Abstract
Introduction
The impact of laparoscopic sleeve gastrectomy (LSG) on gastroesophageal reflux disease (GERD) has not been widely quantified, and the data in the literature remain controversial.
Materials and Methods
Candidates for LSG underwent barium swallow, esophageal manometry, ambulatory 24-h esophageal pH monitoring (APM), and gastric emptying scintigraphy before and after surgery (1 and 18 months). Symptoms were evaluated using a gastroesophageal reflux disease questionnaire (GERDq). Esophagogastroduodenoscopy was performed preoperatively in all patients and at 18 months postoperatively in patients who had suffered from preoperative esophagitis.
Results
Fifty-two patients were included in the study (64.4% women and 34.6% men) with a median age of 46 years (25–63 years) and BMI of 45.0 ± 5.6 kg/m2. The follow-up rates at 1 and 18 months were 82.7% and 80.8%. At 18 months, the percentage of weight loss (%TWL) was 33.6 ± 10.4% and the percentage of excess BMI loss (%EBMIL) was 77.6 ± 25%. Postoperatively, a significant increase in accelerated gastric emptying and impaired esophageal body motility occurred at 1 and 18 months. A significant worsening of all the values obtained at both 1 and 18 months postoperatively becomes evident when comparing the results of the APM. After surgery, 76.4% of patients had developed “de novo” GERD at 1 month and 41% at 18 months. No improvement was found in patients with symptomatic GERD.
Conclusion
Based on the results of this study, LSG led to a considerable rate of postoperative “de novo” GERD. In addition, no improvement was found in patients with symptomatic GERD.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic sleeve gastrectomy (LSG) has become a popular technique as a definitive operation for morbid obesity and has proven to be safe with excellent results in weight loss and long-term resolution of comorbidities [1, 2].
However, despite being a widely implemented surgery, one of the technique’s limitations is the postoperative presence of gastroesophageal reflux disease (GERD). Many studies have evaluated the effects of LSG in GERD, with controversial findings, but few studies have been conducted with an objective analysis. The Bariatric Outcomes Longitudinal Database, which has the highest published casuistry, quantifies the prevalence of GERD as 44.5% for preoperative GERD-related symptoms, 37.4% for postoperative GERD-related symptoms, and 8.6% for “de novo” GERD [3, 4]. The latter percentage is as high as 20% in a recent meta-analysis that discusses the effect of LSG on the prevalence of GERD [5].
Neither the true impact of GERD post-LSG nor the mechanisms affecting the occurrence of reflux after the technique [6, 7] have been discovered. However, there seem to be alterations in the anatomy and physiology of the esophagogastric junction [8,9,10], changes in intragastric pressure [11], and alterations in gastric emptying [12].
The aim of this study was to discover the prevalence of GERD in patients with morbid obesity who underwent LSG, assess the anatomical and functional changes that may occur in the esophagogastric junction postoperatively, and analyze their influence on the physiopathology of GERD.
Materials and Methods
Quasi-experimental study with a within-subject design, pre- and postoperatively, on a 52-patient sample with morbid obesity who met the National Institutes of Health criteria [13] and who underwent LSG surgery between January 2016 and June 2019.
The Ethics Committee of the hospital approved the study, and informed consent was obtained from all patients.
Inclusion and Exclusion Criteria
This study includes morbidly obese patients with or without mild GERD symptoms according to the gastroesophageal reflux disease questionnaire (GERDq) [14].
Patients with severe GERD symptoms, large hiatus hernia, severe esophagitis or Barrett’s esophagus, and/or a history of upper gastrointestinal surgery were excluded.
GERD Assessment
GERD assessment focuses on three pillars: symptoms, endoscopy, and functional testing, and the definition of the clinical phenotype variable of GERD is based on the three main consensuses currently used to define GERD: the Montreal classification that defines the disease by means of its symptoms and its complications [15], the Lyon Consensus that facilitates the interpretation of reflux studies that establish or refute the diagnosis [16], and the Rome IV criteria on functional digestive disorders [17]. Objective evidence of GERD has been defined as esophageal acid exposure time (AET) > 6% or AET 4–6% and adjunctive findings: reflux episodes > 80 and/or endoscopic esophagitis and/or motility findings in GERD.
The clinical phenotypes of GERD have therefore been defined as follows: symptomatic GERD, silent GERD, functional esophageal disorder, and absence of GERD.
Asymptomatic patients without objective evidence of GERD were considered non-GERD cases; asymptomatic patients with objective evidence of GERD were considered silent GERD cases; symptomatic patients with objective evidence of GERD were considered symptomatic GERD cases; and symptomatic patients without objective evidence of GERD were considered cases of functional esophageal disorder.
The presence of symptomatic GERD, silent GERD, or functional esophageal disorder in patients who had not previously suffered from GERD was considered “de novo” GERD cases.
Assessment of GERD was conducted preoperatively and at 1 and 18 months postoperatively by means of the GERDq questionnaire, barium swallow examination, gastric emptying scintigraphy, standard manometry, and ambulatory esophageal pH monitoring (APM). An esophagogastroduodenoscopy with esophageal biopsy was performed preoperatively on all patients included in the study and was repeated at 18 months postoperatively for patients who presented esophagitis before surgery. The presence of esophagitis was determined according to Los Angeles classification [18].
The GERDq questionnaire, designed according to the Montreal definition and based on typical symptoms, was used to establish a quantitative analysis of the symptoms associated with GERD, giving a total GERD score ranging from 0 to 18. The cut-off point was established at 8 [14, 19]. The severity of symptoms was scored as “no symptoms,” “mild symptoms,” or “severe symptoms.”
For APM, the reference values followed were those of the DeMeester score [20], and for manometry, those of the Spanish Group for the study of Digestive Motility [21]. Length and resting pressure of the lower esophageal sphincter (LES) and mean amplitude of the waves in the distal third of the esophagus were analyzed.
All patients with “severe symptoms” (based on the GERDq questionnaire) showed pathological pH-metry results and met the exclusion criterion for sleeve gastrectomy according to the center’s therapeutic protocol; these patients underwent a gastric bypass.
The presence of hiatus hernia and gastric volume were evaluated by means of a barium swallow examination according to a previously described technique using geometric formulas for the gastric body (cylinder) and antrum (truncated cone) [22].
Gastric emptying scintigraphy was conducted after ingestion of TC99m sulfur colloid labeled solid food. Images were obtained using a gamma camera at 0, 30, 60, 120, 180, and 240 min. Calculations were made to determine the gastric emptying half time (T½) and the percent gastric retention at 2 h and 4 h. Gastric emptying rate was classified as normal, delayed, or accelerated according to whether the percentage retained was ≤ 60% in 2 h, > 60% in 2 h, or < 30% in 1 h, respectively.
Surgical Technique
LSG comprises a standardized technique performed by four surgeons from the same facility. A 36 Fr bougie was employed to configure the gastric tract, initiating a transection at 4 cm from the pylorus to the angle of His with an endo-linear stapler and no reinforcement. Crural repair was performed in patients with a preoperative radiological diagnosis of hiatus hernia and at the surgeon’s discretion.
Thirty-day surgical complications were detailed according to the Clavien-Dindo surgical classification [23].
Statistical Analysis
The SPSS Statistics v.22 software was used to calculate the mean and standard deviation, as well as the median with the interquartile range if it did not adhere to a normal distribution.
The results of all tests were compared before and after surgery (at 1 and 18 months). Statistical analysis was carried out by applying McNemar’s test for qualitative variables and the comparison of means in paired quantitative variables with Student’s t-test or the Wilcoxon test as a non-parametric test. Pearson’s correlation test was used for correlations between variables. A p value of < 0.05 was considered statistically significant.
Results
Descriptive Study of the Series
Fifty-two patients (64.4% women and 34.6% men) with a median age of 46 years (25–63 years) were included in the study. Table 1 shows the patients’ clinical characteristics.
One month after the intervention, 43 of the 52 patients (82.7%) completed the study, and at 18 months 42 of the 52 patients (80.8%) remained in the study. Ten patients did not complete the follow-up: six patients dropped out of the study, and four patients left due to postoperative complications that prevented them from completing the study (Fig. 1).
There were six cases of postoperative complications, all of which were classed as major (grade 3b) (leakage and hemoperitoneum), and there was one case of a late complication (stenosis of the gastroplasty that required conversion to bypass at 12 months postoperatively). No deaths were reported.
Tables 2 and 3 show the progression of anthropometric data and comorbidities [24] in the different stages of the study.
Prevalence and Evolution of GERD
Figure 2 shows the changes in the prevalence of GERD according to the different clinical phenotypes.
Among the patients with non-GERD status in the preoperative study (26/52), 76.4% (13/17) were worse at 1 month: seven cases of silent GERD, three cases of symptomatic GERD, and three cases of functional disorder. Fifty-two percent of those who showed worsening symptoms in the first month (7/13) improved after 18 months. A total of nine patients did not complete the follow-up.
None of the patients with symptomatic GERD in the preoperative study (6/52) improved at either 1 or 18 months. At 1 month, five cases presented with silent GERD and the remaining case suffered from symptomatic GERD. Their progression remained unchanged at 18 months. A negative correlation is observed between symptoms (average GERDq score) and BMI (r = − 0.34; p = 0.016) and waist circumference (r = − 0.3; p = 0.03) at 18 months postoperatively.
Only two of the patients with silent GERD in the preoperative study (20/52) improved after 1 month. All the others failed to improve (eleven with silent GERD, five with symptomatic GERD, and two with functional disorder). At 18 months, only six patients had improved.
The percentage of “de novo” GERD was 76.4% (13/17) at 1 month. After 18 months, 54% of the cases had improved. The percentage of “de novo” GERD was 41% (7/17) at 18 months. Five cases suffered from silent GERD, one case from symptomatic GERD, and one case from functional esophageal disorder.
Symptom Assessment (GERDq Questionnaire)
The symptom score is described before and after LSG in Table 4.
The results showed a significant worsening of the symptom score obtained both at 1 and at 18 months postoperatively.
Proton Pump Inhibitor (PPI) Therapy
In the preoperative study, 30.8% of patients were receiving PPI therapy, while these figures were 17.4% at 1 month after surgery and 20.4% at 18 months after surgery, without any statistically significant differences.
No relationship was found between symptoms (average GERDq score) and PPI therapy (dosage of PPI) in any stage of the study (data not shown).
Evolution of pH Monitoring Values
In the preoperative study, 100% of the patients with symptomatic GERD (GERDq ≥ 8) and 43.5% of patients with asymptomatic GERD presented pathological APM. The four patients with esophagitis (grades A and B) in the preoperative esophagogastroduodenoscopy belong to the silent GERD group. At 18 months after surgery, esophagogastroduodenoscopy was only performed on these four patients, and they showed no changes (grade A or B esophagitis) but continued to exhibit pathological APM values (Table 4).
A significant worsening of all the values obtained both at 1 and at 18 months postoperatively becomes evident when comparing the results of the APM (Table 4).
Evolution of Esophageal Manometric Values
No cases of hypomotility were found preoperatively. However, there was a significant increase in hypomotility 18 months postoperatively. The relationship between esophageal motility (median esophageal contraction amplitudes) and DeMeester score was not significant at 1 month and at 18 months after surgery (r = − 0.04; p = 0.81 at 1 month and r = − 0.22; p = 0.6 at 18 months) (Table 4).
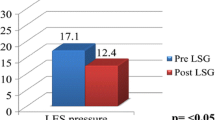
Although the postoperative LES pressures dropped almost 4 points, this decrease is insignificant, and the percentage of hypotensive LES remains at similar levels in all three stages.
In the preoperative phase, half of the patients with GERDq ≥ 8 presented hypotensive LES. None of them showed an improvement after 1 or 18 months (symptoms and/or pathological APM remain unchanged).
Evolution of Gastric Emptying Values
Most of the patients had normal preoperative gastric emptying, showing a significant increase in accelerated gastric emptying after LSG: 15.4% preoperatively, 40% at 1 month, and 35.7% at 18 months (p < 0.05) (Table 4). The relationship between percentage of gastric retention at 2 h and gastric volume was not significant at 18 months (r = 0.26; p = 0.09).
In the seven patients with preoperative delayed gastric emptying, two cases maintained a delayed pattern 1 month and 18 months after the operation. Regarding the four GERD phenotypes, those who maintained a delayed emptying pattern continued to suffer from GERD at 1 month (symptomatic or silent) and at 18 months (silent and functional disorder).
Barium Swallow Examination
In the preoperative study, 35% (18/52) of the patients presented with hiatus hernia in the barium swallow (type I). In the six patients who underwent crural closure, the hiatus hernia was anatomically resolved without any evidence of relapse either at 1 month or at 18 months post-operation. In the twelve remaining cases that did not undergo crural closure, there was no evidence of hiatus hernia in the postoperative barium swallow performed 1 month after the operation in nine cases, although one of these cases showed evidence of hiatus hernia again at 18 months post-operation.
All the patients who underwent crural closure presented preoperative GERD (silent or symptomatic). After 1 month, all the cases continued to present GERD but not hiatus hernia. Meanwhile, at 18 months, only one case had improved, and the rest continued to present silent GERD but not hiatus hernia. However, in the five cases of patients with hiatus hernia and GERD that did not undergo crural closure due to technical difficulties, the evolution from the first to the 18th month was comparable.
Gastric volume decreased significantly during the postoperative period (Table 4).
No relationship was found between postoperative gastric volume and symptoms (average GERDq score), pH-metry, and manometric parameters at 1 month and 18 months post-operation (data not shown).
Discussion
Obesity is associated with GERD, and when there is a decrease in weight, the symptoms of GERD improve [25,26,27]. Studies support the role of abdominal obesity, reflected in a larger abdominal perimeter, as a mediator in the effect that obesity may exert on intragastric pressure and esophageal exposure to acid reflux. Therefore, a reduction in the percentage of postoperative reflux would be expected with optimal weight loss [26].
However, in the series presented here, there is a negative correlation between the mean GERDq score at 18 months after surgery and the anthropometric data (BMI and waist circumference). These findings could be explained from a pathophysiological point of view if the patients with a lower BMI at 18 months were those with a lower gastric volume, i.e., less gastric distensibility, greater gastric pressure, and therefore greater GERD [11]. In this study, intragastric pressure was not measured but gastric volume was, and there was a negative correlation between gastric volume and BMI at 18 months (data not included). Therefore, the mechanisms explaining the evolution of GERD in these patients are influenced by many other factors in addition to weight loss.
As in the literature, studies that use physiological data or invasive techniques to investigate GERD report higher percentages than studies that are only based on symptoms [28, 29]. Consequently, 62% of patients present GERD 18 months postoperatively (57.1% with silent GERD and 4.8% with symptomatic GERD), and 4.8% of patients present functional esophageal disorder. The rate of “de novo” reflux cases was 76.4% 1 month postoperatively and 41% after 18 months.
There are different focuses within the concept of GERD, and the reason they are classified by phenotypes is to provide a broader, more practical understanding of the characteristics of each group. The reason why patients with “functional esophageal disorder” are included in “de novo cases” of GERD is to differentiate them from the “non-GERD” group, since these patients were asymptomatic pre-operation but do present typical symptoms after surgery, despite not presenting objective evidence of reflux according to the Lyon Consensus.
When analyzing the subgroup of symptomatic patients (GERDq score ≥ 8), findings show that 100% of the patients present pathological APM values preoperatively, while the pH monitoring was also pathological in 24 asymptomatic patients (GERDq score < 8); in four of these cases, esophagitis was detected in the esophagogastroduodenoscopy. This last test provides the most representative data since there are scenarios in which the absence of symptoms does not rule out GERD [19].
The preoperative presence of silent GERD may affect the development of GERD, and it is the only group that presents postoperative resolution of GERD, albeit in a low percentage of cases (7.6% after 1 month and 20% after 18 months). These findings are consistent with the work of Thereaux et al., in which the acid exposure value in the preoperative pH-metry is a predictive factor of postoperative GERD, independently of weight loss [30].
It is also a group of patients that requires close follow-up because the absence of symptoms is not enough to rule out pathological acid exposure in the esophagus, with the added risk of developing esophagitis. Genco et al. describe that 26.4% of the patients who underwent LSG presented with Barrett’s esophagus after an average follow-up of 58 months, and they did not display typical reflux symptoms [31].
Analysis of symptomatic progression shows that the average score obtained in the GERDq questionnaire significantly increases at 1 month postoperatively and decreases at 18 months postoperatively, with a significant correlation between anthropometric data and symptoms after 18 months. This tendency in symptom progression is similar to the studies by Himpens et al. [32] and Melissas et al. [33], where the authors observed an initial worsening of GERD symptoms and their subsequent resolution in the following 2–3 years. Similarly to our study, several articles [25, 27] describe a progressive improvement of the symptoms from the first year onwards in parallel with weight loss.
When comparing the results of the APM, a significant worsening of all values obtained at 1 and 18 months postoperatively is observed. These results are in line with the literature review, which also highlights a significant worsening of the outcomes in short-term follow-up [8, 9, 34].
According to the literature, the manometric results are contradictory: some studies indicate an improvement [35], whereas others report a significant worsening in the parameters [9, 36]. The results of this study support a shortening of the sphincter length and a decrease in LES pressure postoperatively, although this last parameter is non-significant.
Regarding the evolution of esophageal peristalsis, the presented results support an increase in esophageal hypomotility, just as Del Genio et al. recognized [8]. This increased hypomotility is also more pronounced 18 months after the operation, with a significant decrease in the average value of the distal wave amplitude of 15.2 points after 1 month and 17.5 points after 18 months. This could promote greater postoperative reflux by reducing the esophagus’s ability to clear itself of acid. However, there was no evidence of a significant correlation between the distal esophageal amplitude and the acid exposure time in the present study.
When analyzing the symptomatic patients in the preoperative phase, half of them presented hypotensive LES, and none of them showed an improvement after 1 or 18 months. Kahrilas et al. describe how sleeve gastrectomy produces an alteration of the anti-reflux gate mechanism by altering the sling fibers engaged in the myoarchitecture of the LES, thus affecting the LES pressure [36,37,38]. For this reason, some authors support the need to conduct functional tests in patients with symptomatic GERD and/or esophagitis since the simultaneous presence of hypotensive LES makes LSG a poor technique for these patients due to the risk of aggravating any reflux progression [36].
As reflected in some studies [11, 39], the increase in intragastric pressure caused by reducing gastric volume could have an adverse effect on GERD after LSG.
The implementation of preventive measures such as crural repair is an effective strategy for the treatment of hiatus hernia, although its implication in improving GERD remains unclear in our results.
The strong point of this study is that it was not only based on symptoms but also that it was an objective study of GERD, in both the preoperative and postoperative stages, with a high rate of follow-up.
Even so, this study has a series of limitations. The first is the small number of patients included in the study due to the difficulty of initially obtaining the patients’ consent to undergo bothersome tests (pH-metry and manometry) pre-operation, 1 month, and 18 months after surgery. The second is the short follow-up time. In the study presented, analyzing the long-term presence of GERD was not considered one of the objectives, but rather evaluating the possible mechanisms involved in its early development after the surgery. Because it is very difficult to follow up with these patients for several years and it is rather bothersome to evaluate their esophageal function objectively through manometry and pH-metry, this study has evaluated the postoperative control 18 months after the surgery at the peak of weight loss (between 1 and 2 years after the operation) [40].
Alkaline reflux, which appears to be frequent after LSG, could not be evaluated [4, 8, 29]. In this study, it was not possible to analyze the presence of non-acid reflux in the patients included since the center where it was carried out did not have the necessary technology to do so.
A postoperative gastroscopy was not performed on all patients 18 months after the operation. Therefore, it has not been possible to evaluate macro- and microscopic alterations of the esophageal mucosa produced by the operation in patients with a normal preoperative endoscopy or modifications resulting from the gastroesophageal reflux evident post-operation. This is because of the center’s logistics, the cost of conducting another gastroscopy on all the patients, and the resulting discomfort. However, based on the recently published long-term results on LSG and Barrett’s esophagus [29, 30], a control endoscopy on all the patients who underwent LSG must be carried out and evaluated throughout their long-term monitoring.
Conclusion
Based on the results of this study, LSG led to a considerable rate of postoperative “de novo” GERD, which was most pronounced 1 month after the operation, and in half of the cases, it persisted despite optimal weight loss. Accordingly, every morbidly obese patient who is offered sleeve gastrectomy should be informed about postoperative GERD and, additionally, that no improvement will be found in patients with symptomatic GERD.
In view of the above, it seems appropriate for all patients scheduled to undergo LSG surgery to have a preoperative study of GERD symptoms based on a targeted anamnesis, with specific questionnaires and esophagogastroduodenoscopy. According to the findings, examinations should be conducted to supplement the esophagogastric junction study.
Since symptoms are not a reliable way to diagnose GERD, our results suggest the need for periodic postoperative esophageal evaluations using pH monitoring and gastrointestinal endoscopy to treat silent GERD and prevent the development of Barrett’s esophagus in the long term.
References
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56. https://doi.org/10.1016/j.amjmed.2008.09.041.
Diamantis T, Apostolou KG, Alexandrou A, et al. Review of long-term weight loss results after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(1):177–83. https://doi.org/10.1016/j.soard.2013.11.007.
Pallati PK, Shaligram A, Shostrom VK, et al. Improvement in gastroesophageal reflux disease symptoms after various bariatric procedures: review of the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2014;10(3):502–7. https://doi.org/10.1016/j.soard.2013.07.018.
Dupree CE, Blair K, Steele SR, et al. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: a national analysis. JAMA Surg. 2014;149(4):328–34. https://doi.org/10.1001/jamasurg.2013.4323.
Oor JE, Roks DJ, Ünlü Ç, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg. 2016;211(1):250–67. https://doi.org/10.1016/j.amjsurg.2015.05.031.
Lazoura O, Zacharoulis D, Triantafyllidis G, et al. Symptoms of gastroesophageal reflux following laparoscopic sleeve gastrectomy are related to the final shape of the sleeve as depicted by radiology. Obes Surg. 2011;21(3):295–9. https://doi.org/10.1007/s11695-010-0339-0.
Laffin M, Chau J, Gill RS, et al. Sleeve gastrectomy and gastroesophageal reflux disease. J Obes. 2013;2013: 741097. https://doi.org/10.1155/2013/741097.
Del Genio G, Tolone S, Limongelli P, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg. 2014;24(1):71–7. https://doi.org/10.1007/s11695-013-1046-4.
Gorodner V, Buxhoeveden R, Clemente G, et al. Does laparoscopic sleeve gastrectomy have any influence on gastroesophageal reflux disease? Preliminary results. Surg Endosc. 2015;29(7):1760–8. https://doi.org/10.1007/s00464-014-3902-2.
Chiu S, Birch DW, Shi X, et al. Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review. Surg Obes Relat Dis. 2011;7(4):510–5. https://doi.org/10.1016/j.soard.2010.09.011.
Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy—volume and pressure assessment. Obes Surg. 2008;18(9):1083–8. https://doi.org/10.1007/s11695-008-9576-x.
Braghetto I, Davanzo C, Korn O, et al. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19(11):1515–21. https://doi.org/10.1007/s11695-009-9954-z.
Gastrointestinal surgery for severe obesity. National Institutes of Health consensus development conference statement. Am J Clin Nutr. 1992;55(2 Suppl):615S-619S. https://doi.org/10.1093/ajcn/55.2.615s.
Jones R, Junghard O, Dent J, et al. Development of the GERDq, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30(10):1030–8. https://doi.org/10.1111/j.1365-2036.2009.04142.x.
Hungin APS, Molloy-Bland M, Scarpignato C. Revisiting Montreal: new insights into symptoms and their causes, and implications for the future of GERD. Am J Gastroenterol. 2019;114(3):414–21. https://doi.org/10.1038/s41395-018-0287-1.
Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67(7):1351–62. https://doi.org/10.1136/gutjnl-2017-314722.
Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23(2):151–63. https://doi.org/10.5056/jnm16214.
Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–80. https://doi.org/10.1136/gut.45.2.172.
Jonasson C, Wernersson B, Hoff DA, et al. Validation of the GERDq questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37(5):564–72. https://doi.org/10.1111/apt.12204.
Ruiz de León San Juan A, Ciriza de los Ríos C, Pérez de la Serna Bueno J, et al. Practical aspects of high resolution esophageal manometry. Rev Esp Enferm Dig. 2017;109(2):91–105. https://doi.org/10.17235/reed.2016.4441/2016.
Grupo español de motilidad digestiva. Asociación Española de Neurogastroenterología y motilidad. En: Manual Técnicas (antiguo tratado). [Internet]. Available form: https://asenem.org/index.php/recursos-bibliograficos/manual-tecnicas. [cited 2021 June 29].
Grifo I, Bruna M, Puche J, et al. Gastrectomia vertical: importancia del volumen gástrico resecado. BMI-J. 2017;7(1):1304–8.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Brethauer SA, Kim J, el Chaar M, et al. ASMBS Clinical Issues Committee. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489–506. https://doi.org/10.1016/j.soard.2015.02.003.
Herbella FA, Sweet MP, Tedesco P, et al. Gastroesophageal reflux disease and obesity. Pathophysiology and implications for treatment. J Gastrointest Surg. 2007;11(3):286–90. https://doi.org/10.1007/s11605-007-0097-z.
El-Serag HB, Graham DY, Satia JA, et al. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100(6):1243–50. https://doi.org/10.1111/j.1572-0241.2005.41703.x.
Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143(3):199–211. https://doi.org/10.7326/0003-4819-143-3-200508020-00006.
Borbély Y, Schaffner E, Zimmermann L, et al. De novo gastroesophageal reflux disease after sleeve gastrectomy: role of preoperative silent reflux. Surg Endosc. 2019;33(3):789–93. https://doi.org/10.1007/s00464-018-6344-4.
Yeung KTD, Penney N, Ashrafian L, et al. Does sleeve gastrectomy expose the distal esophagus to severe reflux?: a systematic review and meta-analysis. Ann Surg. 2020;271(2):257–65. https://doi.org/10.1097/SLA.0000000000003275.
Thereaux J, Barsamian C, Bretault M, et al. pH monitoring of gastro-oesophageal reflux before and after laparoscopic sleeve gastrectomy. Br J Surg. 2016;103(4):399–406. https://doi.org/10.1002/bjs.10089.
Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568–74. https://doi.org/10.1016/j.soard.2016.11.029.
Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16(11):1450–6. https://doi.org/10.1381/096089206778869933.
Melissas J, Leventi A, Klinaki I, et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258(6):976–82. https://doi.org/10.1097/SLA.0b013e3182774522.
Georgia D, Stamatina T, Maria N, et al. 24-h multichannel intraluminal impedance pH-metry 1 year after laparocopic sleeve gastrectomy: an objective assessment of gastroesophageal reflux disease. Obes Surg. 2017;27(3):749–53. https://doi.org/10.1007/s11695-016-2359-x.
Petersen WV, Meile T, Küper MA, et al. Functional importance of laparoscopic sleeve gastrectomy for the lower esophageal sphincter in patients with morbid obesity. Obes Surg. 2012;22(3):360–6. https://doi.org/10.1007/s11695-011-0536-5.
Braghetto I, Lanzarini E, Korn O, et al. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg. 2010;20(3):357–62. https://doi.org/10.1007/s11695-009-0040-3.
Jobe BA, Kahrilas PJ, Vernon AH, et al. Endoscopic appraisal of the gastroesophageal valve after antireflux surgery. Am J Gastroenterol. 2004;99(2):233–43. https://doi.org/10.1111/j.1572-0241.2004.04042.x.
Zifan A, Kumar D, Cheng LK, et al. Three-dimensional myoarchitecture of the lower esophageal sphincter and esophageal hiatus using optical sectioning microscopy. Sci Rep. 2017;7(1):13188. https://doi.org/10.1038/s41598-017-13342-y.
Quero G, Fiorillo C, Dallemagne B, et al. The causes of gastroesophageal reflux after laparoscopic sleeve gastrectomy: quantitative assessment of the structure and function of the esophagogastric junction by magnetic resonance imaging and high-resolution manometry. Obes Surg. 2020;30(6):2108–17. https://doi.org/10.1007/s11695-020-04438-y.
Peterli R, Wölnerhanssen BK, Vetter D, et al. Laparoscopic sleeve gastrectomy versus roux-Y-gastric bypass for morbid obesity-3-year outcomes of the prospective randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS). Ann Surg. 2017;265(3):466–73. https://doi.org/10.1097/SLA.0000000000001929.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• LSG led to a considerable rate of postoperative “de novo” GERD.
• The clearance of acid in the distal esophagus is decreased after sleeve gastrectomy.
• Patients with symptomatic GERD do not experience an improvement after LSG.
• Preoperative study of GERD symptomatology and EGD seems appropriate.
Rights and permissions
About this article
Cite this article
Sancho Moya, C., Bruna Esteban, M., Sempere García-Argüelles, J. et al. The Impact of Sleeve Gastrectomy on Gastroesophageal Reflux Disease in Patients with Morbid Obesity. OBES SURG 32, 615–624 (2022). https://doi.org/10.1007/s11695-021-05808-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05808-w