Abstract
Introduction
Pregnant women with a history of metabolic bariatric surgery (MBS) are at high risk of developing nutrient deficiencies, leading to greater challenges to reach nutritional requirements. This study compared nutrient status of women using specialized “weight loss surgery” multivitamin supplementation (WLS-MVS) to those using standard supplementation (sMVS) during pregnancy following MBS.
Methods
Multicenter observational cohort study including 119 pregnant women at 41.0 (18.5–70.0) months after Roux-en-Y gastric bypass (RYGB, n = 80) or sleeve gastrectomy (SG, n = 39). Routine blood samples were analyzed every trimester (T1, T2, T3), and micronutrient serum levels were compared between WLS-MVS and sMVS users.
Results
During pregnancy after RYGB, WLS-MVS users demonstrated higher serum concentrations of hemoglobin (7.4 [7.2, 7.5] vs. 7.0 [6.8, 7.3] mmol/L), ferritin (23.2 [15.0, 35.7] vs. 13.7 [8.4, 22.4] µg/L), and folic acid (31.4 [28.7, 34.2] vs. 25.4 [21.3, 29.4] nmol/L) and lower serum vitamin B6 levels (T1: 90.6 [82.0, 99.8] vs. 132.1 [114.6, 152.4] nmol/L) compared to sMVS users. Iron deficiencies and elevated serum vitamin B6 levels were less prevalent in the WLS-MVS group. During pregnancy after SG, WLS-MVS users showed higher serum vitamin D concentrations (89.7 [77.6, 101.8] vs. 65.4 [53.3, 77.4] nmol/L) and lower serum vitamin B1 concentrations (T2: 137.4 [124.2, 150.6] vs. 161.6 [149.0, 174.1] nmol/L, T3: 133.9 [120.1, 147.7] vs. 154.7 [141.9, 167.5] nmol/L) compared to sMVS users.
Conclusion
Low maternal concentrations of micronutrients are highly prevalent during pregnancy after MBS. The use of specialized multivitamin supplementation generally resulted in higher serum levels during pregnancy compared to standard supplementation. Future research is needed to investigate how supplementation strategies can be optimized for this high-risk population.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic bariatric surgery (MBS) is the most effective treatment for people with severe obesity, resulting in substantial and long-term weight loss and reduction of obesity-related health risks [1, 2]. More than half of all MBS procedures are performed in women of reproductive age [3], and the Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are the most commonly performed procedures [3]. Undergoing MBS prior to pregnancy significantly reduces the risk of obesity-related complications such as subfertility, gestational diabetes, and hypertensive disorders in pregnancy [4, 5]. However, decreased intake and absorption of nutrients after surgery in combination with the increased demand for nutrients during pregnancy may lead to more pronounced deficiencies [6]. Furthermore, pregnancy symptoms such as morning sickness or hyperemesis gravidarum and abdominal complaints may worsen nutrient status during pregnancy [6, 7]. Overall, low maternal concentrations of vitamins A, B12, and D, folic acid, iron, and zinc are frequently reported during pregnancy after MBS [8,9,10]. Potential neonatal adverse effects that are associated with maternal deficiencies during pregnancy include preterm birth, fetal growth restriction, congenital malformations, and neurological and developmental impairment [6, 7, 9, 11].
Consensus recommendations for prenatal care of these patients have been proposed [12], but evidence-based guidelines regarding optimal nutritional monitoring and supplementation strategies during pregnancy after MBS are lacking. Regular “over-the-counter” or prenatal multivitamin supplements are likely not sufficient to cover the needs of pregnant women who have undergone MBS. Fortunately, specialized “weight loss surgery” multivitamin supplements (WLS-MVS) that are specifically developed for patients after MBS are emerging. The composition of these supplements is often tailored to the type of procedure and varies between brands, but they generally contain high doses of folic acid, vitamins B12 and D, elementary iron, and zinc. Although the superiority of these supplements compared to standard multivitamin supplementation (sMVS) has been demonstrated in the general population after MBS [13,14,15], their efficacy during pregnancy is largely unknown.
Therefore, the aim of this observational cohort study was to explore differences in nutrient status among women using WLS-MVS versus sMVS during pregnancy following MBS.
Methods
Study Design and Participants
The NEWBIE study (Nutritional status of prEgnant Women following BariatrIc surgEry) is a multicenter observational cohort study that was conducted from November 2018 until October 2022 at three general hospitals in the Netherlands (Rijnstate Hospital, Arnhem (RHA), Máxima Medical Center, Veldhoven (MMC), Hospital Gelderse Vallei, Ede (HGV)). Within these hospitals, women with a history of MBS are recommended to postpone pregnancy during the period of rapid weight loss (at least 12 months) and to use specialized WLS-MVS. Antenatal care follows a specific protocol recommending supplementation with WLS-MVS and close monitoring of maternal nutrient status as well as fetal growth and complications (e.g., internal herniation).
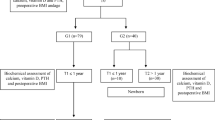
All pregnant women older than 18 years with a medical history of MBS presenting at the obesity or antenatal clinic were eligible for recruitment. Exclusion criteria were elective termination of pregnancy, multiple pregnancy, MBS procedures other than RYGB or SG, reversal of the MBS procedure, and malnutrition due to other causes (e.g., malignancy, alcoholism). Participants were preferably included before 12 weeks of pregnancy and followed up until 2 months post-partum. A total of 129 participants were included of which three women were excluded because of twin pregnancies (n = 2) and history of another MBS procedure (n = 1). During data analysis, seven participants were excluded because of insufficient data about pregnancy (n = 1), unknown MVS use (n = 4), and not using MVS during pregnancy (n = 2). The final population for data analysis consisted of 119 participants of whom 80 women after RYGB (67%) and 39 after SG (33%) (Fig. S1).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were reviewed and approved by the institutional ethics committees of the participating hospitals (ref 2018–1267). Written informed consent was obtained from all subjects.
Data Collection
Clinical Parameters
Maternal characteristics (age, geographic origin, educational level, smoking status, anthropometrics, type of MBS, presence of preexisting diabetes or hypertension) and antepartum variables (time to conception, mode of conception, parity, gestational weight gain, pregnancy complications) were collected from the medical records. Educational level was defined as low (primary education and prevocational secondary education), medium (senior general secondary education, preuniversity education, and secondary vocational education), or high (higher vocational education and university). Smoking status was defined as never, former (stopped before pregnancy), or current (smoked during pregnancy). Anthropometric measurements including height (m) and body weight (kg) were performed during standard visits. Percent total body weight loss (%TWL) at conception was calculated as body weight loss divided by body weight before surgery, multiplied by 100%.
Time from surgery to conception was defined as the period in months between surgery and conception. Mode of conception was classified as spontaneous or assisted (by use of fertility treatment). Gestational weight gain in kilograms was calculated as the difference between late pregnancy weight (weight at the day of delivery or within less than 4 weeks before delivery) and prepregnancy weight (weight at the first antenatal visit or self-reported weight before pregnancy). Subsequently, gestational weight gain was classified as inadequate, adequate, or excessive based on prepregnancy body mass index (BMI) according to the National Academy of Medicine (NAM) recommendations [16]. Evaluated complications during pregnancy included gestational diabetes mellitus (new-onset diabetes diagnosed by glucose monitoring), hypertensive disorders (new-onset hypertension, above 140/90 mm Hg), hyperemesis gravidarum (severe, persistent nausea and vomiting), and internal herniation (small bowel obstruction).
Supplementation Use
All women were advised to use MVS daily, preferably a specialized WLS-MVS that is specifically developed for patients after MBS. Self-reported information on the use of MVS (type, composition, dosage, and compliance) was obtained during each trimester, and participants were categorized as either users of WLS-MVS or sMVS accordingly. sMVS were defined as regular, over-the-counter MVS or prenatal supplements. The composition of the MVS that were most frequently used can be found in Table S1. Participants using both WLS-MVS and sMVS on a daily basis were assigned to the WLS-MVS group, whereas participants who used WLS-MVS and sMVS on alternate days were assigned to the sMVS group. Non-users of MVS were excluded from the analyses.
In addition to daily MVS, all participants were advised to use calcium/vitamin D3 supplementation as part of the standard protocol after MBS. According to general recommendations of the Dutch Health Council [17], supplementation of 400 µg folic acid was also recommended in the preconception period until 8 weeks after conception. In case of observed low micronutrient serum levels during pregnancy, a prescription for the required supplementation was provided according to local protocol.
Laboratory Evaluation
Standard routine laboratory blood tests were performed during each trimester (T1: week 1–12, T2: week 13–26, T3: week 27–42). Evaluated laboratory parameters slightly differed between the centers, but generally included hemoglobin, ferritin, folic acid, vitamins A, B1, B6, B12, and D, and calcium. Calcium levels were corrected for albumin using the following equation: Cacorr = total calcium + 0.02*(40-albumin). A low serum level was defined as a serum level below the local reference value at the time of blood collection (Table S2) as there were no validated standards available for the required levels of micronutrients during pregnancy, except for hemoglobin [18]. Serum ferritin levels below the reference value were used as a marker for iron deficiency.
Statistical Analyses
General characteristics are reported as mean ± standard deviation (normal distribution) or as median (Q1–Q3, non-normal distribution) for continuous variables and as frequency (percentage) for categorical variables.
Differences in serum concentrations across the three trimesters of pregnancy between WLS-MVS users and sMVS users were analyzed using linear mixed-effects models. Serum concentrations of ferritin and vitamin B6 were log-transformed before analysis. The crude model consisted of fixed effects for MVS (WLS-MVS, sMVS), trimester (T1, T2, T3), and their interaction term, plus a random effect for participants. Trimester entered the model as a repeated measure using a first-order autoregressive structure. Log-likelihood ratio tests were performed to explore potential confounders including center, smoking status, surgery-to-conception interval, BMI at conception, timing of sampling, and the use of additional supplementation for iron, folic acid, vitamin B12, and vitamin D during pregnancy. Final models for RYGB included BMI at conception, use of additional supplementation for ferritin and vitamin B12 (yes/no/missing), use of calcium/vitamin D3 supplementation for calcium and vitamin D (yes/no/missing), and timing of sampling for vitamin D (in months). Final models for SG included the use of additional supplementation for ferritin (yes/no/missing) and timing of sampling for vitamin D (in months). Serum concentrations measured after intravenous iron infusions (ferritin) and hydroxocobalamin injections (vitamin B12) were removed from the analyses to prevent biased estimates. Results are presented as estimated (geometric) marginal mean and 95% confidence intervals (CI). Descriptives of the original serum data at each trimester can be found in Table S3.
The prevalence of low and elevated serum levels at each trimester was analyzed using chi-square tests or Fisher’s exact test (if more than 20% of expected counts were less than 5) and presented as frequency (percentage).
All statistical analyses were performed separately for the RYGB and SG group, using IBM SPSS Statistics 25 for Windows (IBM Corp., Armonk USA). A two-sided p value below 0.05 was considered statistically significant.
Results
General Characteristics
Mean age at conception of the total study population was 31.3 ± 4.7 years; the majority of the participants was of West-European origin (92.4%), had a medium educational level (37.8%), and never smoked (61.3%) (Table 1). Median time from surgery to conception was 50.0 (23.4–77.0) months in the RYGB group and 32.2 (16.4–43.8) months in the SG group, and the majority of the participants became pregnant more than 24 months after MBS (RYGB: 75.0%, SG: 59.0%). Mean TWL from surgery to conception was 32.0 ± 9.1% after RYGB and 32.5 ± 8.5% after SG.
Nutrient Status and Supplement use after RYGB
Throughout pregnancy following RYGB, low maternal serum concentrations were frequently observed for hemoglobin (28.7%), ferritin (60.0%), vitamin B12 (43.8%), vitamin A (21.3%), and vitamin D (45.0%) and to a lesser extent for folic acid (12.5%) and calcium (13.8%). Low serum levels of vitamin B1 and B6 were rare (2.5%).
During pregnancy, more participants used WLS-MVS compared to sMVS (T1: 69.6% vs. 30.4%, T2: 75.0% vs. 25.0%, T3: 75.3% vs. 24.7%). Overall, WLS-MVS users had significantly higher serum levels of hemoglobin, ferritin, and folic acid during pregnancy than sMVS users (p < 0.05 for all, Fig. 1a–c). This resulted in less iron deficiencies in the WLS-MVS group compared to the sMVS group during the second (29.6% vs. 55.6%, p = 0.047) and third trimester (36.5% vs. 72.2%, p = 0.01, Table 2). Similarly, anemia tended to be less prevalent in the WLS-MVS group (T1–T3: 11–13% vs. 17–33%, p > 0.05). The prevalence of low serum folic acid levels during pregnancy was comparable between the groups (2–12% vs. 0–6%). There was also a trend towards higher serum vitamin A concentrations in WLS-MVS users compared to sMVS users (1.42 µmol/L, 95% CI [1.27, 1.57] vs. 1.18 µmol/L, 95% CI [0.98, 1.39], p = 0.06). Similarly, the prevalence of low serum vitamin A levels tended to be lower in the WLS-MVS group (T1–T3: 14–22% vs. 25–46%, p > 0.05). Only one participant presented with an elevated serum vitamin A level during pregnancy (WLS-MVS, T2: 3.71 µmol/L). For vitamin B6, there was a significant interaction between MVS and trimester (p = 0.02, Fig. 1g). Compared to WLS-MVS users, sMVS users had higher serum vitamin B6 concentrations in the first trimester (90.6 nmol/L, 95% CI [82.0, 99.8] vs. 132.1 nmol/L, 95% CI [114.6, 152.4], p < 0.001), but levels decreased to similar concentrations in the second and third trimester. Accordingly, the prevalence of elevated serum vitamin B6 levels was significantly lower in the WLS-MVS group compared to the sMVS group during the first and second trimester, but not during the third trimester (T1: 32.6% vs. 61.9%, p = 0.02; T2: 13.0% vs. 43.8%, p = 0.01; T3: 12.5% vs. 22.2%, p = 0.44).
Serum concentrations for WLS-MVS users and sMVS users in the RYGB group across the trimesters of pregnancy (T1, T2, T3). Lines depict estimated marginal means and confidence intervals (error bars). a Hemoglobin. **Significantly higher serum levels for WLS-MVS compared to sMVS (p=0.01). b Ferritin. **Significantly higher serum levels for WLS-MVS compared to sMVS (p=0.003). c Folic acid. **Significantly higher serum levels for WLS-MVS compared to sMVS (p=0.01). d Vitamin B12. e Vitamin A. f Vitamin B1. g Vitamin B6. ***Significantly higher serum levels for sMVS compared to WLS-MVS at T1 (p<0.001). h Vitamin D. i Corrected calcium
We did not find any differences in vitamin B1, vitamin B12, vitamin D, and calcium status between the two supplement groups.
Nutrient Status and Supplement use after SG
Throughout pregnancy following SG, low maternal serum concentrations were frequently observed for ferritin (56.4%), folic acid (20.5%), vitamin B12 (35.9%), vitamin A (30.8%), and vitamin D (43.6%) and to a lesser extent for hemoglobin (12.8%). Low serum levels of calcium (0%) and vitamins B1 and B6 (5.1%) were rare.
During pregnancy, the number of participants using WLS-MVS was comparable to those using sMVS (T1: 51.7% vs. 48.3%, T2: 45.9% vs. 54.1%, T3: 50.0% vs. 50.0%). Overall, WLS-MVS users had significantly higher serum levels of vitamin D during pregnancy compared to sMVS users (89.7 nmol/L, 95% CI [77.6, 101.8] vs. 65.4 nmol/L, 95% CI [53.3, 77.4], p = 0.001, Fig. 2h). Similarly, low serum vitamin D levels tended to be less prevalent in the WLS-MVS group, although not statistically significant (T1–T3: 13–18% vs. 37–39%, p > 0.05, Table 2). For vitamin B1, there was a significant interaction between MVS and trimester (p = 0.02, Fig. 2f). Serum vitamin B1 concentrations were comparable in the first trimester, but slightly decreased over pregnancy in the WLS-MVS group, resulting in lower serum vitamin B1 levels in the second and third trimester compared to the sMVS group (T2: 137.4 nmol/L, 95% CI [124.2, 150.6] vs. 161.6 nmol/L, 95% CI [149.0, 174.1], p = 0.01; T3: 133.9 nmol/L, 95% CI [120.1, 147.7] vs. 154.7 nmol/L, 95% CI [141.9, 167.5], p = 0.03).
Serum concentrations for WLS-MVS users and sMVS users in the SG group across the trimesters of pregnancy (T1, T2, T3). Lines depict estimated marginal means and confidence intervals (error bars). a Hemoglobin. b Ferritin. c Folic acid. d Vitamin B12. e Vitamin A. f Vitamin B1. **Significantly higher serum levels for sMVS compared to WLS-MVS at T2 (p=0.01). *Significantly higher serum levels for sMVS compared to WLS-MVS at T3 (p=0.03). g Vitamin B6. h Vitamin D. **Significantly higher serum levels for WLS-MVS compared to sMVS (p=0.001). i Corrected calcium
We did not find any differences in hemoglobin, ferritin, folic acid, vitamin B12, vitamin A, vitamin B6, and calcium status between the two supplement groups. There were no participants with an elevated serum vitamin A level during pregnancy after SG.
Discussion
The aim of this observational cohort study was to explore differences in nutrient status among women using WLS-MVS versus sMVS during pregnancy following RYGB or SG. During pregnancy after RYGB, WLS-MVS users had higher serum levels of hemoglobin, ferritin, and folic acid and lower serum levels of vitamin B6 compared to sMVS users. Iron deficiencies as well as elevated serum vitamin B6 levels were also less prevalent in the WLS-MVS group. During pregnancy after SG, WLS-MVS users had higher serum levels of vitamin D, but lower serum levels of vitamin B1 compared to sMVS users.
To date, only one other (retrospective) study analyzing supplement use among 197 singleton pregnancies after RYGB has been performed, also showing higher serum levels of hemoglobin and ferritin for WLS-MVS users compared to users of prenatal supplements [19]. They additionally found higher serum vitamin D levels among WLS-MVS users. Despite the similar doses of vitamin D within the MVS used in both studies, differences in the use of additional calcium/vitamin D3 supplementation, season of sampling, and individual differences in supplement adherence and sun exposure could have impacted these findings. To the best of our knowledge, there are no other studies available that report on differences in nutrient status and the efficacy of WLS-MVS during pregnancy after MBS.
Overall, differences between the supplement groups were more pronounced within the RYGB group. Several factors could be involved including the small sample of pregnant women after SG and the higher non-compliance to supplement protocols within this group [20, 21]. Furthermore, pregnancy complications such as hyperemesis gravidarum only occurred in three women who all underwent SG and used WLS-MVS. Persistent vomiting can increase the risk of depleted serum concentrations, and therefore affect our findings. Nonetheless, future research is required as nutritional needs during pregnancy after SG may be different compared to the general SG population.
Our findings are in line with those observed in the general, non-pregnant bariatric patient population. Homan et al. also found higher serum levels of hemoglobin, ferritin, and folic acid and less anemia and iron deficiencies in WLS-MVS users compared to sMVS users 3 years after RYGB [14]. We additionally observed lower serum vitamin B6 levels in the WLS-MVS group, which may be explained by the slightly lower dose of vitamin B6 in the WLS-MVS used in the present study (0.6–0.98 mg (43–70%) RDA vs. 0.98 mg (70%) RDA). Nevertheless, serum vitamin B6 concentrations were near the upper reference limit in both groups. Although exposure to extremely high doses of vitamin B6 (> 50 mg/day) did not appear to be associated with an increased risk of major malformations during pregnancy [22], attention on elevated levels is needed as they may cause maternal peripheral neuropathy [23].
Two observational studies comparing nutrient status between WLS-MVS users and sMVS users in the general SG population also found higher serum vitamin D concentrations in WLS-MVS users [13, 15]. Remarkably, they also found higher serum vitamin B1 levels in the WLS-MVS group compared to the sMVS group, whereas we found the opposite during pregnancy in the present study [13, 15]. This may be explained by the prevalence of hyperemesis gravidarum within this subgroup. Persistent vomiting is a risk factor for thiamine deficiency, which can ultimately result in Wernicke’s encephalopathy [24, 25]. Still, serum vitamin B1 concentrations were far above the lower reference limit in both groups and low serum levels during pregnancy were rare (< 5%).
Consensus on recommended doses for supplementation during pregnancy after MBS has not yet been reached for most micronutrients, evidenced by the lack of evidence-based guidelines as well as the limited consistency across current recommendations [6]. This is concerning as the risk of micronutrient depletion posed by the MBS procedure may be even higher during pregnancy due to the associated physiologic changes. The present study confirmed that low maternal serum concentrations of hemoglobin, ferritin, folic acid, vitamin B12, vitamin D, and vitamin A are prevalent during pregnancy following MBS. Iron deficiency was observed in more than half of the women (RYGB: 60%, SG: 56%), indicating the need for additional iron supplementation in this population. However, oral iron supplements are often poorly tolerated [26]. Alternate day dosing of iron could provide an alternative solution as it significantly increases iron absorption and results in a lower incidence of gastrointestinal side effects compared with dosing iron every day [27, 28]. Intravenous iron administration should be considered in pregnant women with iron deficiency anemia who do not respond to or cannot tolerate oral iron supplementation during the second or third trimester [29]. For folic acid, low serum levels during pregnancy were slightly more prevalent after SG compared to RYGB (21% vs. 13%), but mean serum concentrations remained above the lower limit during pregnancy in all groups. It remains uncertain if additional supplementation for folic acid is required when high-dosed WLS-MVS are used and recommendations in clinical practice are inconsistent. Therefore, a critical review of folic acid requirement in pregnancy post-MBS is needed. Until then, the total supplementation dose should not exceed 1 mg per day if there are no specific medical needs for a high dose in order to prevent potential negative adverse effects from over-supplementation, such as masking of vitamin B12 deficiency [30, 31].
Next to the risks caused by low maternal concentrations of micronutrients, elevated serum levels can also have detrimental consequences for both mother and child. For vitamin A, supplementation with beta-carotene is preferred over the use of retinol during pregnancy due to the well-documented risk of teratogenic malformations [32]. We observed one case of hypervitaminosis A when using WLS-MVS for RYGB containing 800 µg retinol (13 weeks: 3.71 µmol/L). As information on dietary intake was unknown, it is difficult to ascertain whether this elevated level was caused by supplement intake and/or dietary intake. Overall, most WLS-MVS contain about 600–800 µg retinol, which is far below the safe upper level of 3000 µg as indicated by the European Food Safety Authority [33]. Besides, serum vitamin A concentrations markedly decreased within the lower range over the course of pregnancy and low serum vitamin A levels were prevalent in our study population (RYGB: 21%, SG: 31%). Previous research even reports up to 90% of vitamin A deficiencies after MBS [10]. Vitamin A deficiency has been shown to cause night blindness and is associated with fetal growth restriction [6, 7]. Therefore, continuing the use of WLS-MVS during pregnancy after MBS is considered safe and may even be preferred over the use of supplements containing beta-carotene because of its low conversion efficiency [34].
Nevertheless, we do acknowledge that this finding should be regarded with precaution and that regular monitoring of nutrient status during pregnancy is essential to detect any abnormal blood levels (both low and high) at an early stage.
The main strength of the present study is the availability of prospective data on MVS use across the three trimesters of pregnancy, including detailed information on supplement composition. Furthermore, as composition of WLS-MVS may differ per type of MBS procedure, we provided results for RYGB and SG separately.
However, our findings must also be interpreted in light of certain limitations. Most importantly, MVS use differed greatly within and between participants. Because of the relatively small study sample, we were limited to categorizing all MVS as either WLS-MVS or sMVS. Particularly for sMVS, differences in the type of MVS (prenatal vs. regular), composition, and dosing may have impacted the daily administered dose of nutrients. Greater sample sizes are required in order to obtain sufficient statistical power to address these variations. Moreover, underlying motivation or preferences regarding the use of MVS were not addressed in the present study.
We only used pregnancy-specific cut-off values for hemoglobin as uniform, evidence-based pregnancy-specific cut-offs for other micronutrients are lacking [35]. Due to the physiological decrease in serum levels caused by hemodilution and increasing demands of the growing fetus, the number of deficiencies in the present study may have been overestimated [12]. Although some guidelines on laboratory values in healthy pregnant women are available [36, 37], differences in used assays and population groups may limit their transferability to other centers and populations. Ideally, laboratories should provide locally validated reference ranges for pregnant women to recognize normal changes in laboratory values induced by pregnancy. Measuring direct or functional biomarkers (e.g., holotranscobalamin or methylmalonic acid for vitamin B12) could also increase our understanding with respect to functional deficiencies as the used assays in the present study might not have been sensitive enough to pick up deficiencies at lower levels, therefore, possibly underestimating its true prevalence.
Another limitation of the present study is the lack of comprehensive information on the preconception period, as women were only enrolled once they were pregnant. Furthermore, other factors including compliance with supplement protocols, dietary intake, and presence of severe complaints or complications (e.g., abdominal pain, internal herniation) during pregnancy may have also impacted maternal nutrient status during pregnancy and should be taken into account in future research.
Last, it should be noted that all study participants received secondary or tertiary obstetrician-led care which may limit the generalizability of our study results to women receiving primary midwife-led care.
To conclude, our study confirmed that low maternal concentrations of micronutrients are highly prevalent during pregnancy after MBS. This leads to greater challenges to reach nutritional requirements in these pregnancies, making optimal supplementation essential. Overall, we found a general trend towards higher serum levels over the course of pregnancy for women using specialized WLS-MVS compared to those using standard, prenatal supplementation. However, both low and elevated serum levels were still observed in both groups, emphasizing the need for regular assessment and monitoring of nutrient status at each trimester to detect abnormal levels at an early stage.
Future research is needed to investigate how supplementation strategies can be optimized individually for this high-risk population. Ideally, these studies should start before pregnancy, employ pregnancy-specific cut-off values, include direct or functional biomarkers of nutrient status, and take contributing factors as underlying motivation and preferences regarding multivitamin supplementation, compliance with supplement protocols, dietary intake, and complications during pregnancy into account.
Supplementary Information.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;22(347):f5934.
Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34.
Brown WA, Shikora S, Liem R, Holland J, Campbell AB, Sprinkhuizen SM, et al. Seventh IFSO Global Registry Report. 2022.
Johansson K, Cnattingius S, Näslund I, Roos N, Trolle Lagerros Y, Granath F, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372(9):814–24.
Kwong W, Tomlinson G, Feig DS. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am J Obstet Gynecol. 2018;218(6):573–80.
Vanheule G, Ceulemans D, Vynckier AK, De Mulder P, Van Den Driessche M, Devlieger R. Micronutrient supplementation in pregnancies following bariatric surgery: a practical review for clinicians. Obes Surg. 2021;31(10):4542–54.
Slater C, Morris L, Ellison J, Syed AA. Nutrition in pregnancy following bariatric surgery. Nutrients. 2017;9(12):1338.
Chapmon K, Stoklossa CJ, Benson-Davies S, Integrated Health Clinical Issues Committee of the American Society for M, Bariatric S. Nutrition for pregnancy after metabolic and bariatric surgery: literature review and practical guide. Surg Obes Relat Dis. 2022;18(6):820–30.
Jans G, Matthys C, Bogaerts A, Lannoo M, Verhaeghe J, Van der Schueren B, et al. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: a systematic review. Adv Nutr. 2015;6(4):420–9.
Rottenstreich A, Elazary R, Goldenshluger A, Pikarsky AJ, Elchalal U, Ben-Porat T. Maternal nutritional status and related pregnancy outcomes following bariatric surgery: a systematic review. Surg Obes Relat Dis. 2019;15(2):324–32.
Milman N, Paszkowski T, Cetin I, Castelo-Branco C. Supplementation during pregnancy: beliefs and science. Gynecol Endocrinol. 2016;32(7):509–16.
Shawe J, Ceulemans D, Akhter Z, Neff K, Hart K, Heslehurst N, et al. Pregnancy after bariatric surgery: consensus recommendations for periconception, antenatal and postnatal care. Obes Rev. 2019;20(11):1507–22.
Heusschen L, Berendsen AAM, Deden LN, Hazebroek EJ, Aarts EO. Nutritional deficiencies 3 years after sleeve gastrectomy can be limited by a specialized multivitamin supplement. Obes Surg. 2022;32(11):3561–70.
Homan J, Schijns W, Aarts EO, van Laarhoven C, Janssen IMC, Berends FJ. An optimized multivitamin supplement lowers the number of vitamin and mineral deficiencies three years after Roux-en-Y gastric bypass: a cohort study. Surg Obes Relat Dis. 2016;12(3):659–67.
Smelt HJM, van Loon S, Pouwels S, Boer AK, Smulders JF, Aarts EO. Do specialized bariatric multivitamins lower deficiencies after sleeve gastrectomy? Obes Surg. 2020;30(2):427–38.
Rasmussen KM, Yaktine AL. Weight gain during pregnancy: reexaming the guidelines. Institute of Medicine Washington DC: National Academies Press; 2009.
Health Council of the Netherlands. Towards an optimal use of folic acid. The Hague: Health Council of the Netherlands; 2008.
Jans S, Beentjes M. Anemie in de verloskundige praktijk - Aanbevelingen voor preventie, diagnostiek en behandeling. The Royal Dutch Organisation of Midwives., 2010.
Snoek K, van de Woestijne N, Willemsen S, Klaassen R, Galjaard S, Laven J, et al. The impact of preconception gastric bypass surgery on maternal micronutrient status before and during pregnancy: a retrospective cohort study in the Netherlands between 2009 and 2019. Nutrients. 2022 Feb 9;14(4).
Smelt HJM, Heusschen L, Theel W, van Rutte PWJ, Nijboer T, Pouwels S, et al. Factors affecting patient adherence to multivitamin intake after bariatric surgery: a multicentre survey study from the patient’s perspective. Obes Surg. 2021;31(10):4316–26.
Coupaye M, Legardeur H, Sami O, Calabrese D, Mandelbrot L, Ledoux S. Impact of Roux-en-Y gastric bypass and sleeve gastrectomy on fetal growth and relationship with maternal nutritional status. Surg Obes Relat Dis. 2018 Jul 20.
Shrim A, Boskovic R, Maltepe C, Navios Y, Garcia-Bournissen F, Koren G. Pregnancy outcome following use of large doses of vitamin B6 in the first trimester. J Obstet Gynaecol. 2006;26(8):749–51.
Alsabah A, Al Sabah S, Al-Sabah S, Al-Serri A, Al Haddad E, Renno WM. Investigating factors involved in post laparoscopic sleeve gastrectomy (LSG) neuropathy. Obes Surg. 2017;27(5):1271–6.
Bahardoust M, Eghbali F, Shahmiri SS, Alijanpour A, Yarigholi F, Valizadeh R, et al. B1 vitamin deficiency after bariatric surgery, prevalence, and symptoms: a systematic review and meta-analysis. Obes Surg. 2022;32(9):3104–12.
Gasmi A, Bjorklund G, Mujawdiya PK, Semenova Y, Peana M, Dosa A, et al. Micronutrients deficiences in patients after bariatric surgery. Eur J Nutr. 2022;61(1):55–67.
Anvari S, Samarasinghe Y, Alotaiby N, Tiboni M, Crowther M, Doumouras AG. Iron supplementation following bariatric surgery: a systematic review of current strategies. Obes Rev. 2021;22(9):e13268.
Stoffel NU, Cercamondi CI, Brittenham G, Zeder C, Geurts-Moespot AJ, Swinkels DW, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4(11):e524–33.
Stoffel NU, Zeder C, Brittenham GM, Moretti D, Zimmermann MB. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105(5):1232–9.
Api O, Breyman C, Cetiner M, Demir C, Ecder T. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: iron deficiency anemia working group consensus report. Turk J Obstet Gynecol. 2015;12(3):173–81.
Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures - 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175–247.
Vynckier AK, Ceulemans D, Vanheule G, De Mulder P, Van Den Driessche M, Devlieger R. Periconceptional folate supplementation in women after bariatric surgery-a narrative review. Nutrients. 2021;13(5):1557.
Bastos Maia S, Rolland Souza AS, Costa Caminha MF, da Silva Lins S, Callou Cruz R, Dos Santos Carvalho C, et al. Vitamin A and pregnancy: a narrative review. Nutrients. 2019;11(3):681.
EFSA Scientific Committee on Food. Tolerable upper intake levels for vitamins and minerals. European Food Safety Authority, 2006.
Tang G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am J Clin Nutr. 2010;91(5):1468S-S1473.
Lamers Y. Approaches to improving micronutrient status assessment at the population level. Proc Nutr Soc. 2019;78(2):170–6.
Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114(6):1326–31.
Larsson A, Palm M, Hansson LO, Axelsson O. Reference values for clinical chemistry tests during normal pregnancy. BJOG. 2008;115(7):874–81.
Acknowledgements
The authors would like to thank Mirja de Lange, Sofie van Weelden, Gysèle Bleumink, Christianne Cardinaal-Cremers, and Renate Olde Wolbers for their support in participant inclusion.
Funding
This work was financially supported by the research funds of Rijnstate Hospital and Hospital Gelderse Vallei. They had no role in the design, analysis, or writing of this article.
Author information
Authors and Affiliations
Contributions
Conceptualization: LH and EK. Methodology: LH and AB. Formal analysis and investigation: LH. Resources: AvB, JvL, IK, and EH. Data curation: LH. Writing—original draft: LH and AB. Writing—review and editing: LH, AB, AvB, JvL, IK, and EH. Visualization: LH. Supervision: AB and EH. Project administration: LH. Funding acquisition: LH, IK, and EH.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
LH and EH received research funding (paid to institution) from FitForMe. They had no role in the design, analysis, or writing of this article. All others declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Low maternal micronutrient levels are highly prevalent during pregnancy after MBS.
• Using specialized WLS-MVS results in higher micronutrient serum levels during pregnancy.
• Regular monitoring during pregnancy is essential to detect abnormal serum levels early.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heusschen, L., Berendsen, A.A.M., van Bon, A.C. et al. Nutrient Status and Supplement Use During Pregnancy Following Metabolic Bariatric Surgery: A Multicenter Observational Cohort Study. OBES SURG (2024). https://doi.org/10.1007/s11695-024-07446-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11695-024-07446-4