Abstract
Introduction
Laparoscopic sleeve gastrectomy (LSG) is associated with postoperative nausea and vomiting (PONV). We aimed to compare the effects of aprepitant on the incidence of PONV after LSG.
Methods
In this double-blind, randomized controlled trial, the case group received the standard care regimen for PONV (dexamethasone 10 mg, ondansetron 4 mg, and metoclopramide 10 mg) plus prophylactic oral aprepitant 80 mg 1 h preoperatively. The control group received standard care plus a placebo. Comparative analyses using the Rhodes index were performed at 0, 6, 12, and 24 h postoperatively.
Results
A total of 400 patients (201 in the aprepitant group and 199 in the placebo group) underwent LSG. The groups were homogeneous. The aprepitant group experienced less PONV: early, 69 (34.3%) vs. 103 (51.7%), p ≤ 0.001; 6 h, 67 (33.3%) vs. 131 (65.8%), p ≤ 0.001; 12 h, 41 (20.4%) vs. 115 (57.8%), p ≤ 0.001; and 24 h, 22 (10.9%) vs. 67 (33.7%), p ≤ 0.001. Fewer patients in the aprepitant group vomited: early, 3 (1.5%) vs. 5 (2.5%), p = 0.020; 6 h, 6 (3%) vs. 18 (9%), p = 0.020; 12 h, 2 (1%) vs. 17 (8.5%), p = 0.006; and 24 h, 1 (0.5%) vs. 6 (3%), p = 0.040. Patients in the aprepitant group required less additional PONV medication: early, 61 (30.3%) vs. 86 (43.2), p = 0.008; 6 h, 7 (3.5%) vs. 34 (17%), p = 0.001; 12 h, 6 (3%) vs. 31 (15.6%), p ≤ 0.001; and 24 h, 5 (2.5%) vs. 11 (5.5%), p ≤ 0.001.
Conclusions
Prophylactic aprepitant improved PONV between 0 h (early) and 24 h postoperatively in patients undergoing LSG.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, a chronic condition with numerous manifestations, has become a global epidemic [1].
Over the past decade, significant progress has been made in laparoscopic sleeve gastrectomy (LSG) for the treatment of morbid obesity. However, LSG is associated with postoperative nausea and vomiting (PONV) in approximately 48% of cases [2]. PONV may prolong a patient’s discharge from the post-anesthesia care unit, increasing medical costs [3]. The use of anesthetics and opioids to treat postoperative pain, type of surgery, and patient characteristics contribute to PONV. PONV can lead to many complications, such as increased intragastric pressure, hemorrhage, suture dehiscence, leakage, dehydration, and electrolyte imbalances, which lengthen the hospital stay due to delayed recovery [4, 5].
PONV remains one of the most significant causes of patient dissatisfaction with the perioperative experience. PONV rates are predicted to decrease with the use of various pharmacological agents [6]. Aprepitant is a selective neurokinin-1 (NK1) receptor antagonist with a half-life of 9–12 h that effectively prevents opioid-induced vomiting. It is used to prevent PONV and chemotherapy-induced nausea and vomiting [7]. It is also safe for use in patients with obesity undergoing anesthesia and surgery [8].
This randomized, double-blind study aimed to evaluate the efficacy of a combination of ondansetron, metoclopramide, dexamethasone, and aprepitant versus ondansetron, metoclopramide, dexamethasone, and placebo as PONV prophylaxis in patients with obesity undergoing LSG.
Materials and Methods
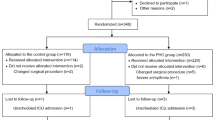
This randomized, double-blind (participants and care providers) study included patients who underwent LSG at a private center. The candidates were recruited between December 2022 and January 2023. This study aimed to determine the effects of aprepitant on PONV from the early postoperative period to 24 h postoperatively. A total of 400 consecutive patients were enrolled and randomized in a 1:1 ratio. The case group received a standard care regimen for PONV during surgery (dexamethasone 10 mg, ondansetron 4 mg, and metoclopramide 10 mg) plus a single oral dose of 80 mg of aprepitant 1 h preoperatively. The control group received the same standard of care and an oral placebo. A comparative analysis of the demographic, anthropometric, and perioperative factors was performed. The primary objective was to evaluate PONV using the Rhodes index at 0, 6, 12, and 24 h postoperatively (Appendix Table 6). The Rhodes index, which was designed to measure nausea frequency and duration, and vomiting quantity, is an eight-item instrument that uses a five-point Likert scale and consists of three subscales: nausea (range, 0–12), vomiting (range, 0–12), and retching (range, 0–8). These ranges provide a severity rating of 0–4 for each item [9]. Rescue medications were administered to patients with moderate or severe PONV at the discretion of the postoperative care physician. The treatment options included ondansetron 4 mg, dexamethasone 8 mg, and diphenidol 40 mg.
The sample size calculation aimed to detect a clinically significant difference in PONV incidence between the two groups. We anticipated a 20% reduction in PONV incidence in the intervention group compared to the control group, based on preliminary findings and previous studies suggesting a potential decrease in PONV with aprepitant use [6]. Assuming a standard deviation (SD) of 0.5 for the Rhodes index score, to detect this difference with 95% confidence and 80% power, the calculation required a minimum of 278 participants, or 139 per group. We performed this calculation using G*Power software, version 3.1.9.7, selecting the sample size analysis for independent means tests with specific parameters: an interest mean difference (d = 0.4), derived from the expected 20% difference and the SD, a significance level (α) of 0.05, and a statistical power (1-β) of 0.80.
Randomization was conducted using a computer-generated sequence obtained from the website www.randomizer.org. For blinding, we used a placebo capsule closely resembling the active medication in appearance. To further ensure the integrity of the blinding process, the administration of both the study drug and the placebo was carried out by hospital staff who were not involved in the study, thus maintaining impartiality, and minimizing bias. The placebo consisted of a commercially sourced inert capsule, matched in color, size, and shape to the aprepitant capsule, and filled with 10 mg of fructose to mimic the weight and consistency of the active drug. The commercial name is “Vegan Empty Pill Capsules”, manufactured by XPRS Nutra, white color and size of 14 × 5x5 mm. The inclusion criteria were adults aged 18–65, non-smoking status, and a body mass index (BMI) > 35 kg/m2, with or without comorbidities. Exclusion criteria included documented hypersensitivity to any component of the study regimen, treatment with pimozide, terfenadine, astemizole, or cisapride, allergies to drugs used in the protocol, a history of drug or alcohol abuse, and a history of prior bariatric procedures. Smoking status was an exclusion criterion due to scientific evidence suggesting that smoking may influence the incidence of PONV. Specifically, the protective effect of smoking on PONV has been recognized in several studies, with research indicating that smokers have a reduced risk of PONV, potentially due to the pharmacokinetic effects associated with smoking [10]. This study was approved by the local ethics committee and registered at www.ClinicalTrials.gov (NCT05772676).
Surgical Technique
The patient was placed in the supine position under general anesthesia, and access to the peritoneal cavity was initiated 20 cm below the xiphoid process. The five-trocar technique was used. The gastroepiploic vessels were located 2–5 cm from the pylorus, the short gastrosplenic vessels were identified on the greater curvature, and the fundus was dissected using a harmonic scalpel until the left pillar was visualized. A 36-Fr calibration bougie was used, and stapling was performed every 60 mm based on the gastric wall thickness. The staple line was reinforced with 2-0 non-absorbable sutures.
Statistical Analysis
The results are expressed as percentages, means, and standard deviations. Normal data distribution was confirmed using the Kolmogorov-Smirnov test. Comparative analysis of continuous variables was performed using the Student's t-test, while categorical variables were analyzed using the chi-squared test. Statistical significance was set at p < 0.05. Statistical analyses were performed using the SPSS for Mac (version 25; SPSS Inc., Chicago, IL, USA).
Results
A total of 400 patients fulfilled the inclusion criteria and underwent LSG; 201 received aprepitant, and 199 received a placebo. The baseline demographic information was homogeneous between the groups (Table 1). Most of the patients (94%) were women. The mean age of the patients was 38 years. The mean BMI was 42.2 kg/m2 in the aprepitant group and 42 kg/m2 in the placebo group. The results of the perioperative analysis are presented in Table 1. Overall, complications occurred in six patients (1.5%); there were no cases of mortality, conversion to open surgery, or reoperation.
In the early postoperative evaluation (T0), the aprepitant group displayed significantly reduced rates of nausea (34.3% vs. 51.7%, p < 0.001) and vomiting (1.5% vs. 2.5%, p = 0.020) and rescue medication use (30.3% vs. 43.2%, p = 0.008) (Table 2). At the 6-h assessment, patients continued to experience less nausea (33.3% vs. 65.8%, p < 0.001), vomiting (3% vs. 9%, p < 0.001), and the need for rescue medication (3.5% vs. 17%, p < 0.001) (Table 3). Similarly, at 12 h postoperatively, the aprepitant group showed lower levels of nausea (20.4% vs. 57.8%, p < 0.001), vomiting (1% vs. 8.5%, p < 0.001), and rescue medication requirements (3% vs. 15.6%, p < 0.001) (Table 4). Finally, at the 24-h evaluation, the aprepitant group continued to experience less nausea (10.9% vs. 33.7%, p < 0.001), vomiting (0.5% vs. 3%, p < 0.001), and the need for rescue medication (2.5% vs. 5.5%, p < 0.001) (Table 5). Complete analyses of each time point and all the measured parameters are shown in Tables 2, 3, 4, and 5.
Discussion
In this randomized controlled trial involving patients who underwent LSG, the use of prophylactic aprepitant significantly reduced the symptoms and discomfort associated with PONV, thereby reducing postoperative complications. Furthermore, appropriate use decreases the need for rescue medications to manage PONV and contributes to a more comfortable and less distressing patient recovery process. PONV is associated with significant perioperative morbidity in the form of dehydration, electrolyte imbalance, venous hypertension, and potential broncho-aspiration, with a higher risk of rehospitalization and emergency department visits [11]. Compared to the general surgical population, patients undergoing bariatric surgical procedures have a much higher incidence of PONV [11]. Moreover, patients undergoing LSG are twice as likely to develop PONV than those undergoing laparoscopic Roux-en-Y gastric bypass, attributable to factors such as stomach manipulation, patient-specific characteristics, and the anesthetic techniques [12]. Involvement of stomach pace disruption and inflammation might be implicated, but there is a lack of scientific data supporting this. Nonetheless, the inclusion of aprepitant in antiemetic regimens can significantly decrease the occurrence of PONV in this population. In our study, 42.2% of patients experienced PONV within the 24-h follow-up, a lower proportion than in previous studies on bariatric surgery [13,14,15].
Multimodal antiemetic therapy involves the simultaneous use of different antiemetics acting through different physiological pathways to produce effective synergistic antiemetics with fewer side effects [16]. In our study, we added aprepitant, a long-acting antagonist of the substance P/neurokinin 1 receptor with little or no affinity for selective serotonin receptors, corticosteroid receptors, or dopamine receptors, and targets of existing antiemetics for PONV. However, it has a high selective affinity for the human substance P NK1 receptor antagonist present in the brainstem regions involved in emesis [17, 18]. The mechanisms of chemotherapy-induced nausea and vomiting (CINV) and postoperative nausea and vomiting (PONV) after bariatric surgery differ significantly. CINV is primarily attributed to the activation of the serotonin and substance P/neurokinin-1 (NK1) pathways, leading to stimulation of the chemoreceptor trigger zone [19]. Aprepitant acts as an NK1 receptor antagonist, effectively managing CINV by blocking substance P signaling. In contrast, PONV, particularly after bariatric surgery, involves factors like surgical manipulation, anesthesia, and individual patient characteristics. Despite these differences, the involvement of NK1 receptors in both CINV and PONV underlies the effectiveness of aprepitant in managing these conditions [20, 21]. We noted a significant reduction in episodes of nausea and vomiting compared to the traditional multimodal ondansetron plus dexamethasone scheme. These results are similar to those reported in a systematic review and meta-analysis [22] that analyzed 15 clinical trials, including four on laparoscopic surgery and one on bariatric surgery, and showed a clear advantage of aprepitant (alone or in combination) in reducing PONV on postoperative day 1.
Diemunsch et al. [4] reported that aprepitant was more effective using five evaluation criteria: absence of significant nausea (56.4% vs. 48.1%); no nausea (39.6% vs. 33.1%); no vomiting (86.7% vs. 72.4%); no nausea or vomiting (38.3% vs. 31.4%); and no nausea, vomiting, or rescue medication use (37.9% vs. 31.2%). Our study used the Rhodes index, which includes six evaluation criteria: the presence and number of retching episodes (4.7% vs. 11.6%), nausea (24.7% vs. 52.25%), vomiting (1.5% vs. 5.7%), and discomfort caused by each (4.7% vs. 11.6%, 24.3% vs. 51.8%, and 1.5% vs. 5.7%, respectively). We found superiority of aprepitant for all parameters over the traditional regimen; however, not all differences were statistically significant (p < 0.05).
A similar study [23] reported that aprepitant was not inferior to palonosetron in terms of complete response at 0–48 h postoperatively (74% vs. 77%). At 0 and 2 h after administration, nausea severity was significantly lower with aprepitant 40 mg than palonosetron 75 mg. In contrast, we found a statistically significant reduction in the use of rescue medication at every time point of analysis, considering that 90% of the patients in our study were at high risk according to Apfel’s criteria [6] (greater use of rescue antiemetics). Sinha et al. [24] found that in populations with obesity, the cumulative incidence of vomiting at 72 h was significantly lower in the aprepitant 80 mg group (3%) than in the ondansetron 4 mg group (15%). The combined results of the parameters in our study showed a lower incidence of nausea and vomiting episodes in the aprepitant versus the traditional regimen group with a 2.1- and 3.8-times lower probability of nausea and vomiting, respectively.
Most studies of PONV and aprepitant have used doses ranging from 40 to 125 mg without significant adverse effects [8, 25, 26]; however, one study reported dizziness using 125 mg versus 80 mg [26]. Other side effects include asthenia, hiccups, dehydration, diarrhea, gastritis, elevation in liver function tests, and thrombocytopenia. Such effects are challenging to evaluate in a patient undergoing a sleeve gastrectomy since the primary surgery itself can induce these problems. A limitation of our study includes a lack of information in terms of laboratory tests and specific information about related side effects. Despite the above, our finding aligns with the literature indicating that aprepitant is generally well-tolerated. A meta-analysis by Liu et al. (2023) [27] supports the safety of aprepitant, showing no significant difference in the incidence of major side effects compared to control groups. A homogeneous dose of 80 mg was administered to all patients in this study. For comparison, another group of patients should be randomized to receive a 125 mg dose in a future study. When comparing our findings with the established global benchmarks for Sleeve Gastrectomy (SG) [28], which are a 90-day complication rate of 6.2% and a readmission rate of 5.5%, our study contributes important insights into one specific aspect of postoperative recovery: the management of PONV. While the benchmark study provides broader surgical outcome metrics, our study’s focus on PONV offers a critical perspective on enhancing patient recovery and comfort, key components of overall surgical success. The observed reduction in PONV rates in our study could potentially contribute to broader goals of reducing postoperative complications and readmissions, as effective PONV management is closely linked with patient well-being and satisfaction in the immediate postoperative period. Our findings suggest that incorporating aprepitant into the postoperative care regimen for SG patients could be instrumental in achieving or even surpassing these global benchmarks, thereby enhancing the quality of care in bariatric surgery.
The limitations of this study include the timing of aprepitant administration and the start of the surgical procedure, which may have varied in some cases owing to logistics. Some assessments were performed early in the morning, which interrupted the patients’ sleep and had unknown effects. Our study also acknowledges the cost of aprepitant as a potential limitation. The total costs, including the intervention, placebo, and other associated expenses such as data collection and analysis, were entirely covered by the research team. The cost for one dose of aprepitant was approximately 45 USD, resulting in a total of 9,045 USD for the 201 participants. Additionally, the cost for the placebo and other research-related expenses were also fully funded by the investigators. It is important to note that our study did not receive any sponsorship or support from any pharmaceutic, ensuring an unbiased approach in our research methodology and analysis. This financial aspect is an important consideration for the practical application of our findings in clinical settings. Another limitation is that smoking status and cannabis consumption reduced PONV. These were among the exclusion criteria; this decision was based on scientific evidence suggesting that smoking may influence the incidence of PONV. The protective effect of smoking on PONV has been recognized in several studies. For instance, a study published by Habib AS et al. [10] showed that smokers have a reduced risk of PONV, which might be attributed to the pharmacokinetic effects associated with smoking. Therefore, including smokers in our study could have introduced a significant confounding variable, potentially skewing the results and affecting the validity of our conclusions regarding the efficacy of the intervention in preventing PONV. By excluding smokers, our study aimed to provide a clearer assessment of the intervention's effectiveness in a population not influenced by this protective factor; however, they were based only on patient interrogation, and no special laboratory tests were performed. Despite these limitations, this is the first randomized study to demonstrate the substantial improvement of prophylactic aprepitant for PONV in patients undergoing LSG. This approach has significantly transformed our routine clinical practice, leading to a more comfortable postoperative recovery for patients, characterized by fewer surgical discomforts. Additionally, it has facilitated an enhanced tolerance to a liquid diet post-surgery.
Conclusions
Compared to the standard of care, preoperative aprepitant significantly improved PONV in patients who underwent LSG. This effect was observed in the early postoperative period and persisted for up to 24 h postoperatively.
References
Camilleri M, Acosta A. Combination therapies for obesity. Metab Syndr Relat Disord. 2018;16:390–4.
Ashoor TM, Kassim DY, Esmat IM. A randomized controlled trial for prevention of postoperative nausea and vomiting after laparoscopic sleeve gastrectomy: aprepitant/dexamethasone vs. mirtazapine/dexamethasone. Anesthesiol Res Pract. 2022;2022:3541073.
Groene P, Eisenlohr J, Zeuzem C, Dudok S, Karcz K, Hofmann-Kiefer K. Postoperative nausea and vomiting in bariatric surgery compared to non-bariatric gastric surgery. Wideochir Inne Tech Maloinwazyjne. 2019;14:90–5.
Moon HY, Baek CW, Choi GJ, Shin HY, Kang H, Jung YH, et al. Palonosetron and aprepitant for the prevention of postoperative nausea and vomiting in patients indicated for laparoscopic gynaecologic surgery: a double-blind randomised trial. BMC Anesthesiol. 2014;14:68. https://doi.org/10.1186/1471-2253-14-68.
Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131:411–48. https://doi.org/10.1213/ANE.0000000000004833.
Sinha AC, Singh PM, Williams NW, Ochroch EA, Goudra BG. Aprepitant’s prophylactic efficacy in decreasing postoperative nausea and vomiting in morbidly obese patients undergoing bariatric surgery. Obes Surg. 2014;24(2):225–31. https://doi.org/10.1007/s11695-013-1065-1.
Okafor D, Kaye AD, Kaye RJ, Urman RD. The role of neurokinin-1 (substance P) antagonists in the prevention of postoperative nausea and vomiting. J Anaesthesiol Clin Pharmacol. 2017;33:441–5. https://doi.org/10.4103/0970-9185.222511.
Gan TJ, Apfel CC, Kovac A, Philip BK, Singla N, Minkowitz H, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104:1082–9. https://doi.org/10.1213/01.ane.0000263277.35140.a3
Rhodes VA, McDaniel RW. The index of nausea, vomiting, and retching: a new format of the lndex of nausea and vomiting. Oncol Nurs Forum. 1999;26:889–94.
Habib AS, Chen YT, Taguchi A, Hu XH, Gan TJ. Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Curr Med Res Opin. 2006;22(6):1093–9. https://doi.org/10.1185/030079906X104830.
Schumann R, Ziemann-Gimmel P, Sultana A, Eldawlatly AA, Kothari SN, Shah S, et al. Postoperative nausea and vomiting in bariatric surgery: a position statement endorsed by the ASMBS and the ISPCOP. Surg Obes Relat Dis. 2021;17:1829–33. https://doi.org/10.1016/j.soard.2021.08.005.
Qiu T, Men P, Xu X, Zhai S, Cui X. Antiemetic regimen with aprepitant in the prevention of chemotherapy-induced nausea and vomiting: an updated systematic review and meta-analysis. Med (Baltim). 2020;99:e21559. https://doi.org/10.1097/MD.0000000000021559.
Bataille A, Letourneulx JF, Charmeau A, Lemedioni P, Léger P, Chazot T, et al. Impact of a prophylactic combination of dexamethasone-ondansetron on postoperative nausea and vomiting in obese adult patients undergoing laparoscopic sleeve gastrectomy during closed-loop propofol-remifentanil anesthesia. Eur J Anaesthesiol. 2016;33:898–905. https://doi.org/10.1097/EJA.0000000000000427.
Benevides ML, Oliveira SS, de Aguilar-Nascimento JE. The combination of haloperidol, dexamethasone, and ondansetron for prevention of postoperative nausea and vomiting in laparoscopic sleeve gastrectomy: a randomized double-blind trial. Obes Surg. 2013;23:1389–96. https://doi.org/10.1007/s11695-013-0923-1.
Ho KY, Chiu JW. Multimodal antiemetic therapy and emetic risk profiling. Ann Acad Med Singap. 2005 Mar;34(2):196–205. https://pubmed.ncbi.nlm.nih.gov/15827668/
Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993;73:229–308. https://doi.org/10.1152/physrev.1993.73.2.229.
Van Laere K, De Hoon J, Bormans G, Koole M, Derdelinckx I, De Lepeleire I, et al. Equivalent dynamic human brain NK1-receptor occupancy following single-dose i.v. fosaprepitant vs. oral aprepitant as assessed by PET imaging. Clin Pharmacol Ther. 2012;92:243–50. https://doi.org/10.1038/clpt.2012.62.
Singh PM, Borle A, Rewari V, Makkar JK, Trikha A, Sinha AC, et al. Aprepitant for postoperative nausea and vomiting: a systematic review and meta-analysis. Postgrad Med J. 2016;92:87–98. https://doi.org/10.1136/postgradmedj-2015-133515.
Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008 Jun 5;358(23):2482–94. https://doi.org/10.1056/NEJMra0706547.
Roila F, Molassiotis A, Herrstedt J, et al. MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting. Ann Oncol. 2016;27(suppl 5):v119–33. https://doi.org/10.1093/annonc/mdw270.
Therneau IW, Martin EE, Sprung J, Kellogg TA, Schroeder DR, Weingarten TN. The role of aprepitant in prevention of postoperative nausea and vomiting after bariatric surgery. Obes Surg. 2018;28(1):37–43. https://doi.org/10.1007/s11695-017-2797-0.
Diemunsch P, Apfel C, Gan TJ, Candiotti K, Philip BK, Chelly J, et al. Preventing postoperative nausea and vomiting: post hoc analysis of pooled data from two randomized active-controlled trials of aprepitant. Curr Med Res Opin. 2007;23:2559–65. https://doi.org/10.1185/030079907X233115.
Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999 Sep;91(3):693–700. https://pubmed.ncbi.nlm.nih.gov/17236637/.
Jung WS, Kim YB, Park HY, Choi WJ, Yang HS. Oral administration of aprepitant to prevent postoperative nausea in highly susceptible patients after gynecological laparoscopy. J Anesth. 2013;27:396–401. https://doi.org/10.1007/s00540-012-1529-9.
Suh S, Helm M, Kindel TL, Goldblatt MI, Gould JC, Higgins RM. The impact of nausea on post-operative outcomes in bariatric surgery patients. Surg Endosc. 2020;34:3085–91. https://doi.org/10.1007/s00464-019-07058-5.
Lim CS, Ko YK, Kim YH, Park SI, Kim JK, Kim MJ, et al. Efficacy of the oral neurokinin-1 receptor antagonist aprepitant administered with ondansetron for the prevention of postoperative nausea and vomiting. Korean J Anesthesiol. 2013;64:212–7. https://doi.org/10.4097/kjae.2013.64.3.212.
Liu Y, Chen X, Wang X, et al. The efficacy of aprepitant for the prevention of postoperative nausea and vomiting: a meta-analysis. Medicine (Baltimore). 2023;102(29):e34385. https://doi.org/10.1097/MD.0000000000034385.
Gero D, Raptis DA, Vleeschouwers W, van Veldhuisen SL, Martin AS, Xiao Y, et al. Defining global benchmarks in bariatric surgery: a retrospective multicenter analysis of minimally invasive roux-en-y gastric bypass and sleeve gastrectomy. Ann Surg. 2019;270(5):859–67. https://doi.org/10.1097/SLA.0000000000003512.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
Authors Elías Ortiz, Alberto I. González, Valeria Jaime, José A Guzmán, Isaac Esparza, José O. Orozco, Manuel A Guerrero, Almino Ramos and Carlos Zerrweck declare no commercial associations or conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Point
• Laparoscopic sleeve gastrectomy is associated with postoperative nausea and vomiting.
• The study compared aprepitant to placebo in a blinded-randomized fashion.
• Aprepitant improved postoperative nausea and vomiting between 0 and 24 h post-LSG.
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ortiz, E., González, A.I., Jaime, V. et al. The impact of Aprepitant on Nausea and Vomiting following Laparoscopic Sleeve Gastrectomy: A Blinded Randomized Controlled Trial. OBES SURG 34, 1316–1323 (2024). https://doi.org/10.1007/s11695-024-07129-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-024-07129-0