Abstract
Introduction
The inflammatory state that accompanies adiposity and the metabolic syndrome (MetS) is called “low-grade” inflammation. White blood cell count (WBC) has been proposed as an emerging biomarker for predicting future cardiovascular events, MetS and mortality. Bariatric surgery (BS) improves comorbidities associated with obesity and the MetS and the surgically induced weight loss is known to improve inflammatory status.
Objectives
To analyze the improvement of low-grade inflammation associated to obesity in patients with metabolically healthy severe obesity (MHSO) and patients with metabolically unhealthy obesity (MUSO) (severe obesity with MetS) after primary bariatric surgery as well as the protective effect of BS against the development of MetS in patients with MHSO by reducing the WBC.
Materials and methods
Retrospective analysis of prospectively collected data of patients undergoing laparoscopic primary BS (gastric by-pass or sleeve gastrectomy) from January 2004-December 2015. Outcomes included changing of low-grade inflammation in terms of leukocytes, neutrophils, lymphocytes, and platelets.
Results
Twenty-one patients with MHSO and 167 patients with MUSO underwent laparoscopic primary BS. The preoperative values of leukocyte and platelet were statistically higher in the group of patients with MHSO. In both groups, there was significant postoperative decrease of inflammatory markers. The greatest drop in WBC occurred in the second postoperative year. No patient of the group of patients with MHSO developed MetS within five postoperative years.
Conclusions
Surgically induced weight loss plays an important role for improvement in chronic inflammation associated to obesity because of reduction of visceral fat mass. MHSO associates a low-grade chronic inflammatory status comparable to MUSO. The improvement or decrease of low-grade inflammation in patients with metabolically healthy severe obesity after bariatric surgery could have a protective effect against the development of MetS and medical conditions associated with severe obesity.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction/Purpose
Obesity is associated with increased mortality and morbidity, which is assumed to be mediated mainly by insulin resistance (IR), diabetes, hypertension and lipid disturbances [1].
The inflammatory state that accompanies adiposity and the metabolic syndrome (MetS) is often called “low-grade” chronic inflammation, meaning metabolically triggered inflammation [2]. Recent studies have suggested a link between obesity and inflammation, as increased mass of adipose tissue may activate the immune process in the white adipose tissue (WAT) and liver and immune cells [3]. WAT is the source of some pro-inflammatory cytokines such as tumor necrosis alpha (TNF-a) and interleukin-6 (IL-6) [3]; both inflammatory markers raise the white blood cell count (WBC) and they induce the hepatic expression of protein genes in the acute phase such as C reactive protein (CRP) [4].
MetS is defined a condition of high risk for cardiovascular disease and previous studies showed associations of WBC with IR which is a central mechanism of MetS [4, 5].
WBC is an objective marker of systemic inflammation [4,5,6]. Relative leukocytosis has been positively associated with obesity, high blood pressure, high serum triglycerides [7], type 2 diabetes [5, 7], and the progression of atherosclerosis [4, 5] while the healthy practice such as maintaining normal weight is negatively associated with WBC, and cultivating healthier practices would lead to lower WBC, or the prevention of low-grade inflammation [4]. Thus, there are reasons to believe that leukocyte is not simply a marker of chronic inflammation, but directly contributes to the pathogenesis of MetS and atherosclerosis [7].
The WBC has been proposed as an emerging biomarker for predicting future cardiovascular events, MetS, and mortality [5].
Bariatric and metabolic surgery (BS) improves medical conditions associated with severe obesity and the MetS, and the surgically induced weight loss is known to improve inflammatory status [2, 3].
The aim of the present study was to analyze the improvement of low-grade inflammation associated to obesity in patients with metabolically healthy severe obesity (MHSO) and patients with metabolically unhealthy obesity (MUSO) (severe obesity with MetS) after primary bariatric surgery as well as the protective effect of bariatric surgery against the development of MetS in patients with metabolically healthy severe obesity by reducing the WBC.
Materials and Methods
We performed a retrospective analysis of prospectively collected data of patients undergoing laparoscopic primary BS (gastric by-pass (LGBP) or sleeve gastrectomy (LSG)) from January 2004 to December 2015.
The eligibility criteria were as follows:
-
Body mass index (BMI) > 35 kg/m2 and metabolic syndrome.
-
BMI > 40 kg/m2 without medical conditions associated with severe obesity.
-
Patients aged 18–65 years.
-
Laparoscopic primary bariatric surgery.
The exclusion criteria were as follows:
-
Revisional surgery.
-
Laparotomy approach.
Data collection included gender, age, weight, BMI (Kg/m2), medical conditions associated with severe obesity [type 2 diabetes (T2D), arterial hypertension (AHT), dyslipidemia (DL), obstructive sleep apnea syndrome (OSAS) and non-alcoholic fatty liver disease (NAFLD)], % Excess BMI Loss (%EBMIL), % Total Weight Loss (%TWL), and laboratory measurements [leukocytes (× 103/µL), neutrophils (× 103/µL), lymphocytes (× 103/µL), and platelets (× 103/µL)] obtained in the preoperative period (T0) and 1st postoperative year (T1), 2nd postoperative year (T2), and 5th postoperative year (T5).
Routine laboratory measurements were done using automated chemical analyses at our center.
Patients with metabolically healthy severe obesity (MHSO) were those with BMI > 40 kg/m2 without medical conditions associated with severe obesity neither MetS and patients with metabolically unhealthy obesity (MUSO) were those with BMI > 35 kg/m2 and MetS.
MetS was defined in accordance with the criteria of the International Diabetes Federation [8], subjects were regarded as having MetS when they had three or more of the following components:
-
Elevated waist circumference for European individuals (> 94 cm and > 80 cm in men and women, respectively).
-
Elevated triglycerides (> 150 mg/dL) or drug treatment for elevated triglycerides.
-
Low concentrations of high-density lipoprotein cholesterol (HDL-c) [< 40 mg/dL in men and < 50 mg/dL in women] or drug treatment for low HDL-c.
-
Elevated blood pressure (systolic > 130 and/or diastolic > 85 mm Hg) or antihypertensive drug treatment.
-
Elevated fasting glucose (> 100 mg/dL) or drug treatment for elevated glucose.
Primary outcome measure was the changing of low-grade chronic inflammation in terms of leukocytes, neutrophils, lymphocytes, and platelets in patients with MHSO. Secondary outcome measures were to compare the changing of low-grade chronic inflammation between patients with MHSO and patients with MUSO and the possible protective effect of BS against the development of MetS in patients with severe obesity by reducing the WBC.
Statistical analysis was performed using the SPSS 22.0. (IBM SPSS Statistics Base). Kolmogorov–Smirnov statistics were used to evaluate sample normality distribution. Continuous variables were reported as mean and standard deviation (SD) when normally distributed data and as median and range [minimum–maximum] when not normally distributed data. Data were analyzed in subgroups according to patients with MHSO and patients with MUSO. Paired t test or Wilcoxon test were used to compare pre- and post-surgery data when appropriate. Intergroup differences were tested by a t test for normally distributed data or a Mann–Whitney U test for non-normally distributed data. Spearman’s rank correlation coefficient was calculated to correlate quantitative variables. A multivariate analysis was performed. The dependent variable was WBC or platelets (T0, T1, T2, and T5), while independent variables were (1) age; (2) gender; (3) weight; (4) BMI; (5) %EBMIL; (6) %TWL; (7) medical conditions associated with severe obesity; and (8) bariatric procedure.
A p value < 0.05 was statistically significant.
The study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical permission was obtained from the hospital ethics committee (reference number HULP: PI-2963).
Results
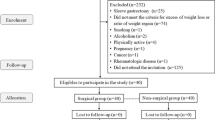
A total of 478 patients underwent bariatric surgery. The medical records of 377 patients who underwent laparoscopic primary BS were analyzed. After exclusion, 21 patients met the criteria for MHSO and 167 patients met the criteria for MUSO.
Baseline Characteristics and Postoperative Weight Evolution
The baseline demographic data and type of bariatric surgery of both groups and the prevalence of comorbidities prior to surgery of the patients with MUSO are shown in Table 1 and Fig. 1, respectively. There was statistically significant difference between the two groups regarding the age and the prevalence of NAFLD; the patients with MHSO were younger and had a lesser prevalence of NAFLD than the patients with MUSO (p < 0.001 and p = 0.032, respectively).
The postoperative weight evolution of both groups was represented in Fig. 2 and Fig. 3; the weight loss of the MHSO group was greater than in the MUSO group but without any statistically significant difference between them.
Preoperative WBC and Platelet Count in Relation to Baseline Characteristics
A weak inverse correlation was found between age and preoperative values of leukocytes (rho = − 0.262), neutrophils (rho = − 0.182), and platelets (rho = − 0.253) and a moderate inverse correlation was found between age and preoperative lymphocyte count (rho = − 0.307).
No correlation was found between preoperative values of inflammatory markers and initial weight, BMI, and waist circumference.
In the MUSO group, when performing the multivariate analysis for leukocytes, significant interaction was found regarding age (p = 0.001) and NAFLD (p = 0.011); in the multivariate analysis for neutrophils, significant interaction was found regarding age (p = 0.040); in the multivariate analysis for lymphocytes, significant interaction was found regarding age (p < 0.001) and NAFLD (p = 0.023); and in the multivariate analysis for platelets, significant interaction was found regarding age (p = 0.005) and gender (p = 0.003).
Changing of Low-Grade Chronic Inflammation in Patients with MHSO
The evolution of inflammatory markers MHSO group is shown in Table 2.
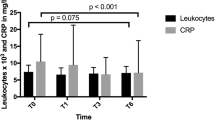
Primary BS significantly lowered leukocyte count at T1 (9.47 vs 7.9 [× 103/μL], p = 0.010), T2 (9.47 vs 7.1 [× 103/μL], p < 0.001), and T5 (9.47 vs 7.28 [× 103/μL], p = 0.015); neutrophil count at T1 (5.56 vs 4.65 [× 103/μL], p = 0.011), T2 (5.56 vs 4.03 [× 103/μL], p = 0.001), and T5 (5.56 vs 4.24 [× 103/μL], p = 0.038); lymphocyte count at T2 (2.6 vs 2.42 [× 103/μL], p = 0.046) and T5 (2.6 vs 2.13 [× 103/μL], p = 0.028); and platelet count at T1 (311 vs 263 [× 103/μL], p = 0.001) and T2 (311 vs 247 [× 103/μL], p = 0.036).
In the multivariate analysis for WBC and platelets, no statistically significant interaction was found regarding weight, BMI, %EBMIL, and %TWL.
The greatest drop in WBC occurred in the second postoperative year.
Changing of Low-Grade Chronic Inflammation in Patients with MUSO
The evolution of inflammatory markers MUSO group is shown in Table 2.
Primary BS significantly lowered leukocyte count at T1 (8.53 vs 6.94 [× 103/μL], p < 0.001), T2 (8.53 vs 6.44 [× 103/μL], p < 0.001), and T5 (8.53 vs 6.2 [× 103/μL], p < 0.001); neutrophil count at T1 (5.09 vs 3.58 [× 103/μL], p < 0.001), T2 (5.09 vs 3.42 [× 103/μL], p < 0.001), and T5 (5.09 vs 3.4 [× 103/μL], p < 0.001); lymphocyte count at T1 (2.48 vs 2.3 [× 103/μL], p < 0.001), T2 (2.48 vs 2.33 [× 103/μL], p < 0.001) and T5 (2.48 vs 2.19 [× 103/μL], p < 0.001); and platelet count at T1 (276.82 vs 249.5 [× 103/μL], p < 0.001), T2 (276.82 vs 242 [× 103/μL], p < 0.001), and T5 (276.82 vs 245 [× 103/μL], p = 0.044).
In the multivariate analysis for leukocytes, a significant interaction was found regarding weight at T1 (p = 0.019) and %EBMIL at T5 (p = 0.039).
In the multivariate analysis for neutrophils, a significant interaction was found regarding %TWL at T5 (p = 0.014).
In the multivariate analysis for lymphocytes, a significant interaction was found regarding BMI at T2 (p = 0.038).
In the multivariate analysis for platelets, no statistically significant interaction was found regarding weight, BMI, %EBMIL, and %TWL at T1, T2, and T5.
The greatest drop in WBC and platelet count occurred in the second postoperative year.
Differences in Low-Grade Chronic Inflammation Between Patients with MHSO and Patients with MUSO
The evolution and comparative analysis of inflammatory markers between the MHSO and MUSO groups are shown in Table 2.
In both groups, there was significant decrease of inflammatory markers after primary BS.
The preoperative values of leukocyte and platelet were statistically higher in the group of patients with MHSO (9.47 vs 8.53 [× 103/μL] (p = 0.040) and 311 vs 276.82 [× 103/μL] (p = 0.007), respectively) and at T1 and T5, the median values of leukocytes and neutrophils continued to be significantly higher in the group of patients with MHSO.
Once the maximum weight loss was achieved (in the second postoperative year), no significant differences were found in the other inflammatory parameters between the two groups.
Effect of Bariatric Surgery on the Resolution of Medical Conditions Associated with Severe Obesity and Against the Development of MetS
In the group of patients with MUSO, primary BS achieved resolution of T2D, DL, OSAS, and MetS in the 75.6%, 92%, 66.2%, and 95.3% of patients affected by these comorbidities, respectively. An improvement or resolution of AHT was achieved in 73.9% of patients with AHT.
No patient of the group of patients with MHSO developed any comorbidity neither MetS in the first five postoperative years.
In the multivariate analysis for WBC and platelets, no statistically significant interaction was found regarding the bariatric procedure and the resolution of medical conditions associated with severe obesity.
Follow-Up Rates
Follow-up rates were 100% (n = 168/168) at T1, 96.3% (n = 181/188) at T2, and 74.5% (n = 140/188) at T5. Drop-out rate was higher in the MUSO group.
Discussion
Although a role for proinflammatory cytokines in mediating insulin resistance in obesity had been established since the 1990s, it was not until 2003 that these observations captured the full attention of the research community [9]. New findings in the pathophysiology of adipose tissue support the intimate relationship between the adipose tissue and the hematopoietic system [10].
Adipose tissue is an active endocrine and paracrine organ that releases a large number of cytokines and bioactive mediators, which influence body weight homeostasis as well as a number of metabolic and coagulative parameters [11]. Dysregulated secretion of cytokines and adipokines from adipose tissue contribute to the pathogenesis of obesity-related disorders [12]. Recent studies have been confirming the association between obesity indexes and inflammatory markers, mainly CPR in women [2], but also other inflammatory markers such as WBC are elevated [2]. CPR is a useful marker of systemic inflammation and an independent risk factor of T2D, cardiovascular disease, and MetS [4]. In clinical practice, CRP and leukocyte count are widely used to evaluate the degree of subclinical inflammation in patients with coronary artery disease and MetS [13]; however, WBC is more useful than CPR in clinical settings because WBC is routinely measured [4] and the association between WBC and MetS has been demonstrated in several studies [4]. WBC counts can be regarded as markers of an increased production of cytokines and acute-phase reactants, and the activation of the inflammatory signaling network involved in the pathogenesis of insulin resistance and atherogenic dyslipidemia, the main metabolic disorders underlying MetS [5]. Tsai et al. show that peripheral total and differential leukocyte counts, even within the normal range, were independently associated with clustering of MetS [7].
The assessment of association between the WBC and the development of the MetS may help a better understanding of the pathophysiology of the MetS because chronic subclinical inflammation has been implicated in the genesis of MetS, and the leukocyte count may be seen as an inflammatory marker that is related to this condition [5].
The prevention and treatment of obesity are crucial to reduce the morbidity and mortality related to obesity-related cardiometabolic diseases and to reduce obesity-related healthcare costs [12].
The aim of this study therefore is to analyze the improvement of low-grade inflammation associated to obesity in patients with MHSO and patients with MUSO after primary BS as well as the protective effect of BS against the development of MetS in patients with MHSO by reducing the WBC.
Following BS, inflammatory mediators and cytokines closely associated with obesity and IR are expected to gradually decline [14]. Although the biological mechanisms by which bariatric surgery resolves inflammatory conditions are mostly unknown, weight loss (by reduction of adipocyte mass) followed by the surgery has been shown as one of the most important mechanism that explain the reduction in levels of inflammatory factors [3, 11]. The amelioration of chronic inflammation appears to be independent of surgical procedure [15]; Chiappetta et al. [2] found that CRP decreased significantly in three surgical groups (sleeve gastrectomy, Roux-en-Y gastric bypass, and one anastomosis gastric bypass), but no significant difference was seen between the groups. In line with these results, we have seen that gastric bypass and sleeve gastrectomy achieve a significant postoperative decrease in total leukocyte and platelet counts without statistically significant differences between the two surgical procedures [16].
Patients examined in the present study show the typical inflammatory profile seen in bariatric population (WBC and platelet count within the upper limit of normal), improving it after BS [6, 11, 17]. The greatest drop in WBC occurs in the second postoperative year when the maximum weight loss is achieved in both groups. These findings are in line with the study carried out by Dixon et al. who found the greater fall in BMI over 2-year period was associated the greater fall in WBC [6].
Between 10 and 40% of people with a BMI > 30 kg/m2 do not have any metabolic alteration (Metabolically Healthy Obese), but over time they tend to develop metabolic alterations (50% will develop metabolic syndrome at 10 years) [18]. In our study, the percentage of patients with metabolically healthy obesity is 5.57%.
The Predimed Study [5] shows that total WBC and subtype counts are directly and significantly associated with the incidence of MetS and an increase in the WBC during the follow-up was also related to a higher risk of MetS; participants with MetS had higher WBC (median 6.5, IR [5.5–7.7] × 103/μL) than those without MetS (6.0 [5.1–7.0]; p < 0.001). In this line of research, Twig et al. [19] found WBC level above 6.9 × 103/μL was associated with a twofold increase in the risk for coronary artery disease. In our study, we found that patients with MHSO had a preoperative total WBC higher than patients with MUSO because of the inverse correlation and interaction between age and inflammatory markers. Stepanova et al. [20] describe that the average age of those patients without chronic disease is 36.85 ± 0.26 years, with a chronic disease it is 42.76 ± 0.30 years, and of those with two or more chronic diseases it is 54 0.50 ± 0.31 years and therefore older age is a strong demographic predictor of the number of chronic diseases; they also observe a higher total leukocyte count in patients with two or more chronic diseases (7.509 × 103 /μL + / − 0.036) compared to healthy patients (7.070 × 103 /μL + / − 0.039) or with a chronic disease (7.366 × 103 /μL + / − 0.040) (p < 0.001) and after adjusting the inflammatory markers for age and BMI, an association between the total leukocyte count, and the number of chronic diseases in the study patients. If we corrected for age, MHSO associates a low-grade chronic inflammatory status comparable to MUSO. So, in clinical practice, patients with metabolically healthy severe obesity, reflecting chronic inflammation, should be prioritized for obesity and metabolic surgery and chronic inflammation should count as a weight related disease.
Surgery for obesity and related diseases has proven to be highly effective in controlling obesity and MetS; the results of this surgery are expressed not only in terms of weight loss but also in terms of resolution of comorbidities [21, 22] and improved quality of life and complications [21], and it leads to an improvement of the chronic inflammation status [10]. In our study, the percentage of resolution or improvement of comorbidities in the MUSO group was greater than 70% and the global weight loss was found within the quality standards in bariatric surgery [21]. In both groups, the significant decrease of inflammatory markers after BS improved in parallel to the metabolic status.
Several studies report that chronic systemic inflammation in obesity originates from local immune responses in visceral adipose tissue, and chronic inflammation directly promotes insulin resistance and type 2 diabetes mellitus, cardiovascular disease, and increased cancer risk [10]. Patients with obesity and who are metabolically healthy cannot be considered truly metabolically healthy because they still have a higher risk of cardiometabolic diseases than patients who are metabolically healthy and who have a normal weight [12]. Thus, surgery for obesity and related diseases leads to an improvement of the chronic inflammation status and in a prevention of inflammation-triggered diseases [10].
In line with the hypotheses and conclusions of the previously articles cited [2, 5, 10, 19], by reducing the inflammatory burden could be regarded as no patient of our MHSO group developed MetS neither comorbidities in the first five postoperative years.
These results suggest that the improvement or decrease of low-grade inflammation in patients with metabolically healthy severe obesity after BS could have a protective effect against the development of MetS and medical conditions associated with severe obesity.
This paper has some limitations:
-
The small sample size might not reflect a high number of patients with MHSO, and results must be interpreted carefully.
-
Only WBC and platelet count were measured; the measurement of, for example, CPR, TNF-a, and IL-6 could have brought some more detailed results.
-
The postoperative waist circumference was not measured.
-
The study design was a retrospective analysis of prospectively collected data and the follow-up rate was 74.5% at the fifth postoperative year.
Conclusion
Surgically induced weight loss play an important role for improvement in chronic inflammation associated to obesity because of reduction of visceral fat mass.
Metabolically healthy severe obesity associates a low-grade chronic inflammatory status comparable to metabolically unhealthy severe obesity.
The improvement or decrease of low-grade inflammation in patients with metabolically healthy severe obesity after bariatric surgery could have a protective effect against the development of MetS and medical conditions associated with severe obesity.
Abbreviations
- IR:
-
insulin resistance
- MetS:
-
metabolic syndrome
- WAT:
-
white adipose tissue
- TNF-a:
-
tumor necrosis alpha
- IL-6:
-
interleukin-6
- WBC:
-
white blood cell count
- CRP:
-
C reactive protein
- BS:
-
bariatric and metabolic surgery
- MHSO:
-
metabolically healthy severe obesity
- MUSO:
-
metabolically unhealthy obesity
- LGBP:
-
laparoscopic gastric by-pass
- LSG:
-
laparoscopic sleeve gastrectomy
- BMI:
-
body mass index
- T2D:
-
type 2 diabetes
- AHT:
-
arterial hypertension
- DL:
-
dyslipidemia
- OSAS:
-
obstructive sleep apnea syndrome
- % EBMIL:
-
% Excess BMI Loss
- % TWL:
-
% Total Weight Loss
- FU:
-
follow-up
- PO:
-
postoperative
- T0:
-
preoperative time
- T1:
-
first postoperative year
- T2:
-
second postoperative year
- T5:
-
fifth postoperative year
References
Sjöström L, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. https://doi.org/10.1056/nejmoa035622.
Chiappetta S, et al. The impact of obesity and metabolic surgery on chronic inflammation. Obes Surg. 2018;28:3028–40. https://doi.org/10.1007/s11695-018-3320-y.
Askarpour M, Khani D, Sheikhi A, Ghaedi E, Alizadeh S. Effect of bariatric surgery on serum inflammatory factors of obese patients: a systematic review and meta-analysis. Obes Surg. 2019;29:2631–47. https://doi.org/10.1007/s11695-019-03926-0.
Odagiri K, Uehara A, Mizuta I, Yamamoto M, Kurata C. Longitudinal study on white blood cell count and the incidence of metabolic syndrome. Intern Med. 2011;50:2491–8. https://doi.org/10.2169/internalmedicine.50.5877.
Babio N, et al. White blood cell counts as risk markers of developing metabolic syndrome and its components in the PREDIMED study. PLoS One. 2013;8:e58354. https://doi.org/10.1371/journal.pone.0058354.
Dixon JB, O’Brien PE. Obesity and the white blood cell count: changes with sustained weight loss. Obes Surg. 2006;16:251–7. https://doi.org/10.1381/096089206776116453.
Tsai JCR, Sheu S-H, Chiu H-C, Chung F-M, Chang D-M, Chen M-P, Shin S-J, Lee Y-J. Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2007;23:111–8. https://doi.org/10.1002/dmrr.647.
Zimmet P, Alberti KGMM, Serrano Ríos M. Una nueva definición mundial del síndrome metabólico propuesta por la Federación Internacional de Diabetes: fundamento y resultados. Rev Española Cardiol. 2005;58:1371–6. https://www.revespcardiol.org/es-una-nueva-definicion-mundial-del-articulo-13082533.
Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Physiol Behav. 2017;47:406–20. https://doi.org/10.1016/j.immuni.2017.08.009.
De Luca M, et al. Indications for surgery for obesity and weight-related diseases: position statements from the international federation for the surgery of obesity and metabolic disorders (IFSO). Obes Surg. 2016;26:1659–96.
Minervino D, et al. Leukocyte activation in obese patients: effect of bariatric surgery. Med (United States). 2015;94:1–7. https://doi.org/10.1097/md.0000000000001382.
Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616–27. https://doi.org/10.1016/s2213-8587(20)30110-8.
Bahadır A, et al. Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anadolu Kardiyol Derg. 2015;15:816–22. https://doi.org/10.5152/akd.2014.5787.
Biobaku F, Ghanim H, Monte SV, Caruana JA, Dandona P. Bariatric surgery: remission of inflammation, cardiometabolic benefits, and common adverse effects. J Endocr Soc. 2020;4:1–17. https://doi.org/10.1210/jendso/bvaa049.
Fenske WK, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9:559–68. https://doi.org/10.1016/j.soard.2012.03.009.
Recarte M, Corripio R, De Cos AI, Vesperinas G, Díaz, and Joaquín. Low-grade systemic inflammation: gastric bypass and sleeve gastrectomy. Obes Surg. 2019;29, 347–1720: 180–181. https://doi.org/10.1007/s11695-019-04101-1
Santos J, et al. Effect of bariatric surgery on weight loss, inflammation, iron metabolism, and lipid profile. Scand J Surg. 2014;103:21–5. https://doi.org/10.1177/1457496913490467.
Acín GD, Baltar J. Capítulo 1: Conceptos básicos. Manual del VIII Curso On-line para Residentes: Iniciación a la Cirugía Bariátrica y Metabólica. (Editor: Asociación Española de Cirujanos, 2021).
Twig G, et al. White blood cell count and the risk for coronary artery disease in young adults. PLoS ONE. 2012;7:1–8. https://doi.org/10.1371/journal.pone.0047183.
Stepanova M, Rodriguez E, Birerdinc A, Baranova A. Age-independent rise of inflammatory scores may contribute to accelerated aging in multi-morbidity. Oncotarget. 2015;6:1414–21. https://doi.org/10.18632/oncotarget.2725.
Sabench F, et al. Criterios de calidad en cirugía bariátrica: revisión de conjunto y recomendaciones de la Asociación Española de Cirujanos y de la Sociedad Española de Cirugía de la Obesidad. Cir Esp. 2017;95:4–16. https://doi.org/10.1016/j.ciresp.2016.09.007.
Chiappetta S, Stier C, Weiner RA. The Edmonton Obesity Staging System predicts perioperative complications and procedure choice in obesity and metabolic surgery—a German Nationwide Register-Based Cohort Study (StuDoQ|MBE). Obes Surg. 2019;29:3791–9. https://doi.org/10.1007/s11695-019-04015-y.
Acknowledgements
Gregorio Vesperinas participated in collecting data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to Participate
“For this type of study, formal consent is not required.”
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Low-grade inflammation associated to obesity
•Metabolically healthy severe obesity
• Metabolically unhealthy severe obesity
• Primary bariatric and metabolic surgery.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Recarte, M., Corripio, R., Palma, S. et al. Improvement of Low-Grade Inflammation in Patients with Metabolically Healthy Severe Obesity After Primary Bariatric Surgery. OBES SURG 33, 38–46 (2023). https://doi.org/10.1007/s11695-022-06345-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06345-w