Abstract
Background
Gastric sleeve stenosis (GSS) occurs in up to 4% of patients after laparoscopic sleeve gastrectomy (LSG). Typical symptoms include reflux, abdominal pain, dysphagia, and regurgitation. Serial pneumatic balloon dilation (PBD) is a successful treatment in many cases obviating the need for revisional surgery, but the potential for weight regain is unknown. The aim of the current study was to assess weight trends following serial pneumatic dilation for GSS.
Methods
Retrospective analysis of a prospectively maintained database of patients undergoing serial PBD for GSS at one institution. Primary outcome was change in BMI before and after serial PBD. Secondary outcomes included complication rates and need for revisional surgery. Sub-group analyses were performed to determine the relationship of patient and procedural factors to BMI after PBD.
Results
Forty-four patients met inclusion criteria, 34 (84.1%) women. Mean age was 46.7 (SD 11.9). Mean pre-sleeve BMI was 47.8 (SD 9.2), and mean BMI prior to first dilation was 34.2 (SD 6.8). Median follow-up was 395 days (range 48–571). Mean BMI at time of last follow up was 33.7 (SD 6.7). There was no statistical difference in BMI pre- or post-PBD (p 0.980). The lowest 10th and highest 90th BMI percentile trended toward a higher and lower BMI after PBD, respectively, though not significant.
Discussion
As the prevalence of sleeve gastrectomy continues to rise, an increasing number of patients will require treatment for GSS. Stenosis is effectively treated with serial PBD in most patients without any impact on weight gain, making this an effective and appealing option for many patients.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The vertical sleeve gastrectomy has been increasing in popularity since the 1980s, when it was initially reported as a first-stage operation for a biliopancreatic diversion with duodenal switch (BPD-DS) [1, 2]. The weight loss outcomes were so successful, and the side effect profile so reasonable, that many patients did not continue with the second stage after sleeve gastrectomy alone [3]. With the advent of laparoscopy in the 1990s, the prevalence of laparoscopic sleeve gastrectomy (LSG) dramatically increased [4]. The LSG provides weight loss outcomes and comorbidity resolution similar to the bypass, with lower morbidity. As a result, it is now the most commonly performed bariatric operation worldwide, with more than 150,000 sleeve gastrectomies performed in the USA each year [5, 6].
Despite the favorable side effect profile, the sleeve has potential complications. The most serious of these are staple-line leak, which occurs in 1–3% of patients [7], and sleeve stenosis, occurring in 0.5–3.5% [8,9,10,11]. Gastric sleeve stenosis (GSS) occurs due to a narrowing of the tubularized stomach, most commonly at the incisura, from edema or ischemia in the early postoperative period, or from torsion, kinking, or scarring along the staple line [12]. Patients frequently present with abdominal pain, nausea/vomiting, dysphagia, or reflux.
While considerable data exists regarding treatment of gastric sleeve leak, optimal treatment of stenosis after sleeve gastrectomy is less well established [11]. The definitive treatment for severe or refractory stenosis after sleeve gastrectomy is surgical revision with conversion to Roux-en-Y gastric bypass. Endoscopic dilation is now emerging as a first-line therapy for GSS that may prevent the need for revisional surgery. Several case series have been published demonstrating the safety and efficacy of endoscopic dilation for GSS in cohorts of between 10 and 33 patients. Symptom resolution occurs in 44–100% of patients, with low or zero reported complications [8, 10, 11, 13,14,15,16,17,18].
While the safety and efficacy of endoscopic pneumatic balloon dilation (PBD) for GSS have been demonstrated, none of these reports includes data on weight change following either successful or unsuccessful endoscopic management. The loss of restriction due to a larger sleeve stomach has been proposed as a mechanism of weight regain following sleeve gastrectomy [19], and revisional sleeve gastrectomy to narrow a dilated sleeve can result in improved weight loss [20]. The impact on weight of PBD in the setting of sleeve stenosis is unknown. The objective of this study is to analyze changes in weight after endoscopic treatment for gastric sleeve stenosis.
Materials and Methods
Patient Population
We performed a retrospective analysis of a prospectively maintained database at a single tertiary care center. Included patients were referred to a trained bariatric endoscopist for suspected GSS and underwent diagnostic endoscopy with through-the-scope (TTS) balloon dilation followed by PBD. Inclusion criteria included prior sleeve gastrectomy, suspicion on symptoms or imaging of sleeve stenosis, age > 18, confirmation of the stenosis by endoscopy, and treatment with balloon dilations. Patients with concurrent sleeve leaks were excluded from the analysis. The study was approved by the Institutional Review Board.
Endoscopic Protocol
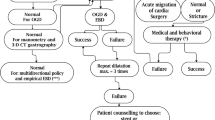
A single bariatric endoscopist performed all the included endoscopic procedures. This protocol has been previously described [21]. A diagnostic upper endoscopy was performed to confirm the diagnosis of GSS and obtain standard upper gastrointestinal landmarks. Severity of stenosis was assessed at the time of endoscopy using previously described features such as luminal diameter and distensibility characteristics and pooling of bilious fluid [22]. Once stenosis was confirmed, patients were treated with balloon dilation using a 20-mm hydrostatic balloon (Cook Medical, Bloomington, IN) at the level of the gastric stenosis followed by successive PBD (Boston Scientific, Marlborough, MA). Serial dilations were performed with through-the scope balloon dilation followed by PBD occurred every 2–4 weeks with increasing balloon sizes (30 mm, 35 mm, 40 mm) and/or filling pressure (maximum pressure per square inch [PSI] of 20) until resolution of symptoms was achieved or patient was referred for surgical revision. Care was taken to avoid the pylorus and gastroesophageal junction. Surgical referral was performed in patients who did not respond to serial endoscopic dilation.
Outcomes
Primary outcome was change in body mass index (BMI) from the initial balloon dilation to time of last follow-up. Secondary outcomes included complication rates and need for revisional surgery. Descriptive statistics were assessed as counts (%) means and standard deviations. Paired Student’s t-test was used to compare pre-dilation BMI and current BMI. We performed sub-group analyses to determine the relationship of patient and procedural factors to BMI after dilation. These included the following: stratification by stenosis severity, comparison of TTS versus PBD, pre-dilation BMI below the 10th percentile or higher than the 90th percentile, and those requiring supplemental nutrition. All statistical analyses were performed using StataMP v14.1.412 (StataCorp LLC, College Station, TX).
Results
Patient Demographics
Patient demographics are summarized in Table 1. A total of 44 patients met inclusion criteria. The mean age of the cohort was 46.7 (± 11.2 SD). Women represented 84.1% of the patients and 90.9% underwent laparoscopic sleeve gastrectomy. Patients were followed for a median of 395 days (interquartile range [IQR] 48–571). Severity of stenosis varied with 13 (29.5%) patients with mild stenosis, 16 (36.4%) with moderate stenosis, and 15 (34.1%) with severe stenosis.
Treatment Outcomes
Treatment outcomes are shown in Table 2. Patients underwent a mean of 2.4 (± 1.3) dilations. Two patients (4.5%) had complications, both of which were lacerations that were treated endoscopically. Thirty-nine patients (88.6%) had resolution of symptoms following serial PBD, and five patients (11.4%) ultimately underwent surgical revision for incomplete or lack of response.
Weight Trends
Weight trends are presented on Fig. 1. Mean pre-sleeve BMI was 47.8 (± 9.2 SD) and mean nadir BMI was 30.3 (± 5.1 SD). The mean pre-dilation BMI was 33.8 (± 6.9 SD) and the mean BMI at time of last follow-up was 33.7 (± 6.7 SD). There was no statistical difference in BMI pre- or post-dilation (p = 0.95).
Subgroup Analyses
When stratified by stenosis severity, there was no significant difference in pre-dilation BMI and BMI at last follow-up in either the mild, moderate, or severe subgroups (Table 3). Similarly, for patients who received only TTS dilation or only PBD dilation, there was no difference in pre- or post-dilation BMI. While there was a trend for patients who were in the lowest 10th percentile to have higher BMI after dilation and for those in the highest 90th percentile to have lower BMI after dilation, this was not statistically significant. Finally, there was no difference in BMI before and after dilation in patients who required enteral or parenteral nutrition.
Discussion
While the safety and efficacy of endoscopic pneumatic balloon dilation for stenosis after sleeve gastrectomy has been demonstrated, data on weight change is lacking. There are two main findings in this study. First, this is the first study to examine weight outcomes after endoscopic dilation for GSS. In our cohort, there is no significant change in BMI before and after dilation. Additionally, we performed sub-group analyses to look at changes in BMI stratified by stenosis severity, type of dilation, and pre-operative BMI. There were no significant differences in any of the sub-groups.
GSS is the most common serious complication after LSG, impacting up to 4% of patients [8,9,10,11]. As the popularity of the LSG continues to increase, a growing population of patients will face this challenge [6]. GSS can result from an anatomical stricture or twisting of the gastric lumen resulting in functional obstruction most commonly at the level of the gastric mid-body or incisura. The presentation often varies depending on the severity of the obstruction and may include symptoms such as reflux, dysphagia, nausea, vomiting, regurgitation, and/or inability to tolerate oral nutrition. Risk factors for GSS are mainly related to surgical technique and include bougie size, tissue edema or ischemia which may progress into fibrotic scarring, gastric adhesions, imbrication of the staple-line, sharp angulations of the stapler, or progressive rotation of the staple line [8, 23].
The work-up commonly entails an upper gastrointestinal series (UGIS) and upper endoscopy; however, there is currently no clear definition of GSS in the current literature. Studies have demonstrated a significant treatment delay and increased healthcare utilization in patients with symptoms due to GSS, likely due to a delay in the diagnosis [24]. Our group has sought to determine objective quantitative criteria for making the diagnosis and to establish an algorithm for the work-up of this condition. While UGIS is generally recommended as part of the evaluation, we have shown that it has a low sensitivity and low negative predictive value in making the diagnosis and predicting response to treatment when compared to endoscopic findings [21]. However, endoscopic evaluation may also be misleading, especially to the less experience endoscopist, due to easy passage of the endoscope into the distal antrum, as well as the fact that air insufflation may relieve gastric torsion [14]. We have also evaluated alternative measurement strategies such as endoluminal functional impedance planimetry (EndoFLIP), which provides objective measurement of gastric lumen diameter and distensibility [25]. Our group demonstrated that EndoFLIP may be a useful tool to characterize GSS and predict response to pneumatic dilation [25]. Furthermore, other quantifiable endoscopic criteria, such as ratio between the narrowest and widest gastric lumen diameters, and the presence of pooling of bilious fluid, have also been investigated and may further help confirm the diagnosis [22]. Given these current limitations, an upper endoscopy performed by a trained bariatric endoscopist could expedite the diagnosis and management of GSS.
After establishing the diagnosis, various approaches to management of GSS have been described in the literature. In a recent systematic review, clinical success of endoscopic therapy (82%) was found to be superior to surgical treatment (75%) and medical management (58%), while carrying fewer adverse events compared with surgery (4.7% vs. 15%) [26]. Among endoscopic modalities, pneumatic dilation appears to have the best clinical efficacy, and has emerged as first-line therapy for GSS. Hydrostatic balloon dilation has demonstrated lower efficacy but is commonly performed prior to pneumatic dilation as was done in this study to minimize the risk of perforation (Reviewer #2, Comment #1). For refractory cases, placement of self-expandable metallic stent has shown to have high success rates. Ultimately, conversion to Roux-en-Y is the preferred modality as a rescue surgery.
While numerous studies have demonstrated the safety and efficacy of endoscopic dilation for treatment of GSS [8, 10, 11, 27], efficacy is balanced against weight outcomes for many patients. There is a fear that dilation can result in a larger sleeve stomach, loss of restriction, and potential weight gain. This fear is supported by studies showing weight loss after revisional narrowing of a dilated stomach [20, 28, 29], but currently there is no good data to help counsel patients on expected changes in weight after dilation.
There are limitations to these findings. First, this is a small, single-center case series. The endoscopic dilations were performed by a single endoscopist. The sub-group analyses must be interpreted within the confines of the sample size. However, the existing literature in this field is also in the form of case series, and this represents one of the largest reports of endoscopic treatments of GSS to date.
While further study is certainly warranted, this is the first study to show that weight regain does not occur after endoscopic dilation for GSS. This finding can help counsel patients on options for GSS and likely outcomes.
Conclusion
Endoscopic dilation for GSS is safe and effective in most patients, and in this cohort has no impact on weight gain.
References
Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8(3):267–82. https://doi.org/10.1381/096089298765554476.
Marceau P, Biron S, Bourque RA, et al. Biliopancreatic diversion with a new type of gastrectomy. Obes Surg. 1993;3(1):29–35. https://doi.org/10.1381/096089293765559728.
Silecchia G, Boru C, Pecchia A, et al. Effectiveness of laparoscopic sleeve gastrectomy (first stage of biliopancreatic diversion with duodenal switch) on co-morbidities in super-obese high-risk patients. Obes Surg. 2006;16(9):1138–44. https://doi.org/10.1381/096089206778392275.
Gagner M, Deitel M, Kalberer TL, et al. The second international consensus summit for sleeve gastrectomy, March 19–21, 2009. Surg Obes Relat Dis. 2009;5(4):476–85. https://doi.org/10.1016/j.soard.2009.06.001.
ASMBS. Estimate of bariatric surgery numbers, 2011–2018. Available from: asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes Surg. 2019;29(3):782–95. https://doi.org/10.1007/s11695-018-3593-1.
Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surg Endosc. 2020;34(1):396–407. https://doi.org/10.1007/s00464-019-06782-2.
Parikh A, Alley JB, Peterson RM, et al. Management options for symptomatic stenosis after laparoscopic vertical sleeve gastrectomy in the morbidly obese. Surg Endosc. 2012;26(3):738–46. https://doi.org/10.1007/s00464-011-1945-1.
Lacy A, Ibarzabal A, Pando E, et al. Revisional surgery after sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2010;20(5):351–6. https://doi.org/10.1097/SLE.0b013e3181f62895.
Deslauriers V, Beauchamp A, Garofalo F, et al. Endoscopic management of post-laparoscopic sleeve gastrectomy stenosis. Surg Endosc. 2018;32(2):601–9. https://doi.org/10.1007/s00464-017-5709-4.
Manos T, Nedelcu M, Cotirlet A, et al. How to treat stenosis after sleeve gastrectomy? Surg Obes Relat Dis. 2017;13(2):150–4. https://doi.org/10.1016/j.soard.2016.08.491.
Levy JL, Levine MS, Rubesin SE, et al. Stenosis of gastric sleeve after laparoscopic sleeve gastrectomy: clinical, radiographic and endoscopic findings. Br J Radiol. 2018;91(1089):20170702. https://doi.org/10.1259/bjr.20170702.
Shnell M, Fishman S, Eldar S, et al. Balloon dilatation for symptomatic gastric sleeve stricture. Gastrointest Endosc. 2014;79(3):521–4. https://doi.org/10.1016/j.gie.2013.09.026.
Ogra R, Kini GP. Evolving endoscopic management options for symptomatic stenosis post-laparoscopic sleeve gastrectomy for morbid obesity: experience at a large bariatric surgery unit in New Zealand. Obes Surg. 2015;25(2):242–8. https://doi.org/10.1007/s11695-014-1383-y.
Rebibo L, Hakim S, Dhahri A, et al. Gastric stenosis after laparoscopic sleeve gastrectomy: diagnosis and management. Obes Surg. 2016;26(5):995–1001. https://doi.org/10.1007/s11695-015-1883-4.
Donatelli G, Dumont JL, Pourcher G, et al. Pneumatic dilation for functional helix stenosis after sleeve gastrectomy: long-term follow-up (with videos). Surg Obes Relat Dis. 2017;13(6):943–50. https://doi.org/10.1016/j.soard.2016.09.023.
Nath A, Yewale S, Tran T, et al. Dysphagia after vertical sleeve gastrectomy: evaluation of risk factors and assessment of endoscopic intervention. World J Gastroenterol. 2016;22(47):10371–9. https://doi.org/10.3748/wjg.v22.i47.10371.
Al Sabah S, Al Haddad E, Siddique I. Endoscopic management of post-laparoscopic sleeve gastrectomy stenosis. Surg Endosc. 2017;31(9):3559–63. https://doi.org/10.1007/s00464-016-5385-9.
Weiner RA, Weiner S, Pomhoff I, et al. Laparoscopic sleeve gastrectomy–influence of sleeve size and resected gastric volume. Obes Surg. 2007;17(10):1297–305. https://doi.org/10.1007/s11695-007-9232-x.
Noel P, Nedelcu M, Nocca D, et al. Revised sleeve gastrectomy: another option for weight loss failure after sleeve gastrectomy. Surg Endosc. 2014;28(4):1096–102. https://doi.org/10.1007/s00464-013-3277-9.
Bhalla S, Yu JX, Varban OA, et al. Upper gastrointestinal series after sleeve gastrectomy is unnecessary to evaluate for gastric sleeve stenosis. Surg Endosc. 2021;35(2):631–5. https://doi.org/10.1007/s00464-020-07426-6.
Yu JX, Dolan RD, Bhalla S, et al. Quantification of gastric sleeve stenosis using endoscopic parameters and image analysis. Gastrointest Endosc. 2021;93(6):1344–8. https://doi.org/10.1016/j.gie.2020.12.009.
Iannelli A, Martini F, Schneck AS, et al. Twisted gastric sleeve. Surgery. 2015;157(1):163–5. https://doi.org/10.1016/j.surg.2014.01.018.
Park JV, Sievers MT, Rollins PD, et al. Quantifying healthcare utilization and delay in the treatment of gastric stenosis following sleeve gastrectomy. Obes Surg. 2022;32(1):90–5. https://doi.org/10.1007/s11695-021-05704-3.
Yu JX, Baker JR, Watts L, et al. Functional lumen imaging probe is useful for the quantification of gastric sleeve stenosis and prediction of response to endoscopic dilation: a pilot study. Obes Surg. 2020;30(2):786–9. https://doi.org/10.1007/s11695-019-04105-x.
Brunaldi VO, Galvao Neto M, Zundel N, et al. Isolated sleeve gastrectomy stricture: a systematic review on reporting, workup, and treatment. Surg Obes Relat Dis. 2020;16(7):955–66. https://doi.org/10.1016/j.soard.2020.03.006.
Chang SH, Popov VB, Thompson CC. Endoscopic balloon dilation for treatment of sleeve gastrectomy stenosis: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91(5):989-1002.e4. https://doi.org/10.1016/j.gie.2019.11.034.
Saliba C, El Rayes J, Diab S, et al. Weight regain after sleeve gastrectomy: a look at the benefits of re-sleeve. Cureus. 2018;10(10):e3450. https://doi.org/10.7759/cureus.3450.
AlSabah S, Alsharqawi N, Almulla A, et al. Approach to poor weight loss after laparoscopic sleeve gastrectomy: re-sleeve vs. gastric bypass. Obes Surg. 2016;26(10):2302–7. https://doi.org/10.1007/s11695-016-2119-y.
Author information
Authors and Affiliations
Contributions
Study concept and design: LM, JXY, ARS. Acquisition, analysis, or interpretation of data: LM, JXY, LW, SV, ARS. Statistical analysis: JXY, ARS. Drafting of the manuscript: LM, JXY, ARS. Critical revision of the manuscript for important intellectual content: LM, JXY, SB, KP, ARS. Study supervision: ARS.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement of Informed Consent
Does not apply.
Conflict of Interest
Laura Mazer, Jessica X Yu, Sean Bhalla, Kevin Platt, Lydia Watts, and Sarah Volk have no conflicts to disclose. Allison R. Schulman is a Consultant for Apollo Endosurgery, Boston Scientific, MicroTech, and Olympus, and receives Research/Grant support from GI Dynamics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
- Gastric sleeve stenosis (GSS) following sleeve gastrectomy most commonly at the incisura and has an increasing prevalence
- Patients frequently present with abdominal pain, nausea/vomiting, dysphagia, or reflux
- Endoscopic pneumatic balloon dilation is emerging as a first-line therapy for GSS that may prevent the need for revisional surgery, but data on weight change is lacking
- Here, we demonstrate that there is no significant change in body mass index (BMI) before and after dilation
Rights and permissions
About this article
Cite this article
Mazer, L., Yu, J.X., Bhalla, S. et al. Pneumatic Balloon Dilation of Gastric Sleeve Stenosis Is Not Associated with Weight Regain. OBES SURG 32, 1–6 (2022). https://doi.org/10.1007/s11695-022-05957-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-05957-6