions was used as received.
Copolymer hydrogels synthesis
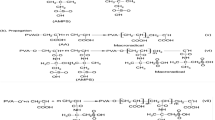
Hydrogels of AAm and MA were prepared by free radical crosslinking copolymerization procedure in distilled water which is the solvent for all components of the primary mixture. Aqueous solutions of monomers (AAm and MA) and crosslinking agent (NBisAAm) were prepared in 10 mL of distilled water at different mass percentages of AAm and MA, respectively: (100, 0%), (90, 10%), (80, 20%) and (70, 30%). The weight ratios of AAm/MA in the initial mixture were (g/g): (1/0), (0.9/0.1), (0.8/0.2) and (0.7/0.3) for samples noted MA00; MA10; MA20 and MA30, respectively. Concentration of the crosslinking agent was typically 1.0 wt%. Amounts of KPS (0.6 wt%) and TEMED were added to solution mixture. The solution mixture was prepared under N2 atmosphere. The reaction was kept out for 24 h in the glass tubes at 25 °C. After the reaction, crosslinked copolymers were cut into portions of about 5 mm length, then washed continually with distilled water for 3 days and finally dried in a vacuum oven at 35 °C.
Determination of the acid value of hydrogels (AVm)
The acid value of hydrogel samples is measured by volumetric titration method where the amount of carboxylic acids (mg) per gram (g) of polymer was determined. Mainly, the carboxylic functions (−COOH) were titrated by alkali solution: the dried samples (25 mg) were swollen in 50 mL distilled water and treated with 0.1 N NaOH solution in the presence of phenolphthalein. The acid values of hydrogels (AVm ) are summarized in Table 1.
Swelling studies
It is known that the swelling is the main characteristic for hydrogels. For this reason, we will use this parameter to study the immobilization of BSA in hydrogel.
The synthesized hydrogels were placed in the following liquids distilled water, physiological saline (0.9% NaCl), 2 g L−1 BSA solution in distilled water (BSA-water) and 2 g L−1 BSA solution in physiological saline (BSA-NaCl 0.9%), to swell until equilibrium at room temperature. The swelling degree, at a time interval t, is assessed by weighing the samples after and before immersing in solutions. All the experiments were carried out in triplicate and the average values have been reported in the data.
The mass swelling degree was calculated using the following equation (Saraydin et al. 1995)
where M 0 and M t are the weights of the dry gel at time 0 and the swollen gel at time t, respectively.
The influence of initial concentration of BSA on hydrogel swelling
The variation of equilibrium swelling degree of hydrogels as a function of the BSA concentration was studied. The dried samples were placed in 25 mL of BSA solution at various concentrations varied from 0 to 8 g L−1 and allowed to swell until their equilibrium value, at room temperature. The hydrogels equilibrium swelling was determined for each BSA concentration and for each sample; results are shown in Fig. 2.
Effect of initial BSA concentration and MA content on BSA adsorption; adsorption isotherm analysis
During tests of BSA immobilization, the hydrogels may undergo cycles of swelling and adsorption of the protein at the same time. In order to determine the maximum amount of BSA adsorbed, a study of adsorption at fixed temperature was carried out on hydrogels synthesized. Practically, weighed hydrogels are placed, at room temperature, in 25 mL BSA solution at corresponding concentration (0–8 g L−1). The hydrogels swell and adsorb the BSA up to a fixed value. The amounts of adsorbed BSA are evaluated for each hydrogel and concentration. Results are shown in Fig. 3.
Adsorption of BSA
For the adsorption measurements, the dried hydrogels (0.1 g) were swollen in 2 g L−1 aqueous BSA solution (BSA-water) and in 2 g L−1 saline BSA solution (BSA-NaCl 0.9%), at 25 °C until equilibrium.
The total amount of BSA loaded in the hydrogels can be determined by the mass change of BSA in the liquid before and after adsorption, using the Bradford method at 595 nm (Bradford 1976). It was calculated by the following equation (Demirel 2007):
C 0 and C e are the initial and equilibrium concentration of BSA after adsorption (mg mL−1), V volume of the BSA solution (mL) and W p mass of dried hydrogel (g), respectively.
Modeling of the adsorption isotherms
Modeling of the adsorption isotherms enable the reading of the q t = f(C e) curves. The data were analyzed by using conventional models equations such as Langmuir and Freundlich models. The linear forms of Langmuir and Freundlich equations are (Foo and Hameed 2010):
where q max, K L and C e are the amount of adsorption at equilibrium (mg g−1), the Langmuir constant (L g−1) and equilibrium BSA concentration (g L−1), respectively. And:
K F and n are Freundlich constants. 1/n is the heterogeneity factor of the surface, which is a measure of the deviation from linearity of the adsorption; it specifies the intensity of adsorption; higher is 1/n value, more favorable is the adsorption.
One essential feature of the Langmuir isotherm can be expressed in terms of a dimensionless constant called separation factor R L (also called equilibrium parameter), which is defined by the following equation (Hall et al. 1966):
C 0 is the initial BSA concentration (g L−1).
The value of R L indicates that the shape of the isotherms is unfavorable (R L > 1), linear (R L = 1), favorable (0 < R L < 1), or nonreversible (R L = 0) (Weber and Chakravorti 1974).
Determination of the standard adsorption free energy (ΔG 0ads )
The bonding nature of molecules to the surface of a solid via adsorption is evaluated by the change in the thermodynamic parameters such as Gibbs free energy, enthalpy and entropy of adsorption. The standard adsorption free energy (ΔG 0ads ) is the fundamental parameter which provides a complete thermodynamic description of the system. This parameter determines whether the process is spontaneous or not. It was given by the following equation:
ΔG 0ads is the change of standard free energy of adsorption (J mol−1); R, T and K 0 are the universal gas constant (8314 J mol−1 K−1), the absolute temperature in Kelvin and the thermodynamic equilibrium constant, respectively. K 0 is a dimensionless parameter and can be got from the Langmuir isotherm using the correction method described by different authors (Zhou and Zhou 2014; Bentiss et al. 2005). A negative value of ΔG 0ads concluded that the process of adsorption was spontaneous (Chen et al. 2013).
Results and discussion
Copolymer hydrogel synthesis
Copolymeric hydrogels were prepared with different percentages of acrylamide and maleic acid by free radical copolymerization/crosslinking in aqueous solution using the same amounts of crosslinking agent (NBisAAm) and redox system as initiator (KPS + TEMED).
From literature, the amide function of acrylamide monomer has a great affinity and compatibility with carboxyl groups (maleic acid). Therefore, the gel based on acrylamide and maleic acid is obtained and decreases with increase in the amount of diprotic maleic acid. The estimated acid values of our hydrogels indicate that the concentration of carboxylic acid within hydrogels increases with the initial weight of diprotic maleic acid in the total weight of monomers reacting. Correspondingly, they are varying from 14 to 107 mg g−1 with 0 to 300 mg of maleic acid (Table 1). The first value of AVm corresponds to MA00 hydrogel which does not contain maleic acid in its structure. This is probably due to the hydrolysis of amide groups of acrylamide monomer during the polymerization. Same results about hydrolysis of acrylamide during the reaction were reported by Kasgöza et al. (2005).
Swelling studies
The swelling curves of poly (AAm-co-MA) hydrogels in distilled water, physiological saline, BSA-water solution and BSA-NaCl 0.9% solution are shown in Fig. 1 named a, b, c and d, respectively.
The hydrophilicity of hydrogels is ensured by the carboxylic groups attached to amide functions of acrylamide. In this case, the swelling occurs through electrostatic repulsion between anions present within the network and by the polymer elasticity (Mahdavinia et al. 2004, 2012).
The kinetic study of the hydrogels swelling shows a rise of swelling with increase in the acid content, except for the MA30 hydrogel where the swelling was slow with a low rate. But once it reaches its equilibrium state, it exceeds the other values (see Table 1). This must be due to the hydrogen bonding between the carboxyl and amide groups within the network; increase in the amount of MA in the gel increases the hydrogen bonding in the system.
The swelling in saline solution was appreciably decreased comparing to those swelling in distilled water. The counter ions Na+ take up places next the carboxylate sites and limit the formation of hydrogen bonding between (–COO−) and water molecules. This limitation results in a decrease in repulsive forces among (–COO−) groups along polymeric segments, which reduce the osmotic pressure and hence the swollen hydrogels shrink dramatically. These results show the ionic character and pH sensitivity of hydrogels.
The swelling of the poly(AAm-co-MA) in the BSA solution is lower than that in the distilled water because of the difference on osmotic pressure between water and BSA solution. The liquid uptake by the hydrogels increases with time, but after a certain time it reach equilibrium.
The equilibrium swelling degrees (EDS) of poly(AAm) and its copolymer hydrogels in different mediums are determined using the following equation:
M eq and M 0 are the mass of swollen gel at equilibrium and mass of dry gel.
Results are shown in Table 1. As expected, EDS values of the copolymers were higher than that of pure poly(AAm). The presence of BSA and Na+ in external solution decreases the electrostatic repulsion in the anionic matrix and allows physical links between them, resulting in the decrease of hydrogel swelling. The EDS values of all hydrogels decrease in the following order:
Kinetic diffusion studies
To clarify the nature of solute diffusion within hydrogel, Peppas have suggested a semi-empirical equation (Peppas et al. 2000):
This equation is valid for initial swelling, when S t /S max < 0.6.
K S and n are gel specific constant and characteristic exponent for the solute mode transfer.
The diffusion coefficient D (cm s−1) can be assessed by the following relationship (Karadag and Saraydin 2002):
where r is the radius of cylindrical polymer sample (cm). D can be got from the slope of the straight lines of S t versus t 1/2 (Table 2).
Table 2 shows the effect of acid content and nature of media on the kind of solute diffusion within hydrogels. From this table, it is observed that all values of n are more than 0.5, indicating that all samples follow non-Fickian diffusion. In this case, the diffusion of water, NaCl and BSA is similar to the relaxation of polymer chains. In distilled water and for MA20 hydrogel, the value of n is approximately close to 1, corresponding to case-II diffusion. As the diffusion type is case-II mechanism, the relaxation of polymer chains is slower than the diffusion mechanism. The greater n values (n > 0.5) indicate that the swelling ratios were larger and hydrogels will absorb solvent faster.
The D values were affected by the nature of external medium and hydrogel composition; mainly values of D increased with an increase in the amount of MA monomer. This is due to ability of hydrophilic carboxyl groups to stimulate the solvent diffusion within the matrix.
Influence of BSA initial concentration on hydrogel swelling
BSA adsorption onto hydrogels mainly depends on the swelling behavior of the hydrogel; higher swelling degree suggests larger amount of BSA adsorbed. Results analysis of Fig. 2 shows that at 2 g L−1 BSA concentration the equilibrium degree of swelling drop dramatically, and as BSA concentration increase the hydrogels EDS becomes stable.
The copolymer hydrogels show a significant decrease in their degree of swelling when the BSA concentration exceeds 2 g L−1. After this limit, the swelling is consistent and stable for all hydrogels. This concentration (2 g L−1) should be chosen for BSA adsorption onto obtained hydrogels. Whatever the initial BSA concentration, the swelling of hydrogels increases with an increase in the amount of MA comonomer.
Effect of initial BSA concentration and MA content on BSA adsorption; adsorption isotherm analysis
The effect of the initial BSA concentration on the amount of BSA adsorbed onto poly(AAm-co-MA) hydrogels is shown in Fig. 3.
As seen in the figure, the amount of BSA adsorbed increases with the increase in the MA content for all initial BSA concentrations. This may be due to the significant interactions between the anionic hydrogel and BSA biomolecules. These interactions increase with an increase in the number of ionized MA in the hydrogel, which in turn increases the free volume of gel accessible for BSA diffusion. The initial BSA concentrations influence too in the amount of BSA adsorbed onto hydrogels (Mahdavinia and Etemadi 2015; Mahdavinia et al. 2016). The amount of BSA adsorbed found in this study are higher than those in literature (Saraydin et al. 1994, Öztop et al. 2003).
Adsorption isotherms modeling
Langmuir and Freundlich models were used for representing the adsorption isotherms and for easily reading the qt = f (Ce) curves. Analysis of hydrogels adsorption isotherms gives the results presented in Figs. 4 and 5.
The BSA adsorption isotherm shapes onto hydrogels agree the behavior of the L- type of Giles and Smith’s classification (Giles et al. 1960). This kind of isotherm suggests that the attraction forces between molecules of BSA and hydrogel are of physical nature (ionic interaction, hydrogen bonding). Values of the models constants were determined using Eqs. (3) and (4), respectively, and are summarized in Table 3.
From these results, it can be concluded that the Langmuir and Freundlich models were more satisfactory to describe mathematically the experimental curves obtained (R2 > 0.91). The Langmuir constant K L increases with acid content, indicating that the adsorption surface becomes quickly saturated. The adsorption capacity (q max) increases with increase in MA content. This increase in the MA amount in the gel increases the physical interactions between the MA carboxyl groups and amide groups of the protein. In this study, we confirmed that incorporation of MA into poly(acrylamide) increases the swelling of hydrogels; and poly(AAm-co-MA) hydrogels swelled very well in the BSA solution. Higher swelling abilities of these hydrogels allowed retention of more BSA molecules and water within the hydrogel. As shown in Table 3, a higher amount of MA (30%) produces a decrease in the adsorption capacity of copolymer. This decrease is probably due to higher number of carboxyl groups which repulse BSA molecules initiating a decrease in the hydrogels adsorption capacity (Karadag et al. 1994). All adsorption values found in this study are higher than those in the literature.
The values of the exponent (1/n) found were all lower than 1, which confirm that isotherm was of the L-type and that BSA molecules have a significant affinity for these pH-sensitive hydrogels. Values of the adsorption equilibrium parameter (0.467 < R L < 0.933) which are lower than 1, show that the adsorption is favorable (see Fig. 6).
Determination of the standard free energy of adsorption (ΔG 0ads )
From Eq. (6), the thermodynamic parameters of adsorption energy were determined at 293°K. Table 4 shows thermodynamic parameters of BSA adsorption onto hydrogels.
From the table it is evident that, the negative value of free energy change indicated the spontaneous nature of sorption, which confirmed the affinity of the hydrogels for the BSA molecules. The adsorption mechanism was reversible and thermodynamically favorable; the inclusion of BSA within the hydrogel matrix is done by a physical adsorption system.
Another remark observed is that the ΔG 0ads was not influenced by the amount of MA co-monomer in hydrogels.
Adsorption of BSA
The choice of 2 g L−1 concentration of BSA solution was described above in the study. The partial (q i ) and total (q t ) amounts of BSA adsorbed in the polymer matrix are determined for all hydrogels. These values are shown in Table 5.
From the table, the amount of BSA adsorbed increases with acid content of hydrogels. In physiological solution, the values of qi (mg/g) are low compared to these in distilled water, because of the sensitivity of BSA to Na+ cations. These last may develop physical links with BSA molecules which prevent its adsorption onto hydrogels. From the table, values of BSA adsorption vary with the swelling of hydrogels (important swelling involves a significant adsorption) (Kim et al. 1992).
In this work we showed that incorporation of diprotic maleic acid to poly(AAm) hydrogels involves a higher swelling and higher BSA adsorption capacities. These results indicate that these copolymer hydrogels may be used as a support for the protein separation or a superabsorbent material.
Conclusions
In this study, poly(acrylamide-co-maleic acid) hydrogels with high amount of diprotic acid were prepared by copolymerization and chemical crosslinking with methylene bis-acrylamide.
The swelling kinetics of these hydrogels in distilled water, physiological saline and BSA solutions were investigated. The swelling and BSA adsorption of hydrogels were affected by hydrogel composition and nature of external medium. Copolymer hydrogels with higher acid content (MA30) have a higher concentration of anionic groups providing a higher degree of swelling. Additionally, acidity of co-monomer is a key factor for the swelling behavior of the copolymer hydrogels. The presence of BSA and Na+ in swelling media weakly decreased the swelling degree of copolymers because of the physical interactions established between these entities and anionic groups within the hydrogel matrix. In addition, amount of BSA adsorbed decreased also in presence of Na+. Influence of initial BSA concentration on swelling behavior of hydrogels was demonstrated. At a concentration of 2 g L−1, the swelling was regular and stable for all hydrogels. Amount of BSA adsorbed increased with increase in hydrogel acid content. Modeling analysis showed that Langmuir and Freundlich models fit well the BSA adsorption isotherms, negative values of ΔG 0ads explain the favorability and reversibility of BSA adsorption onto hydrogels.
The study advised that these copolymers showed high swelling degrees and high BSA uptakes, suggesting their application in biotechnology and pharmaceutical industry as controlled release drugs system and as protein separation processes.
References
Bentiss F, Lebrini M, Lagrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4 thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931. doi:10.1016/j.corsci.2005.05.034
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Censi R, Martino PD, Vermonden T, Hennink WE (2012) Hydrogels for protein delivery in tissue engineering. J Control Release 161:680–692. doi:10.1016/j.jconrel.2012.03.002
Chen L, Remondetto GE, Subirade M (2006) Food protein-based materials as nutraceutical delivery systems. Trends Food Sci Tech 17:272–283. doi:10.1016/j.tifs.2005.12.011
Chen JJ, Ahmad AL, Ooi BS (2013) Poly (N-isopropylacrylamide-co-acrylic acid) hydrogels for copper ion adsorption: equilibrium isotherms, kinetic and thermodynamic studies. J Environ Chem Eng 1:339–348. doi:10.1016/j.jece.2013.05.012
Demirel G (2007) Adsorption of bovine serum albumine onto poly (Nt-butylacrylamide-co-acrylamide/maleic acid) hydrogels. J Polym Res 14:23–30. doi:10.1007/s10965-006-9076-4
Ekici S (2011) Intelligent poly(N-isopropylacrylamide)-carboxymethyl cellulose full interpenetrating polymeric networks for protein adsorption studies. J Mater Sci 46:2843–2850. doi:10.1007/s10853-010-5158-0
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. doi:10.1016/j.cej.2009.09.013
Giles CH, MacEwan TH, Nakhwa SN, Smith D (1960) Studies in Adsorption. Part XI. System of classifcation of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solid. J Chem Soc:3973–3993
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5:212–223. doi:10.1021/i160018a011
Kan B, Lin B, Zhao K et al (2014) Imprinting of bovine serum albumin in a nonwoven polypropylene membrane supported polyacrylamide/calcium alginate interpenetrating polymer network hydrogel. RSC Adv 4:55846–55852. doi:10.1039/c4ra09364j
Karadag E, Saraydin D (2002) Swelling studies of super water retainer acrylamide/crotonic acid hydrogels crosslinked by trimethylolpropane triacrylate and 1,4-butanediol dimethacrylate. Polym Bull 48:299–307. doi:10.1007/s00289-002-0029-8
Karadaǧ E, Saraydin D, Öztop HN, Güven O (1994) Adsorption of bovine serum albumin to acrylamide–itaconic acid hydrogels. Polym Adv Tech 5:664–668. doi:10.1002/pat.1994.220051006
Kaşgöza H, Aydınb İ, Kaşgöza A (2005) The effect of PEG(400)DA crosslinking agent on swelling behaviour of acrylamide-maleic acid hydrogels. Polym Bull 54:387–397
Kim SW, Bae YH, Okano T (1992) Hydrogels: swelling, drug loading and release. Pharm Res 9:283–290. doi:10.1007/s00289-005-0408-z
Ling Y, Lu M (2008) Preparation and characterization of pH and temperature dual responsive-, Poly(N-isopropylacrylamide-co-itaconic acid) hydrogels using DMF and water as mixed solvents. Polym J 40:592–600. doi:10.1295/polymj.PJ2007213
Mahdavinia GR, Etemadi H (2015) Surface modification of iron oxide nanoparticles with κ-carrageenan/carboxymethyl chitosan for effective adsorption of Bovine serum albumin. Arab J Chem. doi:10.1016/j.arabjc.2015.12.002
Mahdavinia GR, Pourjavadi A, Hosseinzadeh H, Zohuriaan MJ (2004) Modified chitosan 4.Superabsorbent hydrogels from poly (acrylic acid-co-acrylamide) grafted chitosan with salt-and pH-responsiveness properties. Eur Polym J 40:1399–1407. doi:10.1016/j.eurpolymj.2004.01.039
Mahdavinia GR, Massoumi B, Jalili K, Kiani G (2012) Effect of sodium montmorillonite nanoclay on the water absorbency and cationic dye removal of carrageenan-based nanocomposite superabsorbents. J Polym Res 19:1–13. doi:10.1007/s10965-012-9947-9
Mahdavinia GR, Mousanezhad S, Hosseinzadeh H, Darvishi F, Sabzi M (2016) Magnetic hydrogel beads based on PVA/sodium alginate/laponite RD and studying their BSA adsorption. Carbohydr Polym 147:379–391. doi:10.1016/j.carbpol.2016.04.024
Mellott MB, Searcy K, Pishko MV (2001) Release of protein fromhighly cross-linked hydrogels of poly (ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials 22:929–941. doi:10.1016/S0142-9612(00)00258-1
Murty VR, Bhat J, Muniswaran PKA (2002) Hydrolysis of oils by using immobilized lipase enzyme: a review. Biotechnol Bioprocess Eng 7:57–66. doi:10.1007/BF02935881
Öztop HN, Saraydın D, Şolpan D, Güven O (2003) Adsorption of BSA onto radiation-crosslinked poly (AAm/HPMA/MA) terpolymers. Polym Bull 50:183–190. doi:10.1007/s00289-003-0152-1
Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50:27–46. doi:10.1016/S0939-6411(00)00090-4
Pollak A, Blumenfeld H, Wax M, Baughn RL, Whitesides GM (1980) Enzyme immobilization by condensation copolymerization into crosslinked polyacrylamide gels. J Am Chem Soc 102:6324–6336
Pothakamury UR, Barbosa-Cánovas GV (1995) Fundamental aspects of controlled release in foods. Trends Food Sci Tech 12:397–406
Rintoul I, Wandrey C (2005) Polymerization of ionic monomers in polar solvents: kinetics and mechanism of the free radical copolymerization of acrylamide/acrylic acid. Polymer 46:4525. doi:10.1016/j.polymer.2005.04.005
Saraydin D, Karadaǧ E, Oeztop HN, Güven O (1994) Adsorption of bovine serum albumin onto acrylamide-maleic acid hydrogels. Biomaterials 15:917–920. doi:10.1016/0142-9612(94)90117-1
Saraydin D, Karadag E, Guven O (1995) Acrylamide/maleic acid hydrogels. Polym Adv Tech 6:719–726
Sassolas A, Blum LJ, Leca-Bouvier BD (2012) Immobilization strategies to develop enzymatic biosensors. Biotechnol Adv 30:489–511. doi:10.1016/j.biotechadv.2011.09.003
Solpan D, Duran S, Saraydin D, Güven O (2003) Adsorption of methyl violet in aqueous solutions by poly (acrylamide-co-acrylic acid) hydrogels. Radiat Phys Chem 66:117–127. doi:10.1016/S0969-806X(02)00384-5
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238. doi:10.1002/aic.690200204
Zhao K, Lin B, Cui W et al (2014) Preparation and adsorption of bovine serum albumin-imprinted polyacrylamide hydrogel membrane grafted on non-woven polypropylene. Talanta 121:256–262. doi:10.1016/j.talanta.2014.01.010
Zhao K, Chen T, Lin B et al (2015) Adsorption and recognition of protein molecular imprinted calcium alginate/polyacrylamide hydrogel film with good regeneration performance and high toughness. React Funct Polym 87:7–14. doi:10.1016/j.reactfunctpolym.2014.12.001
Zhou X, Zhou X (2014) The Unit Problem in the Thermodynamic Calculation of Adsorption Using the Langmuir Equation. Chem Eng Comm 201:1459–1467. doi:10.1080/00986445.2013.818541
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angar, NE., Aliouche, D. An enhanced immobilization of BSA biomolecule on anionic hydrogels: swelling and adsorption modeling. Chem. Pap. 71, 1389–1397 (2017). https://doi.org/10.1007/s11696-017-0129-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0129-4