Abstract
Background
During the last decade, laparoscopic greater curvature plication (LGCP) has been used as a bariatric procedure for the treatment of obesity, regarded as less invasive and less expensive than other surgical bariatric procedures. We aimed to systematically review the literature and highlight recent clinical data regarding outcomes of LGCP in the treatment of obesity.
Methods
A comprehensive research of Pubmed database on LGCP was performed. The search was conducted on the first of May 2020 and was not limited to any date range. Outcomes of interest were surgical technique, postoperative complications, weight loss outcomes, comorbidities improvement or resolution, and revisional surgeries after technical failure or weight regain.
Results
Fifty-three articles were eligible for inclusion, with 3103 patients undergoing LGCP (mean age: 13.8–55 years). Mean preoperative body mass index (BMI) ranged from 31.2 to 47.8 kg/m2. Mean operative time ranged from 48 to 193 min. Length of hospital stay ranged from 0.75 to 7.2 days. Most studies provided postoperative follow-up up to 12 months. Mean percentage of excess weight loss (%EWL) ranged from 30.2 to 71.1% and 35 to 77.1% at 6 and 12 months post-LGCP, respectively. Only one study followed patients for more than 10 years and mean %EWL at 1, 5, and 10 years was 67%, 55%, and 42%, respectively.
Conclusion
LGCP seems to be an acceptable surgical procedure for the treatment of obesity, especially in centers having a low medical budget. However, most existing comparative studies report superiority of LSG regarding weight loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity represents a major worldwide public health concern, reaching more than 1.7 billion people around the globe. In the USA, obesity prevalence has been increasing since the 1980s among adults: between 2015 and 2016; it is estimated that 39.6% of adults over 20 years old are obese (BMI > 30), with 9.7% of women and 5.6% of men suffering from grade 3 obesity (BMI ≥ 40) [1]. Being the harbinger of many other comorbidities, severe obesity is associated with reduced life expectancy [2]. Bariatric surgery is the most effective therapy available for morbid obesity and can result in improvement or complete resolution of obesity comorbidities [2].
Existing surgical procedures act by reducing the size or capacity of the stomach, by bypassing a portion of the intestine, or by a combination of these two approaches, the ultimate goal being to promote long-term weight loss and beneficial metabolic effects. The exact mechanisms assuring efficacy of bariatric surgery are still unclear, and controversy exists regarding the ideal procedure [3]. With the decrease of the use of laparoscopic adjustable gastric banding (LAGB), laparoscopic sleeve gastrectomy (LSG) has been increasingly adopted worldwide, being the most performed primary bariatric procedure in the USA since 2013–2014 [4]. However, despite its efficacy, this procedure is not devoid of complications such as gastric leaks or bleeding, leading thus to a continuous search for new less invasive bariatric surgery techniques.
Gastric plication is a restrictive bariatric procedure that was first described by open surgery in 1976 by Tretbar et al [5]. Its effect in weight loss had been previously reported in animal studies [6]. Recently, Talebpour and Amoli [7] introduced laparoscopic greater curvature plication (LGCP), which has gained popularity among surgeons and patients due to its similarities to LSG. It reduces gastric volume and food intake by plication of the greater curvature, without resecting the stomach. Reported advantages of LGCP are its potential reversibility, the absence of foreign body placement, low complication rate, and reduced cost. However, current literature lacks long-term data on LGCP, with few existing comparative studies between LGCP and other bariatric procedures.
The aim of this study is to provide an up-to-date systematic review of the literature and highlight recent clinical data regarding outcomes of LGCP in the treatment of severe obesity.
Methods
A systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and in line with the protocol agreed by all authors was conducted, after institutional review board approval (written consent by patients was not needed). The search was conducted on the first of May 2020 and was not limited to any date range. The following MeSH terms were used in all possible combinations: “laparoscopic gastric plication”, “laparoscopic greater curvature plication”, and “vertical gastric plication”. Pubmed database was used. Only studies in English were considered. Papers were identified for review by title and abstract. Additionally, references of each article were reviewed for inclusion. Experimental studies, review articles, and studies combining LGCP to other bariatric procedures were excluded. Two authors independently performed data extraction and quality assessment of included articles. Any disparities were solved by a consensus decision. Outcomes of interest were surgical technique, postoperative complications, weight loss outcomes, and comorbidities improvement or resolution. Each selected study included at least one outcome of interest.
For each eligible study, data were extracted according to the following criteria: demographics, surgical technique, postoperative complications, weight loss outcomes, comorbidities improvement or resolution, and revisional surgery after weight loss failure or weight regain. Extracted demographics included: number of patients, sex, mean age, mean weight, mean preoperative body mass index (BMI), and prior abdominal surgeries. Extracted technical details and intraoperative results included access route, additional number of trocars, mean operative time, mean blood loss, intraoperative complications, rate of conversion to open surgery, and concomitant surgery. Extracted postoperative data included duration of hospital stay, relaparoscopy, postoperative bleeding, postoperative complications, mortality, weight loss, and comorbidities resolution data.
Results
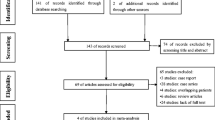
Study Identification (Fig. 1)
Sixty potentially eligible references were identified, of which 4 were excluded after review of their titles and abstracts. After full manuscript assessment, 3 additional references were excluded. Finally, 53 articles were eligible for inclusion in this systematic review, including 24 prospective studies, 6 randomized clinical trials (RCT), 3 non RCT, 14 retrospective studies, and 6 case reports (Fig. 1). Five articles [7,8,9,10,11] were substudies of large series by the same group. Two articles [12, 13] reported short-term and long-term follow-up of the same series. All selected articles were published between 2007 and 2020. Total number of patients included in the reviewed series was 3103. Pooled mean age was 37.1 years (range: 13.8–55 years). Pooled mean preoperative body mass index (BMI) was 41.7 kg/m2 (range: 31.2–47.8 kg/m2).
Surgical Technique (Table 1)
Surgical procedure is performed under general anesthesia, with the patient in reverse Trendelenburg position at 30°- french position (operator between the legs). All authors used four to five trocars placed in the upper abdomen. The procedure involves two main steps: mobilization of the greater gastric curvature followed by plication of the stomach. Currently, there is no standardized technique for LGCP. Most surgical teams begin dissection of the greater curvature 4 to 6 cm from the pylorus and move upward to the left crus of the diaphragm, stopping approximately 2 cm below the angle of His to preserve its anatomy.
The aim of gastric plication is to achieve gastric restriction by packing as much space of the stomach as possible via folds from its own wall. Most surgical teams perform 2 rows of sutures to create plications. Talebpour et al. [8] performed one-row plication during the first 6 years of their experience. A two-row plication using 4 separation points at each transverse level of the stomach representing locations of suture bites was used thereafter. These 4 points are depicted as A and B (anterior gastric wall) and C and D (posterior gastric wall) in Figs. 2, 3, and 4. These points were repeated at many levels from the fundus to the prepyloric area. Bites A and D were 1 cm away from the lesser curvature and in all levels together consist the outer suture row. The inner suture row included different levels of bites B and C, which were 1 cm away from the greater curvature. When pulled, the thread passed across the four points resulted in inward plication of the greater curvature composed of three folds of AB, BC, and CD. The first bite was inserted almost 2 cm above His angle lateral to the angle of His (Figs. 2, 3, and 4). However, some authors such as Fried et al. [12] performed two plication rows only at the beginning of their experience in LGCP to then prefer a single row in their practice, finding no differences between the 2 groups in terms of weight loss and complication rates.
A calibration device was used in all series to adjust gastric volume, except for Talebpour et al. [8], Bara et al. [33], Darabi et al. [25], and Sharma et al. [39] who did not mention this device. Most surgical teams used orogastric tubes with sizes ranging from 32 to 42 French. Brethauer et al. [18] and Fried et al. [12] used intraoperative endoscopy for calibration which provided guidance for the size and shape of the created fold. Buzga et al. [34, 35] carried out the first three procedures under endoscopic observation; thereafter, procedures were performed without endoscopic guidance or bougie. Niazi et al. [27] calculated gastric volume by transient pyloric occlusion with an atraumatic grasper and infusion of liquid into the stomach via a nasogastric tube.
Non-absorbable material was mainly used, except for Mui et al. [26] who used non-absorbable suture in the first row and absorbable suture in the second row, and Skrekas et al. [21], Lese et al. [38] and Morshed and Abdalla [19] who used absorbable suture in the first row and non-absorbable suture in the second row. The type of stitch (interrupted or continuous) remains controversial among studies, with authors reporting 2 rows of continuous sutures [8, 12, 17, 18, 25, 27, 29, 31, 45], a combination of continuous and interrupted sutures [16, 23, 24, 26, 28, 32, 36, 38,39,40, 42, 43, 46, 50] or even 2 rows of interrupted sutures. [20, 21, 49] Gudaityte et al. [48] performed one row of interrupted sutures and a second continuous row for the first 31 patients, then changed to 1.5 row of interrupted sutures for the remaining 30 patients. Gastroscopy was performed 3 years postoperatively in 31 patients to evaluate the integrity of plication fold, with no significant difference in complete or partial disruption rate found between the two plication techniques. Finally, in most studies, distance between sutures was recommended to be no more than 2 cm (1 cm for Talebpour et al. [8] and 1 to 1.5 cm for Skrekas et al. [21]).
Brethauer et al. [18] performed 2 different procedures: LGCP in 6 patients and laparoscopic anterior plication (LAP) in 9 patients. In the latter group, the anterior gastric wall was folded inward from the fundus to the antrum using 2 rows or more of 2–0 polypropylene continuous suture. The greater and lesser curvatures were approximated on the anterior surface of the stomach without greater curvature mobilization.
Weight Loss (Tables 2 and 3)
Weight loss results after LGCP were reported in 41 studies (Tables 2 and 3). Mean percentage of excess weight loss (%EWL) was provided by most studies. Three studies [26, 36, 44, 48] reported weight loss in terms of percentage of excess body mass index lost (%EBMIL), and 2 studies [19, 45] defined weight loss as percentage of total weight loss. Most studies provided postoperative follow-up for 12 months. The mean %EWL ranged from 30.2 to 71.1% at 6 months and 35% to 77.1% at 12 months after LGCP. Six studies [9, 14, 21, 27, 32, 46] provided follow-up until 2 years postoperatively and mean %EWL at 24 months ranged from 37.5 to 74.4%. Follow-up until 3 years after LGCP was reported in 6 studies [7, 10, 37, 39, 43, 48] and mean %EWL at 36 months ranged from 20.5 to 67.3%. Two studies [8, 13] provided 5-year follow-up and mean %EWL ranged from 52.6 to 55%. Only Talebpour et al. [8] followed patients for more than 10 years and mean %EWL at 1, 2, 3, 4, 5 and 10 years was 67%, 70%, 66%, 62%, 55%, and 42% respectively. Brethauer et al. [18] found that mean %EWL after LAP was 23.3% at 12 months compared to 53.4% for LGCP (P = 0.0078).
Fourteen studies compared weight loss results after LGCP and LSG including 5 randomized clinical trials (RCT), 3 non RCT, and 6 retrospective studies (Table 3). Ten studies (5 retrospective, 2 non RTC, 3 RCT) reported significantly higher mean %EWL with LSG after a postoperative time of 3 months [14, 22, 35, 49], 6 months [14, 22, 28, 35, 40, 49], 12 months [22, 28, 31, 35, 37, 42, 49], 18 months [42], 24 months [37, 43], and 36 months [37, 43]. Eight studies (4 retrospective, 1 non RTC, 3 RCT) showed no difference of mean %EWL with LSG at 3 months [28, 37, 40], 6 months [37, 39, 42, 43], 18 months [14, 35], 24 months [14], and 36 months [37, 39]. A meta-analysis by Barrichello et al. [51] included all clinical trials comparing LGCP to LSG (5 RCT and 3 non RCT) with a total of 422 patients and found better mean %EWL after LSG at 3, 6, 12, and 18 months. No significant difference in %EWL was found at 24 and 36 months.
One RCT performed by Darabi et al. [25] compared LGCP to laparoscopic mini-gastric bypass (LMGB) and found no significant difference in mean %EWL between the two groups 1 year after surgery.
Resolution of Comorbidities (Table 4)
Eighteen studies provided postoperative comorbidity outcomes. Sixteen studies reported resolution and improvement of type 2 diabetes (T2D) after LGCP. Eleven studies found that both hypertension and/or hyperlipidemia resolved or improved after LGCP as well [8, 10, 22,23,24,25, 31, 37, 38, 41, 43]. Five studies [8, 22, 24, 37, 43] reported resolution and improvement of sleep apnea, and 4 studies [8, 25, 38, 43] showed reduced joint pain after LGCP. Three studies [10, 25, 37] showed that LGCP had a therapeutic effect on irregular menses, and 2 studies [10, 25] reported improvement or remission of depression. One study [37] reported complete remission of nonalcoholic fatty liver disease.
In a cohort of 13 women with severe obesity and T2D, Bradnova et al. [29] showed statistically significant improvement in fasting hyperglycemia and hyperinsulinemia at 1 and 6 months after LGCP, with improvement in insulin resistance and significant decline of glycated hemoglobin (A1C). Furthermore, significant improvement in postprandial triglyceridemia was also noted.
In a prospective non-randomized study that included 52 patients, Buzga et al. [34] reported significant decrease in serum concentration of glucose, triacylglycerols, leptin, and A1C and significant increase in HDL cholesterol levels and plasma concentrations of ghrelin and adiponectin at 12 months after LGCP. Another study by Buzga et al. [35] comparing LGCP to LSG showed statistically significant decrease of fasting glucose and A1C levels at the 3, 6, 12, and 18-month follow-ups. Abouzeid et Taha [31] showed significant decrease in mean A1C levels after LGCP (6.3 ± 1.6 vs 5.8 ± 1.2; P = 0.0001).
Talebpour et al. [11] performed a prospective study on 60 patients with severe obesity and recently diagnosed T2D and reported significant decline in mean Fasting Blood Sugar (FBS), A1C, total cholesterol, and triglycerides levels as well as mean blood pressure within the first 6 months after LGCP. Mean A1c for the entire cohort decreased from 9.8 to 6.5% during the study, and remission of diabetes was achieved in 92% of patients.
One study by Taha et al. [50] reported little therapeutic effect of LGCP on T2D with mean A1C level of 7.9% at 12 months postoperatively compared to 7.5% preoperatively, with all patients continuing their hypoglycemic medication. Only one study by Dolezalova-Karmanova et al. [13] published in 2017 provided longer follow-up of T2D and reported an improvement rate of 89.7% (52 of 58 patients) at 2 years and 65.5% (38 of 58 patients) at 5 years after LGCP. The same authors already reported their early outcomes in 2012 that showed a significant drop of mean A1C level from 6.4% preoperatively to 5.1% at 6 months after LGCP [12].
Operative Time, Length of Hospital Stay, and Cost
Pooled mean operative time was 84 min (range: 48–193 min). Pooled length of hospital stay was 2.7 days (range: 0.6–7.2 days). Shen et al. [22] reported significant lower total cost of LGCP compared to LSG (USD$3358 versus USD$7826 respectively, P < 0.001). Chouillard et al. [42] reported that the average total operating room cost was 1736 euros for LGCP as compared to 2842 euros for LSG (P < 0.001). None of the included studies focused on additional costs such as readmission, reoperation, or hospital stay.
Postoperative Complications
There was no mortality reported in the reviewed studies. Minor complications included nausea, vomiting, sialorrhea, and gastrointestinal bleeding that resolved within few days with conservative management. Some cases of prolonged vomiting needed readmission for treating dehydration and electrolytes imbalance. Major complications requiring reoperation were reported in 28 series. Gastric outlet obstruction was described in 14 studies (29 patients; 1.4% of LGCP) and was managed by plication revision (reversal with or without secondary plication) or conservatively by endoscopic dilatation. Acute or late gastric prolapse between plication sutures was reported in 10 studies (48 patients; 2.3% of LGCP). These cases were managed with reversal and repeat of plication or fundectomy or conversion to sleeve gastrectomy. Ten of the selected studies reported gastric perforation and leak in 14 patients (0.7% of LGCP). Skrekas et al. [21] reported one case of jejunal necrosis with acute abdomen secondary to portomesenteric thrombosis. Som et al. [52] reported a case of portal vein thrombosis following LGCP. Almulaifi and Mohammad [53] reported a case of obstructive jaundice 6 months after LGCP caused by gastric fold herniation into the duodenum.
Revisional Surgery (Table 5)
Ten reviewed studies reported revisional bariatric surgery following weight regain or weight loss failure in patients with prior LGCP. The largest series of revisional surgeries was published by Heidari et al. [15] and included 102 patients out of a total of 1840 patients who had undergone LGCP from 2000 to 2016. The same group had already reported the first 38 revisional surgeries out of the first 800 patients in their previous paper. [8] Patients with weight loss failure or weight regain underwent 5 main redo procedures: re-plication, LSG, laparoscopic one anastomosis gastric bypass (LOAGB), laparoscopic Roux-en-Y gastric bypass (RYGB), and jejunoileal bypass. One addition of an adjustable gastric band [24] and one Scopinaro biliopancreatic diversion [13] were reported. Albanese et al. [32] performed one fundectomy for upper gastric prolapse found in a patient with loss failure. Six studies provided information about weight loss outcomes of revisional surgery with follow-up from 1 to 48 months.
Re-plication for unsatisfactory weight loss or weight regain was found in 7 studies. Heidari et al. [15] found that the %EWL after re-plication ranged from 40.4 to 92.1% with a follow-up of 3 to 48 months. Re-plication had a greater %EWL at 6 months of follow-up compared to LOAGB, RYGB, and jejunoileal bypass (62.1% vs 55.4%; P = 0.01) and at 3, 6, and 12 months of follow-up compared to LOAGB (40.4% vs 32.4, P = 0.0002; and 62.1% vs 51.6%, P < 0.0001; and 74.6% vs 68.2%, P = 0.01 respectively). There was no significant difference in the %EWL among three surgical procedures in the long term.
Revisional LSG after primary LGCP was found in 5 studies. The mean %EWL after 18 months of follow-up was 61.4% in a study by Zerrweck et al [47] Albanese et al. [32] showed a decrease of the mean BMI from 38 kg/m2 before revisional surgery to 34 kg/m2 1 month after LSG.
Discussion
In March 2011, the American Society for Metabolic and Bariatric Surgery (ASMBS) issued a position statement that gastric plication should be considered investigational because the quantity and quality of available data were insufficient to draw definitive conclusions regarding its safety and efficacy [54]. Since 2011, numerous reports on LGCP have been published. In the reviewed studies, despite important variability in weight loss outcomes, LGCP showed encouraging results in terms of weight loss with a %EWL pooled mean of 53.4 ± 11.6 at 12 months. These results are similar to the First International Consensus summit for sleeve gastrectomy of 2007 that showed a %EWL at 12 months of 47.5 ± 19.5 with a range of 20–100%. Therefore, when considering the initial experience and subsequent worldwide evolution of sleeve gastrectomy, one could argue that the results of LGCP regarding weight loss outcomes can be considered promising. However, most of the reviewed comparative studies reported that significantly higher weight loss rates are observed after LSG when compared to LGCP, with these findings being confirmed by 2 recent meta-analyses. [51, 55]
Several reported studies showed that some patient characteristics, such as preoperative BMI, could be correlated with efficacy and weight loss outcomes of LGCP. Abdelbaki et al. [28] divided patients who underwent LGCP into 2 subgroups based on their preoperative BMI. Subgroup analysis revealed that patients with lower BMI (BMI < 40 kg/m2) had higher %EWL at 1 year when compared to patients with a higher BMI (BMI > 40 kg/m2). This same analysis was previously performed in a study by Sterkas et al. [21] revealing significantly higher overall %EWL for the lower BMI group (BMI < 45 kg/m2) compared to the higher BMI group (BMI > 45 kg/m2) (69.8% versus 55.5%). Gudaityte et al. [48] provided data for 17 patients with a BMI superior to 50 kg/m2 and found that average %EBMIL was lower when compared to 42 patients with BMI < 50 kg/m2, but without statistically significant difference after 1, 2, or 3. Heidari et al. [15] found that the weight loss failure group (weight loss failure was defined as percentage of excess weight loss < 30% during the first 12 postoperative months) had the highest baseline BMI compared to weight regain cases and regain-prone group. These results suggest that weight loss after LGCP may be decreased, and weight loss failure rates may be increased in patients with higher BMI.
LGCP has some potential advantages. First, operative cost is reduced compared to other restrictive bariatric procedures, in particular LSG [41]. LGCP does not require expensive surgical staplers. Furthermore, no significant differences in mean hospital stay and mean operative time in comparison to LSG were found [22, 42, 43]. In addition, Waldrep et al. [56] reported that LGCP may be performed on an outpatient basis with successful ambulatory discharge of 138 of 141 patients and readmission rate of 4%. Second, LGCP can be easily reversed or converted to another bariatric procedure, if needed. Gudaityte et al. [48] and Haidari et al. [15] performed re-plication and revision to LOAGB, RYGB, and jejuno-ileal bypass without major perioperative complications more than 2 years after primary surgery. Similarly, Skrekas et al. [21] reported two cases of plication reversal 3 months after LGCP for acute gastric obstruction. Finally, Talebpour et al. [8] reported that this technique could be easily reversed in the early postoperative period (6 weeks) before formation of dense adhesions. However, it should be noted that most of the abovementioned studies report early revision after LGCP due to early complications, while data for late LGCP revision are scarce. It is therefore extremely important to obtain long-term data to decide whether LGCP is a useful primary bariatric procedure, since performing numerous bariatric procedures could be a major disadvantage in terms of cost and long-term outcomes for patients. Despite the small number of studies that evaluated weight loss outcomes after revisional surgeries, re-plication seems to have encouraging weight loss outcomes after weight loss failure or weight regain and it can be easily performed. [15] In addition, LGCP can be safely performed in severely obese adolescents with acceptable weight loss results [57].
LGCP can lead to lipid profile improvement lasting at least for 1 year [30]. It has the potential to change proteins involved in lipid metabolism, inflammation, and carbohydrate metabolism [58]. LGCP is associated with several postoperative upper gastrointestinal symptoms. Zerrweck et al. [47] reported high number (38%) of severe de novo symptoms (nausea/salivation, epigastric pain, gastroesophageal reflux) that led to reoperation in 11% of patients. Gudaityte et al. [48] reported new onset of gastroesophageal reflux disease (GERD) symptoms in 11 patients (39.3%) up to 3 years after LGCP while only 7 of 24 patients with typical preoperative GERD symptoms continued to have GERD postoperatively. Park and Kim [37] reported one case of refractory GERD after the first postoperative year post-LGCP, treated with long-term proton pump inhibitors.
Our study depicts one of LGCP’s major disadvantages, which is the lack of surgical technique standardization among bariatric surgery teams. Controversies still exist regarding suturing technique, number of rows, bougie size, and bites depth. This lack of surgical technique standardization can be directly related to postoperative complications, weight loss outcomes, and weight regain. Even if it may seem excessive, we preferred to describe all aspects of each surgical technique and report all available data. However, it is extremely hard to correlate all these different techniques with outcomes, since most reported studies reveal different practices for each single aspect of the respective LGCP technique used. It should also be noted that LGCP cannot eradicate the risk of gastric perforation or leak. Some surgical teams recommended to avoid full thickness bites because they could lead to leak when gastric edema occurs postoperatively. Moreover, the distance between bites has been correlated to occurrence of acute or late gastric hernia between sutures with subsequent risk of obstruction, perforation or failure of weight loss.
This review includes a high number of studies focusing on LGCP, with numerous prospective series and 5 clinical trials. In addition, all post-LGCP revisional procedures reported in the literature after weight loss failure or weight regain are reviewed for the first time. On the other hand, the present study has several limitations. First, most of the included studies enrolled a limited number of patients. Only 10 studies included more than 100 patients undergoing LGCP. The same issue should be noted regarding the total number of patients with gastric prolapse, revision LSG, and re-plication, since 2 of the studies that reported these outcomes were performed by the same group and probably include duplicate patients [32, 40]. Third, there is important heterogeneity and variability in weight loss outcomes across different studies. Finally, there is lack of long-term follow-up, with only two studies [8, 13] providing long-term follow-up data for LGCP efficacy.
Conclusions
LGCP seems to be a valuable surgical procedure for the treatment of severe obesity, especially in centers with a low medical budget available. However, a superiority of LSG regarding weight loss is reported by most existing comparative studies. Additional prospective comparative trials, long-term follow-up results, in addition to the standardization of the procedure are necessary to draw definitive conclusions regarding the role of LGCP in the management of severe obesity.
References
Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and ge, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–5.
Buchwald H. Consensus conference panel. Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005;1(3):371–81.
Franco JVA, Ruiz PA, Palermo M, et al. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21(9):1458–68.
Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg. 2014;24(8):1126–35.
Tretbar LL, Taylor TL, Sifers EC. Weight reduction. Gastric plication for morbid obesity. J Kans Med Soc. 1976;77(11):488–90.
Kirk RM. An experimental trial of gastric plication as a means of weight reduction in the rat. Br J Surg. 1969;56(12):930–3.
Talebpour M, Amoli BS. Laparoscopic total gastric vertical plication in morbid obesity. J Laparoendosc Adv Surg Tech A. 2007;17(6):793–8.
Talebpour M, Motamedi SMK, Talebpour A, et al. Twelve year experience of laparoscopic gastric plication in morbid obesity: development of the technique and patient outcomes. Ann Surg Innov Res. 2012;6(1):7.
Talebpour A, Heidari R, Zeinoddini A, et al. Predictors of weight loss after laparoscopic gastric plication: a prospective study. J Laparoendosc Adv Surg Tech A. 2015;25(3):177–81.
Zeinoddini A, Heidari R, Talebpour M. Laparoscopic gastric plication in morbidly obese adolescents: a prospective study. Surg Obes Relat Dis. 2014;10(6):1135–9.
Talebpour M, Talebpour A, Barzin G, et al. Effects of laparoscopic gastric plication (LGP) in patients with type 2 diabetes, one year follow-up. J Diabetes Metab Disord. 2015;14:60.
Fried M, Dolezalova K, Buchwald JN, et al. Laparoscopic greater curvature plication (LGCP) for treatment of morbid obesity in a series of 244 patients. Obes Surg. 2012;22(8):1298–307.
Doležalova-Kormanova K, Buchwald JN, Skochova D, et al. Five-year outcomes: laparoscopic greater curvature plication for treatment of morbid obesity. Obes Surg. 2017;27(11):2818–28.
Talebpour M, Sadid D, Talebpour A, et al. Comparison of short-term effectiveness and postoperative complications: laparoscopic gastric plication vs laparoscopic sleeve Gastrectomy. Obes Surg. 2018;28(4):996–1001.
Heidari R, Talebpour M, Soleyman-Jahi S, et al. Outcomes of reoperation after laparoscopic gastric plication failure. Obes Surg. 2019;29(2):376–86.
Ramos A, Galvao Neto M, Galvao M, et al. Laparoscopic greater curvature plication: initial results of an alternative restrictive bariatric procedure. Obes Surg. 2010;20(7):913–8.
Andraos Y, Ziade D, Achcouty R, et al. Early complications of 120 laparoscopic greater curvature plication procedures. Bariatric Times. 2011;8(9):10–5.
Brethauer SA, Harris JL, Kroh M, et al. Laparoscopic gastric plication for treatment of severe obesity. Surg Obes Relat Dis. 2011;7(1):15–22.
Morshed G, Abdalla H. Laparoscopic gastric plication versus laparoscopic sleeve gastrectomy. Med J Cairo Univ. 2011;79(2):179–82.
Pujol Gebelli J, García Ruiz de Gordejuela A, Casajoana Badía A, et al. Laparoscopic gastric plication: a new surgery for the treatment of morbid obesity. Cir Esp. 2011;89(6):356–61.
Skrekas G, Antiochos K, Stafyla VK. Laparoscopic gastric greater curvature plication: results and complications in a series of 135 patients. Obes Surg. 2011;21(11):1657–63.
Shen D, Ye H, Wang Y, et al. Comparison of short-term outcomes between laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy. Surg Endosc. 2013;27(8):2768–74.
Shen D, Ye H, Wang Y, et al. Laparoscopic greater curvature plication: surgical techniques and early outcomes of a Chinese experience. Surg Obes Relat Dis. 2014;10(3):432–7.
Atlas H, Yazbek T, Garneau PY, et al. Is there a future for laparoscopic gastric greater curvature plication (LGGCP)? A review of 44 patients. Obes Surg. 2013;23(9):1397–403.
Darabi S, Talebpour M, Zeinoddini A, et al. Laparoscopic gastric plication versus mini-gastric bypass surgery in the treatment of morbid obesity: a randomized clinical trial. Surg Obes Relat Dis. 2013;9(6):914–9.
Mui WL-M, Lee DW-H, Lam KK-Y, et al. Laparoscopic greater curve plication in Asia: initial experience. Obes Surg. 2013;23(2):179–83.
Niazi M, Maleki AR, Talebpour M. Short-term outcomes of laparoscopic gastric plication in morbidly obese patients: importance of postoperative follow-up. Obes Surg. 2013;23(1):87–92.
Abdelbaki TN, Sharaan M, Abdel-Baki NA, et al. Laparoscopic gastric greater curvature plication versus laparoscopic sleeve gastrectomy: early outcome in 140 patients. Surg Obes Relat Dis. 2014;10(6):1141–6.
Bradnova O, Kyrou I, Hainer V, et al. Laparoscopic greater curvature plication in morbidly obese women with type 2 diabetes: effects on glucose homeostasis, postprandial triglyceridemia and selected gut hormones. Obes Surg. 2014;24(5):718–26.
Bagheri MJ, Talebpour M, Sharifi A, et al. Lipid profile change after bariatric surgeries: laparoscopic gastric plication versus mini gastric bypass. Acta Chir Belg. 2019;119(3):146–51.
Abouzeid MM, Taha O. Laparoscopic sleeve gastrectomy versus laparoscopic gastric greater curvature plication: a prospective randomized comparative study. Egypt J Surg. 2015;34(1):41–7.
Albanese A, Prevedello L, Verdi D, et al. Laparoscopic gastric plication: an emerging bariatric procedure with high surgical revision rate. Bariatr Surg Pract Patient Care. 2015;10(3):93–8.
Bara T, Borz C, Suciu A, et al. Laparoscopic greater curvature plication for morbid obesity: indications, results. Perspectives Acta Medica Marisiensis. 2015;61(2):142–4.
Bužga M, Holéczy P, Švagera Z, et al. Laparoscopic gastric plication and its effect on saccharide and lipid metabolism: a 12-month prospective study. Wideochir Inne Tech Maloinwazyjne. 2015;10(3):398–405.
Bužga M, Švagera Z, Tomášková H, et al. Metabolic effects of sleeve gastrectomy and laparoscopic greater curvature plication: an 18-month prospective, observational, Open-Label Study. Obes Surg. 2017;27(12):3258–66.
Kim SB, Kim KK, Chung JW, et al. Initial experiences of laparoscopic gastric greater curvature plication in Korea-a review of 64 cases. J Laparoendosc Adv Surg Tech A. 2015;25(10):793–9.
Park YH, Kim SM. Short-term outcomes of laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy in patients with a body mass index of 30 to 35 kg/m2. Yonsei Med J. 2017;58(5):1025–30.
Leşe M, Szasz A, Leşe I. Laparoscopic gastric plication - one year of bariatric surgery in the Emergency County Hospital of Baia Mare. Chirurgia (Bucur). 2015;110(5):440–5.
Sharma S, Narwaria M, Cottam DR, et al. Randomized double-blinded trial of laparoscopic gastric imbrication v laparoscopic sleeve gastrectomy at a single Indian institution. Obes Surg. 2015;25(5):800–4.
Verdi D, Prevedello L, Albanese A, et al. Laparoscopic gastric plication (LGCP) vs sleeve gastrectomy (LSG): a single institution experience. Obes Surg. 2015;25(9):1653–7.
Broderick RC, Fuchs HF, Harnsberger CR, et al. Comparison of bariatric restrictive operations: laparoscopic sleeve gastrectomy and laparoscopic gastric greater curvature plication. Surg Technol Int. 2014;25:82–9.
Chouillard E, Schoucair N, Alsabah S, et al. Laparoscopic gastric plication (LGP) as an alternative to laparoscopic sleeve gastrectomy (LSG) in patients with morbid obesity: a preliminary, short-term, Case-Control Study. Obes Surg. 2016;26(6):1167–72.
Grubnik VV, Ospanov OB, Namaeva KA, et al. Randomized controlled trial comparing laparoscopic greater curvature plication versus laparoscopic sleeve gastrectomy. Surg Endosc. 2016;30(6):2186–91.
Toprak ŞS, Gültekin Y, Okuş A. Comparison of laparoscopic sleeve gastrectomy and laparoscopic gastric plication: one year follow-up results. Ulus Cerrahi Derg. 2015;32(1):18–22.
Casajoana A, Pujol J, Garcia A, et al. Predictive value of gut peptides in T2D remission: randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes Surg. 2017;27(9):2235–45.
Khidir N, Al Dhaheri M, El Ansari W, et al. Outcomes of laparoscopic gastric greater curvature plication in morbidly obese patients. J Obes. 2017;2017:7989714.
Zerrweck C, Rodríguez JG, Aramburo E, et al. Revisional surgery following laparoscopic gastric plication. Obes Surg. 2017;27(1):38–43.
Gudaityte R, Adamonis K, Maleckas A. Laparoscopic gastric greater curvature plication: intermediate results and factors associated with failure. Obes Surg. 2018;28(12):4087–94.
Li Y-H, Wang B-Y, Huang Y-C, et al. Clinical outcomes of laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy: a case-matched control study. Obes Surg. 2019;29(2):387–93.
Taha O. Efficacy of laparoscopic greater curvature plication for weight loss and type 2 diabetes: 1-year follow-up. Obes Surg. 2012;22(10):1629–32.
Barrichello S, Minata MK. García Ruiz de Gordejuela A, et al. laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy treatments for obesity: systematic review and meta-analysis of short- and mid-term results. Obes Surg. 2018;28(10):3199–212.
Som R, Rikabi S, Chang A, et al. Portal vein thrombosis following laparoscopic gastric plication. Ann R Coll Surg Engl. 2017;99(1):e6–7.
Almulaifi A, Mohammad WM. Obstructive jaundice: a rare complication of laparoscopic greater curvature plication. J Surg Case Rep. 2013;2013(8):rjt062.
Clinical Issues Committee. ASMBS policy statement on gastric plication. Surg Obes Relat Dis. 2011;7(3):262.
Ye Q, Chen Y, Zhan X, et al. Comparison of laparoscopic sleeve gastrectomy and laparoscopic greater curvature plication regarding efficacy and safety: a meta-analysis. Obes Surg. 2017;27(5):1358–64.
Waldrep DJ, Pacheco I. Laparoscopic Greater Curve Plication as an Outpatient Weight Loss Procedure. JSLS. 2015;19(3):e2015.00054.
DeAntonio J, Cockrell H, Kang HS, et al. A pilot study of laparoscopic gastric plication in adolescent patients with severe obesity. J Pediatr Surg. 2019;54(8):1696–701.
Savedoroudi P, Bennike TB, Kastaniegaard K, et al. Data from quantitative serum proteomic analysis after laparoscopic gastric plication. Data Brief. 2019;25:104077.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest
Informed Consent
Not applicable.
Human and Animal Rights
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Radwan Kassir and Toni El Soueidy are shared the first authorship
Rights and permissions
About this article
Cite this article
El Soueidy, T., Kassir, R., Nakhoul, M. et al. Laparoscopic Greater Curvature Plication for the Treatment of Obesity: a Systematic Review. OBES SURG 31, 1168–1182 (2021). https://doi.org/10.1007/s11695-020-05112-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-05112-z