Abstract
Background
Obesity and metabolic surgery is known to improve chronic inflammatory status. Whether improvement is related to anatomical changes or weight loss is still to debate.
Objective
The aim of this clinical trial is to compare the different bariatric procedures sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and One-anastomosis gastric bypass (OAGB), pertaining to their effects on inflammation markers.
Methods
Patients who underwent SG, RYGB, or OAGB as a primary treatment for severe obesity were included. The data collected preoperatively (T0) and 1, 3, and 6 (T6) months after surgery included gender, weight, comorbidities and toxic habits at baseline, body mass index (BMI), waist circumference, total body weight loss in % (TBWL), leukocyte count in × 103/μl, C-reactive protein (CRP) in mg/l, HbA1c in %, aspartate transaminase in U/l, alanine transaminase in U/l, gamma-glutamyltransferase in U/l, bilirubin in mg/dl, cholesterol in mg/dl, and triglycerides in mg/dl.
Results
Four hundred sixty-eight patients were included. Drop-out rate was 25.8% at T6. Preoperatively the mean value of leukocytes and CRP was 7.4 × 103/μl ± 2 and 10.5 mg/l ± 8.1. At T6, mean value of leukocytes and CRP was 7.1 × 103/μl ± 1.9 (p = 0.075) and 7.2 mg/l ± 9.5 (p < 0.001). TBWL % at T6 was 24.2 ± 7.6 in the SG, 25.8 ± 5.9 in the RYGB and 25.5 ± 4.6 in the OAGB group. Comparing SG, RYGB, and OAGB in relation to leukocyte count and CRP no significant difference was seen between the groups.
Conclusion
CRP but not leukocyte count decreased after all three bariatric procedures but without any significance between the three groups. Surgically induced weight loss and not anatomical changes might play an important role for improvement in chronic inflammation.
Trial Registration
The National Clinical Trials number was NCT02697695 (https://clinicaltrials.gov/ct2/show/NCT02697695).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The inflammatory state that accompanies adiposity and the metabolic syndrome is often called “low-grade” chronic inflammation or “metaflammation,” meaning metabolically triggered inflammation [1], or “parainflammation” as a term to define an intermediate state between basal and inflammatory states [2].
This chronic inflammation, due to oxidative stress and endoplasmic reticulum stress in adipocytes, leads to macrophage infiltration, abnormal cytokine production, and increased acute-phase reactants [3]. The secretory activity of adipose tissue activates immune signaling pathways; interrupts the tissue homeostasis especially in liver, brain, pancreas, and adipose tissue; and is responsible for the pathogenesis of metabolic syndrome [4, 5].
Different secretory cells include adipokines and inflammatory cytokines and gene expression of the majority of the adipokines is upregulated by obesity [5]. Obese subjects have a higher activity of nuclear transcription factor kappa B (NF-κB) and a higher ribonucleic acid (RNA) expression of proinflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6)) [3, 6], as well as higher chemokine expression on the surface [7].
Recent studies have been confirming the positive association between obesity indices and inflammatory markers, mainly C-reactive protein (CRP) in women [8, 9], but also other inflammatory markers such as calprotectin [10], complement factors C3 and C4, and white blood cell count are elevated [11] .
CRP is an inflammatory and metabolic syndrome parameter [12]. Elevation of CRP predicts the development of type 2 diabetes mellitus (T2DM) [13] and a recent study identified CRP as a marker to detect the risk of coronary heart disease in metabolically healthy persons with abdominal obesity. Van Wijk et al. concluded that the risk of coronary heart disease among metabolically healthy obese persons with CRP levels < 2 mg/l was comparable to that of metabolically healthy non-obese persons [14].
Obesity and metabolic surgery improves insulin resistance, T2DM, and cardiovascular disease [15,16,17]. A crucial role might be the reduction of chronic inflammation. Surgically induced weight loss is known to improve inflammatory status and inflammatory mediators normalize in morbidly obese patients after gastric restrictive surgery [11, 18, 19].
Furthermore, Roux-en-Y gastric bypass (RYGB) was described as an immune restorative procedure, after which elevated levels of eosinophils, monocyte cluster of differentiation 14 (CD 14), and monocyte CD14+/CD16+ subsets reversed rapidly with surgically induced weight loss [20]. Metabolic syndrome markers such as intercellular adhesion molecules (ICAM-1) increase and adiponectin decrease after RYGB, which further decrease the degree of endothelial dysfunction. CRP, which seems to be a key ICAM-1 regulator is positively correlated with ICAM-1. However, the authors announce that changes vary with the amount of weight loss and the type of bariatric procedure performed [12]. Park et al. showed a statistically significant reduction in CRP level in 43 patients, who underwent sleeve gastrctomy (SG) and RYGB with reduction in CRP being an independent factor affecting the improvement in urine albumin-to-creatinine ratio in those patients [21].
Pontiroli et al. found out in 126 morbidly obese patients undergoing laparoscopic gastric banding, that most metabolic abnormalities are associated with visceral fat and that their improvements after weight loss are associated with decrease of visceral fat [22].
Obesity and metabolic surgery, related to the loss of visceral fat, reduces low-grade inflammation (CRP, IL-6) and oxidative stress and beneficially changes the levels of several adipokines, suggesting potential reductions in risk for T2DM and cardiovascular disease [23]. Bypassing the duodenum has also a weight-independent anti-diabetes action with remission of insulin resistance and T2DM [24]. Whether exclusion of the duodenum plays also a role in improvement of chronic inflammation status has to be declared.
Furthermore, the different impact of SG and RYGB on the liver in patients with T2DM and an improvement of non-alcoholic-steatosis hepatitis (NASH) can cause amelioration of inflammation status [25].
On the other hand, also intermittent fasting is known to have an impact on inflammatory markers, including CRP, TNF-α, adiponectin, leptin, and brain-derived neurotrophic factor (BDNF) [26].
Whether the anatomical changes after different bariatric procedures or weight loss, or food restriction plays a crucial role in improvement of inflammatory status has not yet been investigated. Due to the fact that the interface between the adipose tissue, the liver, and the hematopoietic system might play an important part in the development of metabolic diseases, the aim of this clinical trial is to compare the three different bariatric procedures SG, RYGB, and One-anastomosis gastric bypass (OAGB) pertaining to their effects on clinical markers of inflammation postoperative.
Methods
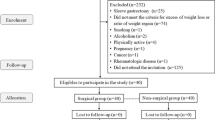
We performed a retrospective analysis of prospectively collected data of patients undergoing laparoscopic sleeve gastrectomy (SG), laparoscopic Roux-en-Y gastric bypass (RYGB), or laparoscopic One-anastomosis gastric bypass (OAGB) as the first surgical treatment for severe obesity. From October 2014 to October 2015, 468 patients underwent SG (n = 241), RYGB (n = 159), or OAGB (n = 68) at Sana Klinikum Offenbach, which is certified as a center of excellence for obesity and metabolic surgery by the European Accreditation Council for Bariatric Surgery. The eligibility criteria were a body mass index (BMI) ≥ 35 kg/m2 and at least one metabolic disease or a BMI ≥ 40 kg/m2, male and female patients aged 18–65 years. The exclusion criterion was acute inflammation during laboratory examination, such as cutaneous, urogenital or pulmonary infection, inflamed joint disease, or influenza referred by the patient. Data collection included the following: gender, age, height in cm, weight in kg, BMI in kg/m2, waist circumference in cm, and excess weight loss (EWL) in % with the calculation of ideal body weight as the equivalent to a BMI of 25 kg/m2 and total body weight loss (TBWL) in %. Laboratory measurements included leukocyte count × 103/μl, C-reactive protein (CRP) in mg/l, HbA1c in %, aspartate transaminase (AST) in U/l, alanine transaminase (ALT) in U/l, gamma-glutamyltransferase (GGT) in U/l, bilirubin in mg/dl, cholesterol in mg/dl, and triglycerides in mg/dl. Patient’s data was recorded 1 day preoperatively (T0), 1 month (T1), 3 months (T3), and 6 months (T6) after surgery.
The laboratory measurements during FU (T1, T3, T6) were performed by the general practitioner of the patients with a distance of ± 2 weeks from FU. Only patients with full laboratory examination were included in the FU. While not every general practitioner performs laboratory measurements, missing data are inevitable in these patients.
Patients data, including comorbidities and toxic status, were collected during admission, 1 day before surgery. The cut-off for HbA1c was < 6.5%, as defined by the American Diabetes Association in 2017 [27].
The surgical techniques for SG, RYGB, and OAGB have been described previously [28]. Routine laboratory measurements were done using automated chemical analyses at our center.
Primary outcome measure was the changing of chronic inflammation in terms of C-reactive protein in mg/l and leukocytes in /nl. Secondary outcome measures were weight loss, expressed in EWL and TBWL, changing of HbA1c, liver enzymes (AST, ALT, GGT, bilirubin), and changing of lipids (cholesterol and triglycerides).
Statistical analysis was performed using the SPSS 25.0 statistical software for Microsoft Windows (SPSS Inc., Chicago, IL, USA). All variables were checked for normal distribution. Data were analyzed in subgroups according to (1) gender; (2) bariatric procedures SG, RYGB, OAGB; (3) weight loss; (4) loss of waist circumference; (5) BMI; (6) HbA1c; (7) liver enzymes (AST, ALT, GGT, bilirubin); and (8) dyslipidemia (cholesterol, triglycerides). Continuous variables, when normally distributed, were reported as mean, standard deviation (SD), and range. A delta (Δ) value was calculated to reflect postoperative change (Δ value = postoperative value − baseline value).
Paired t tests were used to compare pre- and post-surgery data (difference over time) and a repeated measures analysis of variance and post hoc Bonferroni test were used to test for significant differences. Intergroup differences were tested by a two-sample t test for normally distributed data. For comparisons among the groups, a multivariate analysis was performed (two-factor analysis of variance for continuous variables). In the two-factor analysis of variance, the repeated-measure factor was time (T0 and T6), while the dependent quantitative variable was (1) gender; (2) bariatric procedures SG, RYGB, OAGB; (3) weight loss (median split); (4) loss of waist circumference (median split); (5) BMI; and (6) HbA1c (split < 6.5%). The interaction effect was calculated for each group. Intergroup differences were tested by a two-sample t test for normally distributed and a Mann-Whitney U test and Kruskal-Wallis test for non-normally distributed data. Spearman’s rank correlation coefficients were calculated to correlate the different groups. A p value < 0.05 was considered to be statistically significant.
The study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical permission was obtained from the local ethics committee (Landesärztekammer Hessen, Germany, reference number FF 145/2015), and all participants provided written informed consent for data sharing. The National Clinical Trials number was NCT02697695 (https://clinicaltrials.gov/ct2/show/NCT02697695).
Results
Baseline Characteristics
From October 2014 to October 2015, a total of 468 patients underwent SG (n = 241, 51.5%, BMI 53.2 ± 9.1 kg/m2), RYGB (n = 159, 34%, %, BMI 45.3 ± 5.9 kg/m2), or OAGB (n = 68, 14.5%, BMI 49.0 ± 7.2 kg/m2) as a primary treatment for severe obesity. The mean age of the participants was 43.7 ± 10.3 years, and 68% (n = 318) of the participants were women. The baseline demographic data and the comorbidities with the toxic habit smoking prior to surgery are listed in Tables 1 and 2.
Weight, Body Mass Index, and TBWL%
At baseline, there was a statistically significant difference between the three groups regarding body weight (Table 3). The highest body weight was in the SG group (155.8 kg ± 33.3), followed by the OAGB group (143.9 kg ± 26.7) and the RYGB group (128.1 kg ± 21.4). Comparing the different bariatric procedures, no statistical difference was seen in relation to TBWL % at T6 with 24.2 ± 7.6 in the SG group, 25.8 ± 5.9 in the RYGB group and 25.5 ± 4.6 in the OAGB group (SG vs. RYGB p = 0.124; SG vs. OAGB p = 0.509; RYGB vs. OAGB p = 0.848).
Follow-Up and Drop-Out Rates
Drop-out rates were 12.2% (n = 411/468) at T1, 25.6% (n = 270/363) at T3, and 25.8% (n = 193/260) at T6 due to missed follow-up, missed laboratory examination, or acute inflammation during laboratory examination. Drop-out rate was highest in the OAGB group, followed by SG and RYGB. Drop-out rates of this study were quite high, due to missed laboratory examination. In Germany, only few general practitioners perform laboratory examination during FU, since up to date, it is not reimbursed from the insurance.
Leukocyte Count and CRP in the whole Group
Preoperatively, the mean leukocyte count was 7.4 × 103/μl ± 2 and at 6 months (T6) post-surgery, mean leukocyte dropped to 7.1 × 103/μl ± 1.9 (range 3.2–12) (T0 vs. T6 p = 0.075). Preoperatively the mean value of CRP was 10.5 mg/l ± 8.1 and decreased to 7.2 mg/l ± 9.5 after 6 months (T6) post-surgery (T0 vs. T6 p < 0.001) (Fig. 1).
In males, mean CRP was 11 mg/l ± 10.5 at T0 (n = 150) and 8 mg/l ± 14.8 at T6 (n = 56; T0 vs. T6 p = 0.107). Mean CRP in women was 10.3 mg/l ± 6.6 at T0 (n = 318) and 6.8 mg/l ± 5.8 at T6 (n = 137; T0 vs. T6 p < 0.001). No statistical significant interaction was found regarding gender (p = 0.134) in the two-factor analysis of variance.
Leukocyte Count and CRP across the three different groups: SG, RYGB, OAGB
All surgical procedures resulted in a significant decrease in CRP at T6 compared to baseline values (SG p = 0.002, RYGB p < 0.001, OAGB p = 0.028) (Fig. 2). In contrast, no statistically significant decrease in leukocyte count compared to baseline was found (SG p = 0.053, RYGB p = 1.00, OAGB p = 0.338) (Fig. 3).
Comparing the different surgical treatment groups at 6 months (T6), no statistically significant difference was seen regarding leukocyte count and CRP in the two-factor analysis of variance (Table 4, Figs. 2 and 3).
Leukocyte Count and CRP in relation to BMI
Mean preoperative BMI of the 468 patients was 50.1 kg/m2 ± 9 (range 35.5–95). For further interpretations, the study group was divided into three groups according to BMI (group 1: class I and II obesity, group 2: class III obesity and super obesity, and group 3: super-super obese patients, BMI > 60 kg/m2). The cut-off for these weight groups were based on the guidelines of the American Society for Bariatric Surgery, which subdivided obesity in patients having a BMI of 40–50 kg/m2 as morbidly obese, a BMI of 50–60 kg/m2 as super obese, and a BMI > 60 kg/m2 as super-super obese [29].
Group 1 included patients with a BMI < 40 kg/m2 (n = 48, 10.3%), group 2 patients with a BMI 40–60 kg/m2 (n = 364, 77.8%), and group 3 patients with a BMI > 60 kg/m2 (n = 56, 12%).
Mean EWL % in group 1 was 71.5 ± 15.0, 50.1 ± 14.3 in group 2, and 35.8 ± 14.7 in group 3. Mean TBWL % in group 1 was 24.9 ± 4.5, 25.2 ± 6.6 in group 2, and 23.0 ± 9.3 in group 3 (Tables 5 and 6).
No correlation was found between BMI and leukocyte count at baseline (rho = 0.047) and 6 months post-surgery (rho = 0.097). There was no statistically significant interaction in the reduction of leukocyte count between the three groups in the two-factor analysis of variance (p = 0.727).
A weak correlation was found between CRP and BMI at baseline (rho = 0.222) and at 6 months post-surgery (rho = 0.289). There was no statistically significant interaction in the reduction of CRP between the three groups in the two-factor analysis of variance (p = 0.971) (Table 6).
Between CRP and EWL, a weak correlation was found at T6 (rho = − 0.229) and correlation raised with increasing BMI (rho = − 0.500 in BMI > 60 kg/m2), (Tables 5 and 6, Fig. 4).
The study group showed negative correlation coefficients of EWL and CRP with an increasing rho in group 2 (BMI 40–60 kg/m2, rho = − 0.194) and group 3 (BMI > 60 kg/m2, rho = − 0.500). Group 3 showed the lowest EWL of 35.8% with the highest CRP difference (T0–T6) ∆CRP 5.3 mg/l (BMI < 40 kg/m2: ∆CRP = 2.2 mg/l; BMI 40–60 kg/m2: ∆CRP = 3.3 mg/l).
Leukocyte Count and CRP in relation to DM2 and HbA1c
Mean preoperative HbA1c of the 468 patients was 5.9% ± 1 (range 4.6–10.2). For further interpretations, the study group was divided into two groups according to the American Diabetes Association. The cut-off for HbA1c was < 6.5%. One hundred thirty-five of 468 patients (28.8%) had a known DM2 prior to surgery with injectable or oral hypoglycemic treatment. According to the laboratory examination, 342 patients had a HbA1c < 6.5% and 126 had a HbA1c ≥ 6.5%. Preoperatively the mean value of the sub-cohort with HbA1c ≥ 6.5% was 7.7 ± 1.1 (range 6.5–10.2), which decreased to 5.7 mg/l ± 0.7 (range 5.2–7.0) after 6 months (T6) post-surgery (T0 vs. T6 p < 0.001). This sub-cohort (n = 126) had a leukocyte value of 7.9 × 103/μl ± 2.1 (range 2.3–15) and a CRP of 12.8 mg/l ± 8.7 (range 5–41), while the sub-cohort of patients with a HbA1c < 6.5% had a leukocyte value of 7.3 × 103/μl ± 2 (range 2.7–15.1) and a CRP of 9.8 mg/l ± 7.8 (range 4–99) (leukocytes group 1 vs. group 2 p < 0.0047; CRP group 1 vs. group 2 p = 0.004).
Liver Enzymes
Liver enzymes, such as AST, ALT, and GGT decreased in all surgical groups 6 months after surgery, with a lesser linear decline in the OAGB group. Bilirubin was stable in all groups.
Multivariate Analysis
In the multivariate analysis for leukocytes, no statistical significant interaction was found regarding (1) gender (p = 0.621), (2) bariatric procedures (p = 0.4161), (3) loss of waist circumference (p = 0.710), (4) BMI (p = 0.727), and (5) HbA1c ≥ 6.5% (p = 0.234). A significant interaction was found regarding weight loss (p = 0.012).
In the multivariate analysis for CRP, no statistical significant interaction was found regarding (1) gender (p = 0.134), (2) bariatric procedures (p = 0.201), (3) weight loss (p = 0.814), (4) loss of waist circumference (p = 0.527), and (5) BMI (p = 0.971). A statistical interaction was found regarding HbA1c (p = 0.0238).
Discussion
The modulation of CRP varies with the amount of weight loss and the type of bariatric procedure performed [30]. Nutrient sensing and immune signaling are strongly linked and chronic metabolic inflammation plays a key role in the development of metabolic syndrome. The inflammatory status interferes with glucose metabolism and induces insulin resistance [31]. Amelioration of inflammatory status is described in the literature both after purely restrictive surgery such as adjustable gastric banding [11, 19] and metabolic surgery such as RYGB [20].
Obesity and metabolic surgery powerfully improves T2DM [32] as well as other weight-related diseases [33].
A certain gradient of efficiency among the surgical interventions has been reported in several trials in terms of amelioration of glycemic control [34]. Malabsorptive biliopancreatic diversion has been shown to be the most efficient operation in terms of T2DM remission rate (but the most radical in terms of potentially severe side effects) followed by RYGB, SG, and gastric banding [35]. The underlying pathophysiological mechanisms are still insufficiently understood, and whether a gradient of efficiency of different bariatric procedures is also found in amelioration of inflammatory status still remains unclear.
The aim of this study therefore was to reveal the impact of different bariatric interventions (SG, RYGB, and OAGB) on inflammatory status and, further, to examine whether the different anatomical changes or rather amount of weight loss is responsible for the amelioration in clinical inflammation markers, such as leukocyte count and CRP.
Patients examined in the present trial showed the typical profile seen in bariatric populations with elevated baseline CRP, decreasing after bariatric surgery. A mean CRP of 10.5 mg/l (normal range < 5 mg/l) is quite similar to other studies: Hanusch-Enserer et al. observed a significant decrease of CRP after gastric banding with a mean CRP of 12 mg/l at baseline and 8.5 mg/l 6 months post-surgery [11]. Afshar et al. presented a CRP of 5.5 mg/l in 22 patients, decreasing to 1.6 mg/l 6 months after RYGB [36] and Netto et al. reported of 41 patients, whose CRP decreased from 21.36 mg/l to 2.4 mg/l (p < 0.01) 6 months after RYGB [12].
In the present study, CRP showed a positive correlation with increasing BMI while leukocyte count did not correlate with BMI. These findings are in line with a trial carried out by Ilàn-Gòmez et al. who found a moderate correlation between CRP and BMI in 60 obese women 1 year after RYGB (r = 0.40) [37]. Furthermore, CRP showed a positive correlation with HbA1c ≥ 6.5%. While inflammatory status is associated with glucose metabolism and insulin resistance [31, 38], a continued reduction of CRP in the obese diabetic patients might be strongly associated with the amelioration of T2DM. Moreover, Yadav et al. showed a continued reduction of resistin and TNF-α in the obese diabetic group up to 12 months after RYGB and concluded that this may explain the reduction in HOMA-IR [39].
Our study group showed normal leukocyte counts in the preoperative and postoperative setting without any statistically significant reduction pre- vs. postoperatively or across the different surgical groups SG, RYGB, and OAGB.
However, in a study cohort of 477 patients, Dixon et al. demonstrated a statistically significant decrease in leukocyte count from 7.30 × 103/μl ± 1.78 to 6.27 × 103/μl ± 1.69 (p < 0.001) 2 years after adjustable gastric banding [40]. The difference in observation time might explain these contrasting findings.
Interestingly, Thorand et al. performed a cross-sectional analysis among 641 men and 597 women and showed that measures of both total and visceral adiposity were highly correlated with markers of systemic inflammation (CRP, serum amyloid A, fibrinogen, IL-6) in both genders. In a multivariable linear regression analysis, a considerably higher percentage of variability in inflammatory markers was explained by body composition in women compared to men. Furthermore, in women, fat mass in % explained the highest percentage of the variability of circulating acute-phase proteins, whereas in men, waist hip ratio explained the highest percentage of the variability [41].
In our study, we found sex differences in reduction in CRP after 6 months: only in women, a statistically significant reduction of the chronic inflammatory marker CRP was found. Growing evidence has shown that estrogen interacts with many of the inflammatory pathways and the potent anti-inflammatory effect of estrogen might explain these findings [42].
While decrease in CRP after bariatric surgery could be demonstrated in several trials, the underlying cause (lower nutritional intake, weight loss, anatomical changes) has not been elucidated so far. In the present study, CRP decreased significantly in all three surgical groups SG, RYGB, and OAGB, but no significant difference was seen between the groups. This is in line with other trials: Fenske et al. investigated a total of 34 morbidly obese patients 1 and 12 months after RYGB (n = 10), laparoscopic adjustable gastric banding (n = 13), and SG (n = 11) for serum cytokine levels of macrophage migration inhibitory factor, monocyte chemotactic protein-1, and chemokine ligand-18. At 12 months after surgery, the patients in all three treatment arms showed a significant decrease in serum inflammatory markers (all p < 0.001) and there were no differences across the intervention groups in terms of gender, BMI, and obesity-related comorbidities. The study group concluded that these effects appear to be independent of the surgical procedure [43] and thus underline our study results.
Ianelli et al. compared the incidence of low-grade systemic inflammation in 12 patients undergoing RYGB and 10 patients undergoing SG. At 6 months after surgery, there was no significant difference in any of the parameters investigated. One year after surgery, patients in the RYGB group had significantly lower plasma levels of C-reactive protein compared to SG. (2.3 mg/l ± 1.5 vs. 5.1 mg/l ± 4.6; p < 0.05) [44]. However, the small sample size might not reflect a high number of participants and results must be interpreted carefully.
Lips et al. investigated systemic inflammation in age- and BMI-matched morbidly obese T2DM women who underwent RYGB or very low-calorie diets (VLCD). Systemic inflammation was assessed 1 month before and 3 months after RYGB (n = 15) or VLCD (n = 12). An age-matched group of lean women (n = 12) was studied as a control group. RYGB and VLCD had differential effects on the activation status of peripheral leukocytes and levels of cytokines in obese women with T2DM, despite comparable weight loss 3 months after the intervention. VLCD seemed to have more favorable effects on the inflammatory profile as compared to RYGB [45]. It is well-known that chronic nutrient excess is a key underlying mechanism in the pathogenesis of metabolic disease [6, 11] and novel studies underline the importance of dietary restriction and its impact on metabolic and cellular changes. It is known that dietary restriction affects oxidative damage and inflammation, optimizes energy metabolism, and enhances cellular protection [46].
Our study results confirm that decrease in CRP is more likely linked to nutrient restriction and weight loss than to anatomical changes. Moreover, it seems that in patients with a higher BMI, less EWL is necessary to reduce CRP. Our study group showed negative correlation coefficients of EWL and CRP with an increasing rho in group 2 (BMI 40–60 kg/m2) and group 3 (BMI > 60 kg/m2). Group 3 showed the lowest EWL of 35.8% with the highest CRP difference (T0–T6).
Since prevalence of obesity is increasing rapidly, prioritization systems for obese patients are getting more popular. Sharma et al. developed the Edmonton Obesity Score System [47] to offer clinicians a useful approach for identifying obese individuals at elevated risk who may benefit from more attention to obesity and metabolic surgery. In our study group, patients with a BMI > 60 kg/m2 had the highest basal CRP and CRP-difference (T0-T6), and we approve the Obesity Surgery Score by Perez et al. in which BMI > 60 reflects a high degree of obesity severity [48].
The importance of inflammation as a central and reversible mechanism through which obesity promotes cancer risk and progression is another current point of discussion and shows the clinical relevance for improvement of chronic inflammation. Iyengar et al. concluded in their article that metabolic syndrome, including dyslipidemia and insulin resistance, occurs in the setting of adipose inflammation and operates in concert with local mechanisms to sustain the inflamed microenvironment and promote tumor growth. Importantly, adipose inflammation and its protumor consequences can be found in some individuals who are not considered to be obese or overweight by BMI. They concluded that adipose inflammation is a reversible process and represents a novel therapeutic target that warrants further studies to break the obesity-cancer link [49]. A recent study by Afshar et al. could prove that RYGB in obese adults led to a decrease in rectal crypt cell proliferation and reduced systemic and mucosal markers of inflammation. The authors concluded that surgically induced weight loss might therefore lower colorectal cancer risk [36].
A reduction in chronic inflammation not only reduces cancer risk but also decreases the risk of cardiovascular disease. The remission or improvement of T2DM is one of the most important effects of metabolic surgery. Our study results demonstrate that there is a significant interaction between the reduction of CRP and the improvement of HbA1c. In clinical practice, patients with an elevated CRP, reflecting the chronic inflammation, should be prioritized for obesity and metabolic surgery and chronic inflammation should count as a weight-related disease [33].
Our study has some limitations. First, only leukocyte count and CRP were measured as parameters for chronic inflammation. The measurement of, for example, TNF-α, IL-1, and IL-6 and granulocyte macrophage colony-stimulating factor could have brought some more detailed results. On the other hand, leukocyte count and CRP represent the standard chemical analyses, which are evaluated beyond clinical research in most everyday clinical practice.
Second, the study design was retrospective and due to logistic changes CRP and leukocyte count were not evaluated in all patients, the drop-out rate at T6 was quite high with 25.8%.
Third, follow-up of 6 months could be too short to analyze further aspects of the amelioration of chronic inflammation. Since the effect of time since surgery is mostly noticed in the first 6 months, we strictly assume that this time period is short-term but meaningful [39].
Nevertheless, future prospective studies are needed to evaluate whether fasting, caloric restriction, or surgically induced weight loss has the best impact on chronic inflammation.
Conclusion
CRP but not leukocyte count decreased after all three bariatric procedures but without any significant difference between the three groups. HbA1c ≥ 6.5% was associated significantly with a higher CRP prior to surgery. Women showed a significant decrease in CRP at 6 months, and also CRP of super-super obese (BMI > 60 kg/m2) improved more significantly 6 months after surgery compared to obese patients (BMI < 40 kg/m2).
For that, it seems that the surgically induced weight loss and not the post-surgery anatomical changes might play the crucial role of improvement in chronic inflammation.
Change history
27 June 2018
In Table 4 the column labeled “p values” and its data should be deleted.
Abbreviations
- SG:
-
Sleeve gastrectomy
- RYGB:
-
Roux-en-Y gastric bypass
- OAGB:
-
One-anastomosis gastric bypass
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- EWL:
-
Excess weight loss
- TBWL:
-
Total body weight loss
- CRP:
-
C-reactive protein
- ICAM-1:
-
Intercellular adhesion molecules
- RNA:
-
Ribonucleic acid
- NF-κB:
-
Nuclear transcription factor kappa B
- TNF-α:
-
Tumor necrosis factor-α
- IL-1:
-
Interleukin-1
- IL-6:
-
interleukin-6
- BDNF:
-
Brain-derived neurotrophic factor
- T2DM:
-
Type 2 diabetes mellitus
- CD14:
-
Cluster of differentiation 14
- VLCD:
-
Very low-calorie diet
- SD:
-
Standard deviation
- NASH:
-
Non-alcoholic statosis hepatitis
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- GGT:
-
Gamma-glutamyltransferase
- OSAS:
-
Obstructive sleep apnea
References
Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–85.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–35.
Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–9.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7.
Dahlman I, Elsen M, Tennagels N, et al. Functional annotation of the human fat cell secretome. Arch Physiol Biochem. 2012;118(3):84–91.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91.
Krinninger P, Ensenauer R, Ehlers K, et al. Peripheral monocytes of obese women display increased chemokine receptor expression and migration capacity. J Clin Endocrinol Metab. 2014;99(7):2500–9.
Bochud M, Marquant F, Marques-Vidal PM, et al. Association between C-reactive protein and adiposity in women. J Clin Endocrinol Metab. 2009;94(10):3969–77.
Shemesh T, Rowley KG, Jenkins A, et al. Differential association of C-reactive protein with adiposity in men and women in an aboriginal community in Northeast Arnhem Land of Australia. Int J Obes. 2007;31(1):103–8.
Mortensen OH, Nielsen AR, Erikstrup C, et al. Calprotectin—a novel marker of obesity. PLoS One. 2009;4(10):e7419.
Hanusch-Enserer U, Cauza E, Spak M, et al. Acute-phase response and immunological markers in morbid obese patients and patients following adjustable gastric banding. Int J Obes Relat Metab Disord. 2003;27(3):355–61.
Netto BD, Bettini SC, Clemente AP, et al. Roux-en-Y gastric bypass decreases pro-inflammatory and thrombotic biomarkers in individuals with extreme obesity. Obes Surg. 2015;25(6):1010–8.
Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (atherosclerosis risk in communities study): a cohort study. Lancet. 1999;353(9165):1649–52.
van Wijk DF, Boekholdt SM, Arsenault BJ, Ahmadi-Abhari S, Wareham NJ, Stroes ES, et al. C-reactive protein identifies low-risk metabolically healthy obese persons: the European prospective investigation of Cancer-Norfolk Prospective Population Study. J Am Heart Assoc 2016;5(6).
Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641–51.
van Dielen FM, Buurman WA, Hadfoune M, et al. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89(8):4062–8.
Santos J, Salgado P, Santos C, et al. Effect of bariatric surgery on weight loss, inflammation, iron metabolism, and lipid profile. Scand J Surg. 2014;103(1):21–5.
Cottam DR, Schaefer PA, Shaftan GW, et al. Effect of surgically-induced weight loss on leukocyte indicators of chronic inflammation in morbid obesity. Obes Surg. 2002;12(3):335–42.
Park S, Kim YJ, Choi CY, et al. Bariatric surgery can reduce albuminuria in patients with severe obesity and normal kidney function by reducing systemic inflammation. Obes Surg. 2018;28(3):831–7.
Pontiroli AE, Frige F, Paganelli M, et al. In morbid obesity, metabolic abnormalities and adhesion molecules correlate with visceral fat, not with subcutaneous fat: effect of weight loss through surgery. Obes Surg. 2009;19(6):745–50.
Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res. 2012;61(8):789–807.
Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes. 2009;33(Suppl 1):S33–40.
Billeter AT, Senft J, Gotthardt D, et al. Combined non-alcoholic fatty liver disease and type 2 diabetes mellitus: sleeve gastrectomy or gastric bypass?—a controlled matched pair study of 34 patients. Obes Surg. 2016;26(8):1867–74.
Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371–93.
Marathe PH, Gao HX, Close KL. American Diabetes Association Standards of Medical Care in Diabetes 2017. J Diabetes. 2017;9(4):320–4.
Chiappetta S, Stier C, Squillante S, et al. The importance of the Edmonton Obesity Staging System in predicting postoperative outcome and 30-day mortality after metabolic surgery. Surg Obes Relat Dis. 2016;12(10):1847–55.
Guidelines for reporting results in bariatric surgery. Standards Committee, American Society for Bariatric Surgery. Obes Surg. 1997;7(6):521–2.
Nijhuis J, van Dielen FM, Fouraschen SM, et al. Endothelial activation markers and their key regulators after restrictive bariatric surgery. Obesity (Silver Spring). 2007;15(6):1395–9.
Pekala P, Kawakami M, Vine W, et al. Studies of insulin resistance in adipocytes induced by macrophage mediator. J Exp Med. 1983;157(4):1360–5.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–77.
De Luca M, Angrisani L, Himpens J, et al. Indications for surgery for obesity and weight-related diseases: position statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg. 2016;26(8):1659–96.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Surg Obes Relat Dis. 2016;12(6):1144–62.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256.e5.
Afshar S, Malcomson F, Kelly SB, Seymour K, Woodcock S, Mathers JC. Biomarkers of colorectal cancer risk decrease 6 months after Roux-en-Y gastric bypass surgery. Obes Surg 2017.
Illan-Gomez F, Gonzalvez-Ortega M, Orea-Soler I, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22(6):950–5.
Shimobayashi M, Albert V, Woelnerhanssen B, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128(4):1538–50.
Yadav R, Hama S, Liu Y, et al. Effect of roux-en-Y bariatric surgery on lipoproteins, insulin resistance, and systemic and vascular inflammation in obesity and diabetes. Front Immunol. 2017;8:1512.
Dixon JB, O'Brien PE. Obesity and the white blood cell count: changes with sustained weight loss. Obes Surg. 2006;16(3):251–7.
Thorand B, Baumert J, Doring A, et al. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184(1):216–24.
Kassi E, Spilioti E, Nasiri-Ansari N, et al. Vascular inflammation and atherosclerosis: the role of estrogen receptors. Curr Med Chem. 2015;22(22):2651–65.
Fenske WK, Dubb S, Bueter M, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9(4):559–68.
Iannelli A, Anty R, Schneck AS, et al. Inflammation, insulin resistance, lipid disturbances, anthropometrics, and metabolic syndrome in morbidly obese patients: a case control study comparing laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy. Surgery. 2011;149(3):364–70.
Lips MA, van Klinken JB, Pijl H, et al. Weight loss induced by very low calorie diet is associated with a more beneficial systemic inflammatory profile than by Roux-en-Y gastric bypass. Metabolism. 2016;65(11):1614–20.
Brandhorst S, Choi IY, Wei M, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and health span. Cell Metab. 2015;22(1):86–99.
Gill RS, Karmali S, Sharma AM. The potential role of the Edmonton obesity staging system in determining indications for bariatric surgery. Obes Surg. 2011;21(12):1947–9.
Casimiro Perez JA, Fernandez Quesada C, Del Val Groba Marco M, et al. Obesity Surgery Score (OSS) for prioritization in the bariatric surgery waiting list: a need of public health systems and a literature review. Obes Surg. 2018;28(4):1175–84.
Iyengar NM, Gucalp A, Dannenberg AJ, et al. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Informed Consent
Informed consent was obtained from all the individual participants included in the study.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Chiappetta, S., Schaack, H.M., Wölnerhannsen, B. et al. The Impact of Obesity and Metabolic Surgery on Chronic Inflammation. OBES SURG 28, 3028–3040 (2018). https://doi.org/10.1007/s11695-018-3320-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3320-y