Abstract
Three dendritic nickel complexes C1–C3 were synthesized from three poly(amido amine) dendrimers, salicylic aldehyde and nickel chloride hexahydrate via Schiff base condensation reaction and coordination reaction. The structures of the dendritic ligands and nickel complexes were characterized by FT-IR, UV, 1H NMR, ESI–MS, and elemental analysis. When activated with aluminum co-catalysts, three complexes C1–C3 were able to catalyze ethylene oligomerization. The catalytic activities and the product distribution of complexes C1–C3 were depended on the reaction parameter, co-catalyst, solvent, and the structure of the pre-catalyst. When using ethyl aluminum sesquichloride (EASC) as co-catalyst in toluene, the catalytic activity of complex C3 containing the longest bridging methylene groups reached the highest value of 1.63 × 106 g·(mol Ni·h)−1 with 69.15% C11 in the product at 30 min, 25 °C, 0.5 MPa, and Al/Ni ratio of 900.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The properties of dendrimers were first examined with Tomalia’s poly(amido amine) (PAMAM) dendrimers (Tomalia et al. 1985, 1986) and Newkome’s arborols (Newkome et al. 1985). Then dendrimers were widely applied in many fields with unique physics and supramolecular properties, such as nano-material, electrochemistry, and catalysis (Knecht et al. 2006; Arotiba et al. 2007; Astruc et al. 2010, Astruc and Chardac 2001). Among these applications, catalysis stands as one of the most promising fields. In recent years, the dendritic nickel complexes which can produce various types of products and exhibit good tolerance toward functional groups/polar monomers in the field of olefins oligomerization and polymerization (Wang et al. 2014b) have developed by some researchers. Benito et al. (2006) use carbosilane dendritic nickel(II) complexes containing N,N-imino pyridine chelating ligands (Gn-ONNMe2NiBr2, -ONN=-4-oxygen-(2,5-dimethyl phenyl)-imine-2-pyridine) for oligomerization and polymerization of ethylene. The catalytic activity of dendritic nickel (II) catalyst was higher than the mononuclear nickel catalyst. The poly(propylene imine) dendritic multinuclear catalyst was first applied in the polymerization of ethylene by Malgas et al. (2008) and in the polymerization of norbornene by Malgas-Enus et al. (2008) successfully. Martínez-Olid et al. (2014) employed dendritic mononuclear nickel complex [NiBr2(Gn-PBE-ONN)](Gn-PBE=Fréchet style dendritic wedges) in the oligomerization and polymerization of ethylene. In our research group, the PAMAM salicylaldimine nickel chloride (ethylenediamine as core) (Wang et al. 2013) was first synthesized and showed good performance in the oligomerization of ethylene. After that, PAMAM, 3,5-di-tert-butyl-2-hydroxy-benzaldehyde nickel chloride (Wang et al. 2014a) was prepared and we found that substituents on the benzene ring had negative influence on the catalytic activity of the catalyst. In addition, PAMAM bridging pyridine-imine nickel chloride (Wang et al. 2015a, b) was prepared and exhibited good catalytic activity and selectivity for higher carbon number olefins in ethylene oligomerization and 1-pentene oligomerization. Though our effort to change the structure of the peripheral of the dendritic catalyst was effective, recent work on ethylene oligomerization has highlighted that the structure of bridging group may affect catalytic activity and the product distribution (Ivanchev et al. 2012). Thus, three PAMAM salicylaldimine nickel chlorides with different bridging groups were prepared and used to catalyze the ethylene oligomerization herein hoping to explore the effect of the structure of the catalyst on the catalytic activities and product distribution. Besides, co-catalyst, solvent, and reaction parameters, such as Al/Ni mole ratio, ethylene pressure, and reaction temperature in ethylene oligomerization, were also investigated.
Experiment
General
The reaction of air- and moisture-sensitive compounds was performed using the standard Schlenk techniques. The anhydrous methanol, anhydrous sodium sulfate, cyclohexane, and methylene chloride were purchased from Harbin Chemical Plant. 1,4-butanediamine, 1,6-hexanediamine, 1,8-octanediamine, ethylenediamine, and methyl acrylate were provided by Tianjin Kermel Chemical Reagent Company. Salicylic aldehyde, nickel chloride hexahydrate, and EASC were purchased from Aladdin. MAO (methylaluminoxane) (10 wt% in toluene) was purchased from Sigma Aldrich. These agents were used as received without any further purification. Polymer grade ethylene was obtained from Afrox Company Limited. Toluene was dried by being reflowed over sodium/benzophenone.
Synthesis of dendritic ligands
The dendritic ligands 5, 6, and 7 were synthesized via Schiff base condensation between three PAMAM dendrimers (1, 2, 3) and salicylic aldehyde (4) (Scheme 1) according to the literature (Wang et al. 2014a, b).
Synthesis of dendritic complexes
The dendritic nickel complexes 9, 10, and 11 were prepared by the coordination reaction of dendritic ligands (5, 6, 7) with the nickel chloride hexahydrate (8) (Scheme 2). The solution of nickel chloride hexahydrate (2 eq) in anhydrous methanol was added to the solution of ligands (0.5 mmol) in methylene chloride (2 mL) in a Schlenk tube. The mixture was stirred for 24 h at 25 °C and then cooled to the room temperature. The precipitation was filtrated and dried under 50 °C.
Oligomerization reaction
The ethylene oligomerizations were carried out in a 250 mL steel autoclave equipped with a stirrer and temperature control. The steel autoclave was heated 4 h to 150 °C and cooled down to room temperature. The dry nitrogen and ethylene were injected to the reactor three times and vented successively. The appropriate amount of co-catalyst and solvent was injected to the reactor. Then, the system was kept stirring for 10 min. The pressure of the system was raised to the specified value and maintained for a certain time after the addition of the pre-catalyst. After a period time, the free ethylene was released, and the solution was collected and examined by gas chromatography. The reaction was terminated by adding 10 mL of acidified ethanol (90:10 ethanol/hydrogen chloride). All the data of ethylene oligomerization are average value.
Test statistics
Infrared spectra were detected using Nicolet FT-IR750 infrared spectrometer (USA). UV spectra of three ligands and its complexes were obtained on UV-1700 UV–visible spectrophotometer (SHIMADZU). 1H NMR spectra were recorded on American Varian VNMRS 400 MHz spectrometers with CDCl3 as solvent and tetramethylsilane (TMS) as the internal standard. Electrospray ionization mass spectra (ESI–MS) were conducted on the Bruker micro TOF-Q II instrument (Germany). Elemental analysis was carried on the Hereus element analyzer (Germany). Gas chromatography analysis was obtained on HP-5890 apparatus (USA) using Agilent 19091S-001 capillary column and hydrogen flame ionization detector (FID). Characterization data of newly prepared compounds are collected in Supplementary Tables S1 and S2.
Results and discussion
Characterization of the synthesized ligands
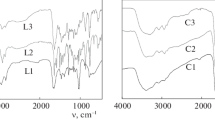
The IR spectra of three ligands are depicted in Fig. 1. The sharp peak at 1632 cm−1 indicates that the amino groups on the terminal of the dendrimer have condensed with the aldehyde and form the relevant imine (Malgas-Enus et al. 2008). The broadband around 3426 cm−1 is the stretching vibration of O–H in the salicylaldimine units. The peak around 1497 cm−1 is the vibration of C=C in the skeleton of benzene.
The UV spectra of three ligands are given in Fig. 2. The band observed at 220 nm is assigned to the π → π* transition of C=O. The band at around 256 nm belongs to band K of the conjugation of benzene and C=N. The band observed at 318 nm is assigned to the n → π* transition of the C=N.
The 1H NMR spectra of three ligands are given in Fig. 3. The imine groups in the ligands showed proton signals at around δ 8.3 (Ivanchev et al. 2012). In addition, the spectra showed proton signals on the benzene at around δ 7.0. The signal at around δ 4.0 is the hydrogen signal of the hydroxy. The signal at around δ 1.3 indicates the methylene groups in the core of the ligand. Several signals at around δ 3.0 are attributed to the methylene groups in the backbone of the ligand and the bridge between the dendrimer and the salicylaldehyde according to different surrounds (Fig. 4).
1H NMR spectra of ligand 5(a), 6(b), and 7(c) (for atom numbering, see Fig. 4)
The ESI–MS spectra of the ligands 5, 6, and 7 are given in Fig. 5. In the spectrum of ligand 5, the peak at m/z 961.5197 is assigned to [L]+ ion, and the peaks at m/z 487.2333, 753.4714, and 857.4976 are attributed to ions of [L-4C7H6NO]+, [L-2C7H6O]+, and [L-C7H6O]+, respectively. In the spectrum of ligand 6, the peak at m/z 989.5535 is assigned to [L]+ ion. In the spectrum of ligand 7, the peak at m/z 1017.5818 is assigned to [L]+ ion, and the peaks at m/z 799.4828, 808.5300, and 913.5604 are attributed to ions of [L-C12H15N2O2]+, [L-2C7H6O]+, and [L-C7H6O]+, respectively. These results provide a powerful evidence that four salicylaldehyde molecules have reacted with one dendrimer.
Characterization of the synthesized complexes
The IR spectra of the nickel complexes are depicted in Fig. 6. Compared to the curve of ligands, the stretching vibration of C=N and C–O has shifted to 1625 and 1201 cm−1, respectively. This phenomenon can be attributed to the metal coordination to the atoms N and O. The reduction of electron density around C=N moves the vibration frequency to low wave. The peak in 631 cm−1 belongs to N–Ni that shows the coordination of ligand and nickel chloride hexahydrate has occurred.
The UV spectra of three complexes are given in Fig. 7. After the nickel coordinated to the –OH and the C=N, the intensity of the band at round 319 nm decreased a lot and the band at round 256 nm has shifted to 242 nm. This phenomenon is due to the destruction of the conjugated system of the C=N with aromatic ring, resulting in the decrease of the maximum absorption wavelength and the reduction of the molar absorption coefficient.
Ethylene oligomerization
Nickel complexes 9 (C1), 10 (C2), and 11 (C3) were evaluated to catalyze ethylene oligomerization using MAO or EASC in toluene or in cyclohexane. Reaction parameters, such as Al/Ni mole ratio, ethylene pressure, and reaction temperature (Ainooson et al. 2011; Yang et al. 2013; Ahamad and Alshehri 2013), were investigated. The ethylene oligomerization reactions were conducted first by complex C1 with MAO in toluene at 25 °C, 0.5 MPa for 0.5 h. The catalytic activity and the product distribution are shown in Fig. 8.
As shown in Fig. 8 for C1, the increase of the Al/Ni ratio from 100 to 700 resulted in enhanced catalytic activity. A further increase of Al/Ni ratio to 900 led to a decrease of catalytic activity. We believed that there was no sufficient MAO to activate the Ni center at low concentration of the MAO; on the other hand, excess MAO around the Ni center would block the ethylene monomer insertion (Ling and Jin 2004). The catalytic activity of complex C1 was 5.48 × 104 g (mol Ni h)−1 at the optimized Al/Ni ratio of 700. Besides, the content of C4 increased at first and then declined with the Al/Ni ratio from 100 to 900, and the content of C8 was at opposite tendency. To our surprise, the content of C8 reached the maximum of 85.45% at the Al/Ni ratio of 300. It is assumed that the C8 was obtained easily at low concentration of the MAO, in accordance with study of Wang et al. (2015b).
Further experiments were taken with different ethylene pressures in Fig. 9. The catalytic activity of complex C1 fluctuated with the change of the ethylene pressure between 0.1 and 0.6 MPa. There was no value of catalytic activity larger than 5.48 × 104 g (mol Ni h)−1 at the ethylene pressure of 0.5 MPa. The result may result from more ethylene participate in the oligomerization at the higher ethylene pressure (Sun et al. 2012). The impact of the ethylene pressure on the product distribution was remarkable. The increase of the ethylene pressure from 0.1 to 0.3 MPa led to improve the content of C8 in the product with the consumption of C4. A further increase of ethylene pressure to 0.6 MPa led to a decrease of the content of C8. The optimized ethylene pressure for C8 was 0.3 MPa, in consistent with our prior study (Wang et al. 2015a).
Reaction temperature also influenced catalytic activity and product distribution. When reaction temperature was raised from 5 to 35 °C (Fig. 10), the catalytic activity of complex C1 improved a lot. A further increase of reaction temperature to 45 °C, the catalytic activities dropped with more C8. This can be ascribed to an optimal temperature existed for C1, such phenomena were also observed by other nickel catalysts (Wang et al. 2014a; Yu et al. 2011). The maximum content of C8 was obtained at 25 °C.
When the solvent was changed from toluene to cyclohexane, the effect of Al/Ni mole ratio on the catalytic activity and product distribution for complex C2 was explored. The experiments were conducted at ethylene pressure of 0.5 MPa at 25 °C for 0.5 h with MAO in cyclohexane. The results are summarized in Fig. 11.
As shown in Fig. 11, the catalytic activity of complex C2 reached 7.63 × 104 g (mol Ni h)−1 at the optimal Al/Ni ratio of 700. Meanwhile, C4 become the main product of oligomerization in all reaction conditions. Interestingly, the content of >C6 of complex C2 at Al/Ni mole ratio of 900 was much bigger than others. This phenomenon was similar to the result of complex C1 at 0.3 MPa in Fig. 8. We believed that these two special reaction conditions of catalytic system could be suit for prepared C8 with high selectivity.
Most importantly, the effect of structure of complex C1–C3 on the catalytic activity and product distribution was explored. The results are shown in Fig. 12.
Effect of structure of catalyst on the catalytic activity and product distribution. Reaction condition: pre-catalyst, 9 μmol; co-catalyst, MAO; solvent, 50 mL. a 0.5 h; 25 °C; 0.5 MPa; n(Al)/n(Ni), 700; toluene. b 0.5 h; 25 °C; 0.3 MPa; n(Al)/n(Ni), 700; toluene. c 0.5 h; 35 °C; 0.5 MPa; n(Al)/n(Ni), 700; toluene. d 0.5 h; 25 °C; 0.5 MPa; n(Al)/n(Ni), 700; cyclohexane
As shown in Fig. 12, complexes C1–C3 were evaluated at several optimized reaction conditions. The catalytic activity of C1 was 5.48 × 104 g (mol Ni h)−1 with 34.09% C8 at 0.5 h, 25 °C, 0.5 MPa, and the Al/Ni ratio of 700 in toluene. At the same condition, the catalytic activity of complex C2 was larger than that of complex C1, and the catalytic activity of complex C3 followed that of complex C2. The content of C8 in the product of C2 was smaller than that of C1, while C3 was better than C2. At 0.5 h, 25 °C, 0.3 MPa, and the Al/Ni ratio of 700 in toluene, the catalytic activity of complex C3 was the largest (5.00 × 104 g (mol Ni h)−1) among three complexes with 11.64% C8. Besides, the trend of catalytic activities with the bridge linkage of three complexes in 35 °C was similar to that in 25 °C at 0.5 h, 0.5 MPa, and the Al/Ni ratio of 700 in toluene, and the catalytic activity of complex C3 reached 1.02 × 105 g (mol Ni h)−1 with 40.12% C8. When the solvent was changed to cyclohexane, the catalytic activity of complex C2 was maximal among three complexes with 0.95% C8. Furthermore, it is unfavorable that the catalytic activity of complexes C1–C3 declined a lot and the content of C4 was larger than 90%. It is supposed that MAO showed better activation performance in the toluene which could activate the Ni metal center more efficiently at the same Al/Ni mole ratio and accelerated the chain growth than in cyclohexane. This may be caused by the solubility factor, because the catalyst showed better solubility in toluene than in cyclohexane and hence the much higher initial rate of conversion of the ethylene monomer to product in toluene.
It is well known that the type of activator may have a profound influence on the catalytic activity and the product distribution (Obuah et al. 2014). The EASC was considered with a higher acid value which could active the catalyst easier, and increased the reaction rate by the ethylene molecule coordinated with the active species. In recent years, this idea was verified in practice (de Souza et al. 2007; Bahuleyan et al. 2010). This prompted us to use EASC as co-catalyst.
As shown in Fig. 13, an interesting observation was the presence of odd-number carbon compound in the product mixture when the ethylene oligomerization was activated by EASC. At the same condition, the odd-number carbon compound did not exist when the reaction solvent was changed to cyclohexane. An explanation for our result was that Friedel–Crafts alkylation of the solvent and toluene occurred in the presence of the short-chain oligomers (C4, C6, and C8) to yield alkylated toluenes (C11, C13, and C15). The Friedel–Crafts alkylation was proved independent. In our prior work (Wang 2013), a small amount of butene, hexane, octene, and most of Friedel–Crafts alkylated product were obtained in the evaluation of 1.0-generation polypropylene imine dendrimer nickel complexes in ethylene oligomerization when using EtAlCl2 as co-catalyst. In addition, Budhai et al. (2013) has found olefins and alkyl benzene product using pyridine-benzene nickel complexes activated by EtAlCl2. Obuah et al. (2014) and Nyamato et al. (2014) have also reported oligomers and alkyl benzene in catalyst system of EtAlCl2 and toluene. It was not coincidence for the results, because EASC is the mixture of ethylaluminum dichloride (EtAlCl2) and diethylaluminum monochloride (Et2AlCl) with equal molar. We suggested that it was the EtAlCl2 in the EASC that behaved alkylation function.
Typical GC product (oligomers and alkylated toluenes) in Fig. 14
From the experiments with MAO in toluene or cyclohexane, complexes C2 and C3 were more active than complex C1 and thus were mainly used for the rest experiments. Complexes C2 and C3 were evaluated on the ethylene oligomerization with co-catalyst EASC. Reaction parameters, such as Al/Ni mole ratio and ethylene pressure, were investigated. As shown in Fig. 14, the catalytic activity of complex C2 was higher nearly one order of magnitude than the result in Fig. 12. In addition, the catalytic activity of complex C2 reached 1.14 × 106 g (mol Ni h)−1 and the content of C11 also attained the highest value of 69.14% at the optimized Al/Ni ratio of 900.
The effect of ethylene pressure on the catalytic activity was remarkable (Fig. 15). The increase from 0.1 to 0.5 MPa led to a sevenfold increase in the catalytic activity of complex C3. The catalytic activity of complex C3 in 0.4 and 0.5 MPa was much higher than that in 0.1–0.3 MPa, which was agreed with the report by Sun et al. (2012).
In addition, the effects of structure of complexes C1–C3 on the catalytic activity and product distribution were also explored. The results are shown in Fig. 16.
In Fig. 16, the catalytic activity of complex C3 get the maximum value of 1.63 × 106 g (mol Ni h)−1 among three complexes at 0.5 h, 25 °C, 0.5 MPa, and the Al/Ni ratio of 900. In addition, the content of C13 and C15 was in descending order of complex C3, complex C1, and complex C2. At the reaction condition of 0.5 h, 25 °C, 0.4 MPa, and the Al/Ni ratio of 900, the catalytic activity of complex C3 was also larger than the other two complexes. At the same time, the content of C13 and C15 in the product of complex C3 was better than that of complexes C1 and C2. In a word, the complex C3 containing most bridging methylene groups showed the highest catalytic activity with maximum potential for high-carbon product. This could be attributed to that the best solubility of complex C3 in toluene for the longest flexible bridging groups (Ivanchev et al. 2012), hence resulting in the highest initial rate of conversion from monomer to product. Meanwhile, the cavity structure of complex C3 was most loose, thus can accommodate the longest chain oligomers and retard β-hydrogen elimination step.
From the results of Figs. 14, 15, and 16, C11 was the dominant product in the ethylene oligomerization with EASC as co-catalyst. Malgas-Enus et al. (2011) reported that the C11 product was formed in relatively high amount in the ethylene oligomerization with EtAlCl2 by generation 1 DAB salicylaldimine nickel complex and proposed the mechanism of the alkylation reaction. The peripheral structure of PAMAM salicylaldimine nickel complex was the same with DAB salicylaldimine nickel complex. A proposed catalytic cycle for oligomerization of ethylene and tandem Friedel–Crafts alkylation was shown in Scheme 3.
In Scheme 3, the first catalytic process was the ethylene oligomerization. Ethylene oligomers (C4, C6, and C8) were generally obtained by ethylene insertion/β-H elimination mechanism (Peitz et al. 2010). The first step involved the alkylation of the Ni complex with EASC, followed by α-hydride elimination to form the Ni–H which was thought to be the active species. Step 2 was the coordination of ethylene at an open site on the metal and insertion into the metal-alkyl bond. Continuous insertion of the ethylene species promoted chain growth. At step 3, either continuous chain propagation to give longer chain oligomer or β-H elimination occurred, as shown in step 4. After the oligomerization process, the Friedel–Crafts alkylation was introduced. Step 5 involved the double bond of the 1-butene attacking free EtAlCl2 to form a pro-active electrical particle carbocation a. After that a typical Friedel–Crafts mechanism ensued. Electrophilic addition reaction proceeded between carbon positive ion a and toluene to give compound b, and the Friedel–Crafts alkylation product c (C11) was released by removing hydrogen of compound b. Meanwhile, the ethylene monomer and EtAlCl2 could react with toluene to obtain ethyl-toluene. When the reaction rate of the alkylation process was greater than or exactly equal to the rate of the oligomerization process, the resulting product was only alkyl-toluenes (Ojwach et al. 2009). On the contrary, both the oligomers and alkylated products (Malgas-Enus and Mapolie 2014) existed. In this experiment, due to the similarity of structure of the three dendritic catalysts, the catalytic activities of three catalysts were nearly in the same order of magnitude (105 g (mol Ni h) −1). At the same time, the resulting product contained both oligomers and alkyl-toluenes.
Conclusions
Three new dendritic ligands and the resulting nickel complexes were synthesized and characterized by FT-IR, UV, 1H NMR, ESI–MS, and elemental analysis. Complexes C1–C3 had shown varying catalytic behavior under typical reaction conditions for the ethylene oligomerization with the addition of MAO or EASC in toluene or in cyclohexane. For the similarity of the structure of three catalysts, the catalytic activities of synthesized C1–C3 were in the same order of magnitude with the same kind of product. Under the same condition, complex C3 containing the longest bridging methylene groups showed the highest catalytic activity with maximum potential for high-carbon product. In addition, the reaction parameters, such as Al/Ni mole ratio, ethylene pressure, and reaction temperature, could change the results. The catalytic activity of complex C3 reached the highest value of 1.63 × 106 g (mol Ni h)−1 at 30 min, 25 °C, 0.5 MPa, and Al/Ni ratio of 900 with 69.15% C11. Besides, the co-catalyst and solvent had dramatic influence on the catalytic activity and the product distribution. Use of MAO in toluene or in cyclohexane displayed relatively lower catalytic activity and produce ethylene oligomers. Activation using EASC in toluene gave enhanced catalytic activity and the product was oligomers and alkyl-toluenes which resulted from Friedel–Crafts alkylation.
References
Ahamad T, Alshehri SM (2013) Synthesis and characterization of polymer metal complexes and their catalytic activity in ethylene oligomerization. Adv Polym Technol 32(3):586–589. doi:10.1002/adv.21350
Ainooson MK, Ojwach SO, Guzei IA, Spencer LC, Darkwa J (2011) Pyrazolyl iron, cobalt, nickel, and palladium complexes: synthesis, molecular structures, and evaluation as ethylene oligomerization catalysts. J Organomet Chem 696(8):1528–1535. doi:10.1016/j.poly.2013.01.018
Arotiba OA, Ignaszak A, Malgas R, Al-Ahmed A, Baker PG, Mapolie SF, Iwuoha EI (2007) An electrochemical DNA biosensor developed on novel multinuclear nickel (II) salicylaldimine metallodendrimer platform. Electrochim Acta 53(4):1689–1696. doi:10.1016/j.electacta.2007.08.016
Astruc D, Chardac F (2001) Dendritic catalysts and dendrimers in catalysis. Chem Rev 101(9):2991–3024. doi:10.1021/cr010323t
Astruc D, Boisselier E, Ornelas C (2010) Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev 110(4):1857–1959. doi:10.1021/cr900327d
Bahuleyan BK, Oh JM, Chandran D, Ha JY, Hur AY, Park DW, Ha CS, Suh H, Kim I (2010) Highly efficient supported diimine Ni (II) and iminopyridyl Fe(II) catalysts for ethylene polymerizations. Top Catal 53(7–10):500–509. doi:10.1007/s11244-010-9478-8
Benito JM, de Jesús E, de la Mata FJ, Flores JC, Gómez R, Gómez-Sal P (2006) Mononuclear and dendritic nickel(II) complexes containing N, N′-iminopyridine chelating ligands: generation effects on the catalytic oligomerization and polymerization of ethylene. Organometallics 25(16):3876–3887. doi:10.1021/om0509084
Budhai A, Omondi B, Ojwach SO, Obuah C, Osei-Twum EY, Darkwa J (2013) Tandem ethylene oligomerization and Friedel-Crafts alkylation of toluene catalyzed by bis-(3, 5-dimethylpyrazol-1-ylmethyl) benzene nickel (II) complexes and ethylaluminium dichloride. Catal Sci Technol 3(12):3130–3135. doi:10.1039/C3CY00334E
de Souza CG, de Souza RF, Bernardo-Gusmao K (2007) Effect of alkylaluminum co-catalyst on ethylene polymerization with nickel-α-diimine complex. Appl Catal A 325(1):87–90. doi:10.1016/j.apcata.2007.03.007
Ivanchev SS, Yakimansky AV, Ivancheva NI, Oleinik II, Tolstikov GA (2012) Ethylene polymerization using catalysts based on binuclear phenoxyimine titanium halide complexes. Eur Polymer J 48(1):191–199. doi:10.1016/j.eurpolymj.2011.10.020
Knecht MR, Garcia-Martinez JC, Crooks RM (2006) Synthesis, characterization, and magnetic properties of dendrimer-encapsulated nickel nanoparticles containing <150 atoms. Chem Mater 18(21):5039–5044. doi:10.1021/cm061272p
Ling YG, Jin GX (2004) Synthesis, structure and ethylene polymerization behavior of titanium complexes [C3H6 (N=CH™ Ar™ O)2] TiCl2. Chin Sci Bull 49(12):1236–1240. doi:10.1360/04wb0041
Malgas R, Mapolie SF, Ojwach SO, Smith GS, Darkwa J (2008) The application of novel dendritic nickel catalysts in the oligomerization of ethylene. Catal Commun 9(7):1612–1617. doi:10.1016/j.catcom.2008.01.009
Malgas-Enus R (2011) The preparation and characterization of multinuclear catalysts based on novel dendrimers: application in the oligomerization and polymerization of unsaturated hydrocarbons. University of Stellenbosch
Malgas-Enus R, Mapolie SF (2014) Nickel metallodendrimers as catalyst precursors in the tandem oligomerization of ethylene and friedel-crafts alkylation of its olefinic products. Inorg Chim Acta 409:96–105. doi:10.1016/j.ica.2013.06.016
Malgas-Enus R, Mapolie SF, Smith GS (2008) Norbornene polymerization using multinuclear nickel catalysts based on a polypropyleneimine dendrimer scaffold. J Organomet Chem 693(13):2279–2286. doi:10.1016/j.jorganchem.2008.03.029
Martínez-Olid F, de Jesús E, Flores JC (2014) Monometallic nickel(II) complexes containing N, N′-iminopyridine chelating ligands with dendritic substituents: the influence of dendrimer topology on the catalytic oligomerization and polymerization of ethylene. Inorg Chim Acta 409:156–162. doi:10.1016/j.ica.2013.07.025
Newkome GR, Yao ZQ, Baker GR, Gupta VK (1985) Micelles. Part 1. Cascade molecules: a new approach to micelles. J Org Chem 50(11):2003–2004. doi:10.1021/jo00211a052
Nyamato GS, Ojwach SO, Akerman MP (2014) Unsymmetrical (pyrazolylmethyl) pyridine metal complexes as catalysts for ethylene oligomerization reactions: role of solvent and co-catalyst in product distribution. J Mol Catal A: Chem 394:274–282. doi:10.1016/j.molcata.2014.07.018
Obuah C, Omondi B, Nozaki K, Darkwa J (2014) Solvent and co-catalyst dependent pyrazolylpyridinamine and pyrazolylpyrroleamine nickel (II) catalyzed oligomerization and polymerization of ethylene. J Mol Catal A: Chem 382:31–40. doi:10.1016/j.molcata.2013.10.024
Ojwach SO, Guzei IA, Benade LL, Mapolie SF, Darkwa J (2009) (Pyrazol-1-ylmethyl)pyridine nickel complexes: ethylene oligomerization and unusual friedel-crafts alkylation catalysts. Organometallics 28(7):2127–2133
Peitz S, Aluri BR, Peulecke N, Müller BH, Wöhl A, Müller W, Al-hazmi MH, Mosa FM, Rosenthal U (2010) An alternative mechanistic concept for homogeneous selective ethylene oligomerization of chromium-based catalysts: binuclear metallacycles as a reason for 1-octene selectivity? Chem-A Eur J 16(26):7670–7676. doi:10.1002/chem.201000750
Sun WH, Song S, Li B, Redshaw C, Hao X, Li YS, Wang F (2012) Ethylene polymerization by 2-iminopyridylnickel halide complexes: synthesis, characterization and catalytic influence of the benzhydryl group. Dalton Trans 41(39):11999–12010. doi:10.1039/C2DT30989K
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1985) A new class of polymers: starburst-dendritic macromolecules. Polym J 17(1):117–132. doi:10.1295/polymj.17.117
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1986) Dendritic macromolecules: synthesis of starburst dendrimers. Macromolecules 19(9):2466–2468. doi:10.1021/ma00163a029
Wang HC (2013) Study on the synthesis and catalysis of dendritic salicyladimine nickel complexes[M]. Northeast Petroleum University, Daqing, pp 30–32
Wang J, Zhang P, Chen S, Li CQ, Li HY, Yang G (2013) The preparation of dendritic nickel complex and performance evaluation in the oligomerization of ethylene. J Macromol Sci Part A Pure Appl Chem 50:163–167. doi:10.1080/10601325.2013.741486
Wang J, Yang G, Li CQ, Shi WG, Wang SH (2014a) Novel dendrimer-based nickel catalyst: synthesis, characterization and performance in ethylene oligomerization. Chem Pap 68(11):1532–1538. doi:10.2478/s11696-014-0603-1
Wang S, Sun WH, Redshaw C (2014b) Recent progress on nickel-based systems for ethylene oligo-/polymerization catalysis. J Organomet Chem 751:717–741. doi:10.1016/j.jorganchem.2013.08.021
Wang J, Huo HL, Li CQ, Ma LL, Shi WG, Chen S (2015a) Synthesis of novel dendritic bridging nickel complex for oligomerization of olefins. Chem J Chin Univ 68(11):1532–1538. doi:10.7503/cjcu20150385
Wang S, Zhang W, Du S, Asuha S, Flisak Z, Sun WH (2015b) Propyl substituted 4-arylimino-1, 2, 3-trihydroacridylnickel complexes: their synthesis, characterization and catalytic behavior toward ethylene. J Organomet Chem 798:408–413. doi:10.1016/j.jorganchem.2015.05.001
Yang Y, Liu Z, Liu B, Duchateau R (2013) Selective ethylene tri-/tetramerization by in situ-formed chromium catalysts stabilized by N, P-based ancillary ligand systems. ACS Catal 3(10):2353–2361. doi:10.1021/cs4004968
Yu JG, Hu XQ, Zeng YN, Zhang LP, Ni CH, Hao X, Sun WH (2011) Synthesis, characterization and ethylene oligomerization behaviour of N-(2-substituted-5,6,7- trihydroquinolin-8-ylidene) arylaminonickel dichlorides. New J Chem 35(1):178–1832011. doi:10.1039/c0nj00516a
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21576048), Innovation Fund of Petro-China (No. 2014D-5006-0503) and the University of Jilin.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Ma, LL., Song, L. et al. Synthesis, characterization, and ethylene oligomerization of three novel dendritic nickel catalysts. Chem. Pap. 71, 895–904 (2017). https://doi.org/10.1007/s11696-016-0009-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0009-3