Abstract
Purpose
Patients with laparoscopic sleeve gastrectomy (LSG) are at high risk of postoperative nausea and vomiting (PONV). Goal-directed fluid therapy (GDFT) has been proven effective in improving postoperative gastrointestinal function in patients with obesity, but its effect on prevention of PONV remains controversial. This study aimed to investigate the impact of GDFT on PONV in high-risk patients with LSG.
Methods
In a randomized, single-blinded, two-arm trial, patients with an Apfel score ≥ 3 and scheduled for LSG were included. Patients in the GDFT group received stroke volume-guided fluid therapy. Patients in the control group received conventional fluid therapy. The primary outcome was the incidence of PONV within 48 h after LSG. The second outcome included intensity of PONV, use of rescue therapy, recovery of gastrointestinal function, and postoperative length of stay (LOS).
Results
A total of 137 patients were analyzed. The incidence of PONV in the GDFT group was lower than that in the control group (47.1% vs. 71.6%; odds ratio [95%CI], 0.35 [0.17–0.72]; P = 0.004). Fewer patients in the GDFT group received rescue therapy (30% vs. 58.2%; P = 0.001). Patients following GDFT protocol had a faster return of flatus (27.5 (19, 31) vs. 31 (20, 48) hours, P = 0.037) and shorter postoperative LOS (6.1 ± 1.0 vs. 6.6 ± 1.1 days; P = 0.007).
Conclusion
GDFT is conducive to deceasing PONV occurrence, restoring intestinal function, and shortening postoperative LOS in high-risk patients undergoing LSG.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is widely used to treat obesity and decrease obesity-related comorbidities. However, adverse events after bariatric surgery deserve attention. Postoperative nausea and vomiting (PONV) is known to be the most common complications, especially for patients with laparoscopic sleeve gastrectomy (LSG) [, , 1, 2, 3]. Despite guidelines and practice to prevent PONV, the incidence can still be higher than 70% [, 4, 5]. In laparoscopic surgery, gastrointestinal hypoperfusion caused by pneumoperitoneum may impair gastrointestinal function and promote the occurrence of PONV. Therefore, adequate intravenous (i.v.) hydration is well accepted as an effective intervention to reduce the risk of PONV [6]. In clinical settings, this goal can be achieved by minimizing perioperative fasting time or using supplementary i.v. fluids to maintain euvolemia.
Goal-directed fluid therapy (GDFT), a useful strategy for optimizing hemodynamics in patients under general anesthesia, has shown considerable advantages in prompting recovery of gastrointestinal function and decreasing occurrence of PONV [, , 7, 8, 9]. There have been some studies on the relationship between fluid therapy and PONV [, , 10, 11, 12]. Nonetheless, the effect of GDFT on reducing PONV after LSG is a topic of much controversy [, 13, 14]. In a before-after intervention study, GDFT induced a 33.7% reduction in PONV incidence compared with conventional fluid therapy [13]. Whereas in another prospective cohort study, the severity of PONV in patients with obesity was not alleviated by GDFT strategy [14].
Considering the controversy over this fluid management strategy and multiple risk factors of PONV in patients undergoing bariatric surgery, this study aimed to evaluate whether GDFT protocol could reduce the occurrence of PONV and accelerate recovery of gastrointestinal function, compared with convention fluid therapy, in high-risk patients after LSG.
Methods
This prospective, randomized, controlled trial was approved by the institutional ethics committee of the First Affiliated Hospital of Chongqing Medical University. The trial was registered with the Chinese Clinical Trial Registry (ChiCTR2100048618) and signed consent was obtained from all individual participants.
Study Population
Patients scheduled for elective LSG under general anesthesia at the First Affiliated Hospital of Chongqing Medical University between July 2021 and February 2022 were included in this study. The inclusion criteria were patients with a body mass index (BMI) > 30 kg/m2, a simplified Apfel score ≥ 3, and an American Society of Anesthesiologists (ASA) classification of II or III. Exclusion criteria included major organ dysfunction, communication difficulties, psychiatric or neurological disease, pre-use medicine (antiemetics, opioids, or glucocorticoids), and revision or combined surgery.
Randomization and Blinding
Participants were randomly allocated to either flow-guided fluid therapy (GDFT group) or pressure-guided fluid therapy (control group) using a computerized random number generator (http://www.randomization.com). Group assignment was revealed only to the anesthesiologist from a sealed envelope when patients arrived at the operating room. The participants and surgeon were both blinded to the group assignment. An anesthesia resident who was unaware of the group assignment and not involved in the anesthesia process conducted the follow-up and data collection.
Standardized Anesthesia and Perioperative Management
Vital signs were continuously monitored when participants entered the operating room (supplementary material). In both groups, a 20-G radial artery catheter was cannulated to monitor arterial pressure, and the last mean arterial pressure (MAP) before induction was considered basal blood pressure.
All patients were preoxygenated for 10 min with a tight facemask. Propofol (1–2 mg/kg total body weight), sufentanil (0.5 μg/kg lean body weight (LBW)) [15], and rocuronium (0.9 mg/kg LBW) were administered for anesthesia induction. Dexamethasone (10 mg i.v.) was given as a prophylactic antiemetic after endotracheal intubation. Ventilator settings were adjusted to maintain normoxia (supplementary material). Anesthesia was maintained with desflurane (MAC 0.8–1.0) and dexmedetomidine (0.5 μg/kg/h), keeping the level of Narcotrend (Monitor Technik, Bad Bramstedt, Germany) between 40 and 55. Additional sufentanil (0.1–0.2 μg/kg LBW) and rocuronium (0.3–0.4 mg/kg LBW) were administered to maintain adequate levels of analgesia and muscle relaxation.
Tropisetron (2 mg i.v.) was given at the start of skin closure. At the end of surgery, all patients received a PCA device for postoperative analgesia (supplementary material). After surgery, patients were transferred to the post-anesthesia care unit (PACU) for recovery and were sent back to ward when the criteria were fulfilled [16]. If patients developed severe nausea or vomiting or requested an antiemetic, 10 mg of metoclopramide was administered intravenously as rescue therapy.
Control Group
Preoperative fluid deficit was estimated by the 4–2-1 rule based on patients’ LBW and an 8-h fasting time [17]. After participants entered the operating room, half of the deficit was given before induction using lactated Ringer’s, and the other half was supplemented until the establishment of pneumoperitoneum. Intraoperative infusion flow of crystalloids was set at 6–8 mL/kg/h and was adjusted by the anesthesiologist. Hypotension (decreased by more than 20% of basal MAP) was first corrected by ephedrine (5 mg) or phenylephrine (50 µg) intravenously during the surgery. If the hypotension recurred, a quick fluid infusion of 250–500 mL colloids (Succinylated Gelatin, B. Braun) was administered until the MAP was restored.
GDFT Protocol
Arterial pressure waveform analysis and stroke volume (SV) were obtained from FloTrac/EV1000™ system device (Edwards Lifesciences Corporation, Irvine, CA, USA). Preoperative fluid deficit was corrected by the same protocol as in the control group. After establishment of pneumoperitoneum, the GDFT algorithm was started and the initial SV was recorded as SV0 (Fig. 1). A fluid challenge of 200 mL of colloids was administered in 10 min, and the SV response was recorded. This step was repeated if the SV increased by more than 10%. Otherwise, 2 mL/kg/h lactated Ringer’s was maintained. The last SV with a response rate above 10% was defined as the maximal SV. If the SV fell below the trigger SV which was set at 10% lower than the maximal SV, another fluid challenge was given until the SV increased above the trigger SV [13]. Intraoperative hypotension was corrected with the same protocol as in the control group.
Surgical Technique
All surgeries were performed by the only surgeon. Three ports were introduced: left sub-costal (optic view), right hypochondrium (left hand), and mesogastrium (right hand). A longitudinal resection from the angle of His to approximately 3–4 cm proximally to the pylorus was performed. The sleeve was calibrated over a 36-French bougie.
Data Collection and Outcome Measures
Patient characteristics were recorded before surgery. Data on operative time, fluid volume, urine volume, blood loss, intraoperative opioid consumption, intraoperative hypotension and the type of vasopressors were extracted from the medical record (supplementary material). The primary outcome was the incidence of PONV in the first 48 h after LSG (supplementary material). Participants were asked by an anesthesia resident at 24 h and 48 h postoperatively using a questionnaire [18]. The second outcome included intensity of PONV, use of rescue therapy, postoperative length of stay (LOS) and other complications (supplementary material). The time of first postoperative water intake, flatus passage and ambulation were also recorded as the effect of GDFT on gastrointestinal function.
Sample Size
The sample size was calculated concerning the primary outcome. Sample size calculation software (www.powerandsamplesize.com) suggested that 60 patients for each group should be enrolled to provide a power of 80% (β = 0.2) with α set at 5%. Accounting for 20% potential loss to follow-up, the sample size was increased to 150 patients.
Statistical Analysis
Data were presented as mean ± SD or median (25th percentile, 75th percentile) depending on distribution. The Mann–Whitney U test was used for group comparisons of continuous variables when the data were non-normally distributed; otherwise, Student’s t test was applied. Repeated measures ANOVA was applied with change from baseline as the dependent variable, and treatment, time, and the treatment multiplied by time interaction as independent variables. Categorical variables were expressed as numbers (percentage) and compared using the chi-square test. Effect size was indicated using mean or median differences or odds ratios, with 95% confidence intervals (CIs). All statistical tests were 2-sided and P values < 0.05 were considered statistically significant. All analyses were performed using IBM SPSS Statistics 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
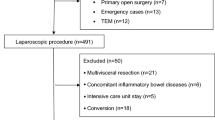
Of the initial 183 participants, 33 were excluded (see Fig. 2 for reasons). The others were randomly assigned to control group or GDFT group in a ratio of 1:1 (n = 75 per group). While 13 subjects were discontinued the study for revision surgery (n = 4), combined surgery (n = 1), translation to the ICU (n = 3), withdrawn from follow-up (n = 4) and blood transfusion (n = 1). In total, 137 patients were analyzed as per protocol, 67 in the control group and 70 in the GDFT group (Fig. 2).
No significant differences were detected in the demographic characteristics and perioperative data except for the colloids volume and crystalloids volume (Table 1). Patients in the GDFT group received more colloids (median (25th, 75th): 500 (475, 650) vs. 500 (0, 500) mL; P < 0.001) and less crystalloids (900 (900, 900) vs. 1300 (1200, 1400) mL; P < 0.001) during the surgery than those in the control group.
Incidence and Intensity of PONV During the First 48 h After LSG
Thirty-three patients (47.1%) receiving GDFT protocol suffered from PONV during the first 48 h after LSG compared to 48 (71.6%) in the control group (OR [95%CI], 0.35 [0.17–0.72]; P = 0.004) (Table 2). There was no significant difference in the PON occurrence between the two groups (P = 0.076), while the incidence of POV was lower in patients with GDFT (40% vs. 58.2%; OR [95%CI], 0.48 [0.24–0.95]; P = 0.033) (Table 2).
The frequency of PON showed no significant difference between the two groups (P = 0.971; Table 2). However, among the 39 patients who suffered from POV in the control group, 37 (94.9%) complained of at least 2 times of POV and 2 (5.1%) reported only once. By contrast, 21 (75%) patients with GDFT protocol suffered from 2 or more episodes of POV and 7 (25%) experienced only once (P = 0.003; Table 2). Rescue therapy in the control group was used nearly twice as often as in the GDFT group (58.2% vs. 30%; P = 0.001).
Postoperative Outcomes
The postoperative LOS in the GDFT group was 6.1 ± 1.0 days, which was shorter than that of the control group (6.6 ± 1.2 days; P = 0.007; Table 3). The time to first postoperative flatus in the GDFT group was 3 h earlier than that in the control group (27.5 (19, 31) vs. 31 (20, 48) hours; P = 0.037; Table 3). While the time to water intake and ambulation showed no significant difference between the two groups (P = 0.067; P = 0.095, respectively).
Discussion
This study demonstrated that GDFT is effective in decreasing the incidence and intensity of PONV after LSG. Moreover, this fluid therapy facilitated recovery of bowel function and shortened postoperative hospital stay.
For patients with LSG, several factors may contribute to the high susceptibility to PONV, including the fact that most of the subjects are younger women and non-smokers, with higher consumption of volatile anesthetics and opioids, impaired splanchnic perfusion during pneumoperitoneum and surgery-related anatomical changes [, , 19, 20, 21]. Unfortunately, most prophylactic antiemetics had poor efficacy in controlling PONV after LSG. In this study, even with double prophylactic antiemetics, 71.6% of subjects in the control group reported PONV episode. While GDFT reduced the incidence of PONV by more than one third. One reasonable explanation for this positive result is that the participants are more susceptible to PONV; thus, our cohort is more likely to reflect the antiemetic effect of GDFT. Another difference between this study and previous studies is the relatively larger sample size. The incidence rather than the severity of PONV was selected as the primary outcome, which may lead to different requirement for the sample size.
This study also revealed that GDFT was effective in accelerating recovery of intestinal function and shortening postoperative LOS. It is supposed that optimization of intravascular volume through SV-guided fluid therapy could minimize the impact of elevated intra-abdominal pressure on gastrointestinal perfusion during pneumoperitoneum [, 8, 22]. In addition, GDFT may also protect against intestinal barrier disorder and intestinal-related lymphoid tissue damage [, 22, 23].
Perioperative fluid management and accurate assessment of volume status have always been challenges for anesthesiologists [, 24, 25]. As an individualized approach to assess volume status and detect fluid responsiveness, GDFT has been recommended for bariatric surgery by several perioperative care guidelines [, 26, 27]. Among the hemodynamic parameters, SV rather than SVV seems to be more suitable for patients with obesity in consideration of increased intrathoracic pressure and low tidal volume during mechanical ventilation, which may influence the evaluation of fluid responsiveness [28]. Under the guidance of these parameters, hypovolemia can be corrected in time and a near-maximal SV can be achieved by intraoperative colloid bolus.
In the GDFT group, we chose colloidal solution other than crystalloid solution as fluid challenge, which may be a potential confounding factor for PONV occurrence and further studies are needed. In fact, the two solution will eventually distribute throughout the entire extracellular volume, but colloids initially remain predominantly within the plasma volume and therefore result in immediate preferential intravascular volume expansion [29]. And colloids was superior for improving oxygen transport, myocardial contractility, and cardiac output [30]. Thus, colloids are commonly used for fluid resuscitation in the GDFT protocol. Moreover, a relevant RCT indicated that PONV occurrence after LSG was independent of the type of solutions [14].
The present study has several limitations. First, there was a gender imbalance within both groups since the high-risk population of PONV is mostly female. This may limit the generalizability of our results to all morbidly obese patients, especially to male. Second, the anesthesiologist who implemented the fluid protocol was aware of the group assignment. This single-blinded study design is prone to inducing subjective bias and causing treatment imbalance between the two groups. However, the anesthesiologist was unaware of the aim and assessment outcomes of the present study, and the postoperative data were collected by another anesthesia resident who was blinded to the group assignment. Fourth, the incidence of PONV may be affected by the type of resuscitation fluid. Thus, a further research is necessary to explore the effect of crystalloids as resuscitation on PONV. At last, PONV was evaluated only once per day according to the study design. This subjective recollection of PONV episodes may not reflect the true incidence of this adverse event over time.
Conclusion
SV-guided fluid therapy can reduce the occurrence and intensity of PONV in high-risk patients undergoing LSG. Moreover, GDFT is conducive to accelerating recovery of intestinal function and shortening postoperative hospital stay.
References
Ponce J, DeMaria EJ, Nguyen NT, et al. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in 2015 and surgeon workforce in the United States. Surg Obes Relat Dis. 2016;12(9):1637–9.
Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149(3):275–87.
Griffith PS, Birch DW, Sharma AM, et al. Managing complications associated with laparoscopic Roux-en-Y gastric bypass for morbid obesity. Can J Surg. 2012;55(5):329–36.
Xiong Q, Min S, Wei K, et al. Transcutaneous electrical acupoint stimulation combined with dexamethasone and tropisetron prevents postoperative nausea and vomiting in female patients undergoing laparoscopic sleeve gastrectomy: a prospective, randomized controlled trial. Obes Surg. 2021;31(5):1912–20.
Zheng XZ, Cheng B, Luo J, et al. The characteristics and risk factors of the postoperative nausea and vomiting in female patients undergoing laparoscopic sleeve gastrectomy and laparoscopic gynecological surgeries: a propensity score matching analysis. Eur Rev Med Pharmacol Sci. 2021;25(1):182–9.
Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–48.
Giglio MT, Marucci M, Testini M, et al. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103(5):637–46.
Gómez-Izquierdo JC, Feldman LS, Carli F, et al. Meta-analysis of the effect of goal-directed therapy on bowel function after abdominal surgery. Br J Surg. 2015;102(6):577–89.
Zheng H, Guo H, Ye JR, et al. Goal-directed fluid therapy in gastrointestinal surgery in older coronary heart disease patients: randomized trial. World J Surg. 2013;37(12):2820–9.
Goodarzi M, Matar MM, Shafa M, et al. A prospective randomized blinded study of the effect of intravenous fluid therapy on postoperative nausea and vomiting in children undergoing strabismus surgery. Paediatr Anaesth. 2006;16(1):49–53.
Maharaj CH, Kallam SR, Malik A, et al. Preoperative intravenous fluid therapy decreases postoperative nausea and pain in high risk patients. Anesth Analg. 2005;100(3):675–82.
Apfel CC, Meyer A, Orhan-Sungur M, et al. Supplemental intravenous crystalloids for the prevention of postoperative nausea and vomiting: quantitative review. Br J Anaesth. 2012;108(6):893–902.
Muñoz JL, Gabaldón T, Miranda E, et al. Goal-directed fluid therapy on laparoscopic sleeve gastrectomy in morbidly obese patients. Obes Surg. 2016;26(11):2648–53.
Cho HJ, Huang YH, Poon KS, et al. Perioperative hemodynamic optimization in laparoscopic sleeve gastrectomy using stroke volume variation to reduce postoperative nausea and vomiting. Surg Obes Relat Dis. 2021;17(9):1549–57.
Hioki H, Watanabe Y, Kozuma K, et al. Risk stratification using lean body mass in patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2018;92(7):1365–73.
Members of the Working P, Nightingale CE, Margarson MP, et al. Peri-operative management of the obese surgical patient Association of Anaesthetists of Great Britain and Ireland Society for Obesity and Bariatric Anaesthesia. Anaesthesia. 2015;70(7):859–76.
Rahbari NN, Zimmermann JB, Schmidt T, et al. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96(4):331–41.
Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br J Anaesth. 2012;108(3):423–9.
Varner KL, March AL. Prevention of nausea and vomiting after laparoscopic sleeve gastrectomy: are we doing enough? AANA J. 2020;88(2):142–7.
Horn CC, Wallisch WJ, Homanics GE, et al. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur J Pharmacol. 2014;722:55–66.
Bataille A, Letourneulx JF, Charmeau A, et al. Impact of a prophylactic combination of dexamethasone-ondansetron on postoperative nausea and vomiting in obese adult patients undergoing laparoscopic sleeve gastrectomy during closed-loop propofol-remifentanil anaesthesia: a randomised double-blind placebo-controlled study. Eur J Anaesthesiol. 2016;33(12):898–905.
Rollins KE, Lobo DN. Intraoperative Goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trials. Ann Surg. 2016;263(3):465–76.
Chong MA, Wang Y, Berbenetz NM, et al. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: a systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35(7):469–83.
Chappell D, Jacob M, Hofmann-Kiefer K, et al. A rational approach to perioperative fluid management. Anesthesiology. 2008;109(4):723–40.
Navarro LH, Bloomstone JA, Auler JO Jr, et al. Perioperative fluid therapy: a statement from the international Fluid Optimization Group. Perioper Med (Lond). 2015;4:3.
Thorell A, MacCormick AD, Awad S, et al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. 2016;40(9):2065–83.
Ruiz-Tovar J, Sanchez-Santos R, Martín-García-Almenta E, et al. Enhanced recovery after bariatric surgery. Cir Esp (Engl Ed). 2019;97(10):551–9.
Kuper M, Gold SJ, Callow C, et al. Intraoperative fluid management guided by oesophageal Doppler monitoring. BMJ. 2011;342: d3016.
Tremblay LN, Rizoli Sb Fau - Brenneman FD, Brenneman FD. Advances in fluid resuscitation of hemorrhagic shock. Can J Surg. 2001;44(3):172–9.
Hankeln K, Rädel C Fau - Beez M, Beez M Fau - Laniewski P, et al. Comparison of hydroxyethyl starch and lactated Ringer's solution on hemodynamics and oxygen transport of critically ill patients in prospective crossover studies. Crit Care Med. 1989;17(2):133–5
Funding
The present work was supported by grants from Chongqing Science and Technology Bureau (project no. CSTC2019jscx-msxmX0214) and Chongqing Health Commission (project no. 2021MSXM146, 2022jstg026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This trial was approved by the institutional ethics committee of the first affiliated hospital of Chongqing Medical University and registered at the Chinese Clinical Trial Registry (ChiCTR2100048618). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Patients with GDFT were associated with lower incidence of PONV.
• Fewer patients received rescue therapy in the GDFT group.
• Patients with GDFT had a faster return of flatus and shorter postoperative LOS.
• GDFT protocol is performed based on the change in stroke volume.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, X., Wei, K., Liu, L. et al. The Impact of Goal-Directed Fluid Therapy on Postoperative Nausea and Vomiting in High-Risk Patients Undergoing Laparoscopic Sleeve Gastrectomy. OBES SURG 32, 3533–3540 (2022). https://doi.org/10.1007/s11695-022-06260-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06260-0