Abstract
Background
To compare changes in bone mineral density (BMD) in patients with morbid obesity and type 2 diabetes (T2D) a year after being randomized to metabolic gastric bypass (mRYGB), sleeve gastrectomy (SG), and greater curvature plication (GCP). We also analyzed the association of gastrointestinal hormones with skeletal metabolism.
Methods
Forty-five patients with T2D (mean BMI 39.4 ± 1.9 kg/m2) were randomly assigned to mRYGB, SG, or GCP. Before and 12 months after surgery, anthropometric, body composition, biochemical parameters, fasting plasma glucagon, ghrelin, and PYY as well as GLP-1, GLP-2, and insulin after a standard meal were determined.
Results
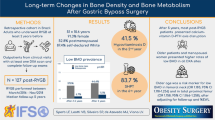
After surgery, the decrease at femoral neck (FN) was similar but at lumbar spine (LS), it was greater in the mRYGB group compared with SG and GCP − 7.29 (4.6) vs. − 0.48 (3.9) vs. − 1.2 (2.7)%, p < 0.001. Osteocalcin and alkaline phosphatase increased more after mRYGB. Bone mineral content (BMC) at the LS after surgery correlated with fasting ghrelin (r = − 0.412, p = 0.01) and AUC for GLP-1 (r = − 0.402, p = 0.017). FN BMD at 12 months correlated with post-surgical fasting glucagon (r = 0.498, p = 0.04) and insulin AUC (r = 0.384, p = 0.030) and at LS with the AUC for GLP-1 in the same time period (r = − 0.335, p = 0.049). However, in the multiple regression analysis after adjusting for age, sex, and BMI, the type of surgery (mRYGB) remained the only factor associated with BMD reduction at LS and FN.
Conclusions
mRYGB induces greater deleterious effects on the bone at LS compared with SG and GCP, and gastrointestinal hormones do not play a major role in bone changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is the most effective treatment for morbid obesity [1]. However, it exerts some negative effects on the bone that can differ among surgical techniques [2,3,4,5,6,7]. This might be especially deleterious in patients with type 2 diabetes mellitus (T2D) that have increased bone fragility attributed to damaged bone matrix by glycation end products [8]. Several mechanisms have been proposed to be responsible for bone loss after bariatric surgery including skeletal unloading, reduction in leptin and adipose tissue aromatase activity, and the rise in adiponectin concentrations [9,10,11]. Also, calcium and vitamin D deficiency caused by malabsorption and decreased intake leads to secondary hyperparathyroidism that contributes to bone loss [9, 12]. More recently, the role of gastrointestinal hormones on bone metabolism has been suggested with scarce evidence coming mainly from animal and in vitro studies [13,14,15,16,17]. In this sense, GLP-1 administration in rodents has shown an anabolic effect on bone, and GLP-2 provoked a reduction in bone resorption markers [14, 15]. In in vitro studies, ghrelin administration resulted in suppression of osteoclastogenesis and enhancement of osteoblast proliferation and differentiation [16]; conversely, PYY has been positively associated with bone resorption [17].
It is reasonable to suppose that the effect on skeleton metabolism might be different between different bariatric techniques. This would depend not only on the amount of weight loss, the degree of calcium, and vitamin D malabsorption but also on the effect of adipose tissue and gastrointestinal hormone changes. The aim of our study was to compare changes in bone mineral density (BMD) and bone remodeling markers in patients with morbid obesity and T2D a year after being randomized to: metabolic gastric bypass (mRYGB), sleeve gastrectomy (SG), and greater curvature plication (GCP), in the setting of a randomized controlled trial (RCT). Also, we analyzed the association of gastrointestinal hormone changes with skeletal metabolism.
Materials and Methods
This study was part of a prospective single-center and non-blinded RCT, including patients with morbid obesity and T2D. Patients were consecutively recruited from the obesity and diabetes outpatient clinic of Bellvitge University Hospital, Barcelona, Spain. The study was conducted according to the principles of the Declaration of Helsinki and all patients signed an informed consent form approved by the institutional ethics committee. Inclusion and exclusion criteria have been published in a previous paper [18]. Buse criteria were used to define T2D remission in each procedure at 1 year of follow-up [19]. The trial was registered at www.controlledtrials.com as ISRCTN14104758.
Randomization
Patients were randomly assigned 1:1:1 to undergo mRYGB, SG, or GCP, with stratification according to baseline levels of HbA1c (greater or lower/equal to 7%). The detailed study protocol has been described previously [18]. After surgery, 800 UI of vitamin D and 1000 mg of calcium were prescribed as an oral supplement in all patients in addition to a multivitamin pill once daily. Patients undergoing mRYGB required higher doses of vitamin D (16,000 units of vitamin D every 15 days).
Anthropometric Parameters
Body weight and height were measured during every visit. Weight change after surgery was reported as total weight loss percentage (TWL%). Body composition (fat mass and lean mass (kg), whole body bone mineral content (BMC) (g), and BMD (g/cm2) at lumbar spine (LS) L2-L4 and proximal femur were measured by DXA (Hologic QDR 4500; Hologic Inc., Waltham, MA, USA) before and a year after surgery. Osteopenia (T score between − 1.0 and − 2.5) and osteoporosis (T score below − 2.5) were defined according to the WHO criteria [20].
Standard Meal Test
The standard meal test (SMT) was performed before bariatric surgery and at months 1 and 12. The SMT consisted of 200 mL of a liquid meal (Edanec®). Blood was drawn immediately before and 15, 30, 60, and 120 min following the SMT for determination of GLP-1, GLP-2, and insulin. Fasting ghrelin, PYY, and glucagon levels were determined before the SMT.
Laboratory Determinations
Phospho-calcium metabolism and markers of bone remodeling were determined before and 1 year after bariatric intervention. Glucose, calcium, phosphorus, and alkaline phosphatase were determined using standard enzymatic methods. 25-hydroxyvitamin D [25(OH)D3] concentrations were determined using a radioimmunoassay (DiaSorin, Stillwater, MN, USA). Intact serum parathyroid hormone (PTH) was measured by a two-site immunoradiometric assay (Diagnostic System Laboratories, Webster, TX, USA). Osteocalcin was measured by electrochemiluminescence immunoassay (E170 Modular System; Roche, Mannheim, Germany). Plasma insulin was analyzed by immunoassay (Coat-A-Count Insulin; Diagnostic Products Corp., Los Angeles, CA). GLP-1 was measured by radioimmunoassay (Millipore, Saint Charles, MO), and plasma ghrelin by enzyme immunoassay (CUSABIO, China). GLP-2, glucagon, and PYY were measured by enzyme immunoassay (Yanahaira Institute Inc., Awakura, Fujinomiya-shi Shizuoka, Japan).
Surgical Procedures
mRYGB combines restriction, creating a small gastric pouch, with malabsorption, accomplished by a 200-cm biliopancreatic limb and an alimentary limb of 100 cm. SG is a restrictive technique consisting of a gastric volume reduction of 75–80% by resecting the stomach over a 36 French catheter beginning 4 cm from the pylorus and ending at the angle of His. GCP is a reversible restrictive technique with no stomach resection, in which an invagination of the greater gastric curvature is performed with two running non-absorbable sutures, calibrated over a 36 French catheter.
Statistical Analysis
Based on preliminary data, the study was designed to detect a 20% difference in GLP-1 secretion (measured by the area under the curve [AUC] after SMT) before and 1 month after bariatric surgery, with a power of 80% and an α risk of 0.05 [18]. The primary outcome of the study was the predictive value of gut hormone dynamics (GLP-1, glucagon, PYY, and ghrelin) on glucose metabolism improvement at 1 and 12 months after surgery for each procedure. The study of BMD was a secondary outcome. Biochemical, hormonal, and body composition parameters were compared between procedures by analysis of variance; categorical variables were compared using chi-square test and quantitative variables using ANOVA test or the Kruskal-Wallis test. GLP-1 area under the curve (AUC) was calculated by the trapezoidal method [21]. Bivariate (Pearson or Spearman) and multivariate linear regression analyses were employed to determine the predicting factors of BMD decrease after bariatric surgery. Relevant clinical variables previously associated were included in the model (type of surgery, changes in weight, gastrointestinal hormones concentrations, phospho-calcium metabolism and metabolic parameters, and the presence of diabetes remission). Statistical analysis was performed using R software version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). A p value < 0.05 was considered statistically significant.
Results
Forty-five morbidly obese patients with T2D, aged 49.4 ± 7.8 years, BMI 39.4 ± 1.9 kg/m2, initial HbA1c 7.7 ± 1.9%, were consecutively randomized to mRYGB (n = 15), SG (n = 15), or GCP (n = 15). Sixty-six percent of patients were women and menopause was present in 62% of those undergoing mRYGB, 60% in SG, and 75% in GCP, p = 0.704. Follow-up compliance at 1 year was 97.78% (n = 44). Initial clinical, biochemical, and body composition characteristics were comparable between groups, except BMI that was higher in GCP (Table 1).
As a summary of previously published data [11], TWL% at 12 months was significantly greater in the mRYGB group than in SG and GCP groups at 12 months, − 35.29 ± 8.17 vs. − 27.26 ± 5.66 vs. − 20.24 ± 7.49%, respectively, p < 0.05. At 1 year of follow-up, HbA1c was significantly lower in the mRYGB group and short-term complete diabetes remission at 1 year was found in 80% of patients in the mRYGB group (n = 12), 53.8% in the SG group (n = 7), and 16.7% in the GCP group (n = 2), p < 0.001.
Changes in biochemical and body composition parameters are shown in Table 2. One year after surgery, greater significant weight loss, metabolic improvement, and lower fat mass values were observed after mRYGB. However, lean mass after the intervention was similar among groups. Serum calcium was significantly lower after mRYGB but within normal values and no differences were observed in phosphate, PTH, or vitamin D concentrations (all within normal ranges).
Changes in BMD Parameters and Bone Remodeling Markers After Bariatric Surgery
Baseline BMD parameters were similar between all groups. A year after surgery, a significant reduction in the femoral neck (FN) BMD was observed in the three groups without differences when comparing mRYGB vs. SG vs. GCP − 10.34 (6.05) vs. − 5.30 (6.17) vs. − 6.69 (5.68)%, p = 0.118. However, at the LS, a greater decrease was observed after mRYGB compared with SG and GCP − 7.29 (4.6) vs. − 0.48 (3.9) vs. − 1.2 (2.7)%, p < 0.001. No significant differences were observed between SG and GCP (Fig. 1). A year after surgery, FN osteopenia was observed in 35.7% of patients mRYGB vs. 30.8% after SG and 9% after GCP, respectively, p = 0.251. At LS, osteopenia was observed in 50% of patients after mRYGB vs. 7.69% and 18.2%, p = 0.098. Osteoporosis developed in 7.14% of patients after mRYGB compared with 7.69% and 9.09%, respectively, p = 0.075. No bone fractures were observed. Bone remodeling markers osteocalcin and alkaline phosphatase were increased after surgery to a greater extent after mRYGB compared with SG and GCP (Fig. 2).
Changes in Gastrointestinal Hormones After Bariatric Surgery
As described in detail previously [11], when analyzing hormone changes after bariatric surgery, the increase in the AUC for GLP-1 and GLP-2 fasting values of PYY and ghrelin were significantly higher in the mRYGB group than in SG and GCP groups p < 0.05, without differences between SG and GCP groups (p > 0.05). Fasting levels of glucagon were not different between the three groups.
Correlations of BMC with Gastrointestinal Hormones
Before surgery, FN BMC correlated with fasting PYY (r = 0.325, p = 0.044) and AUC for insulin (r = 0.378, p = 0.02). Also, LS BMC correlated positively with fasting PYY and fasting glucagon (r = 0.468, p = 0.02) and (r = 0.374, p = 0.018), respectively. Twelve months after surgery, BMC at the LS showed an inverse correlation with fasting ghrelin and AUC for GLP-1 (r = − 0.412, p = 0.01) and (r = − 0.402, p = 0.017), respectively.
Correlations of BMD and Bone Remodeling Markers with Gastrointestinal Hormones
FN BMD at 12 months correlated positively with post-surgical fasting glucagon (r = 0.498, p = 0.04) and insulin AUC (r = 0.384, p = 0.030). LS BMD at 12 months correlated with the AUC for GLP-1 at the same time period (r = − 0.335, p = 0.049). When analyzing the different surgical techniques, the correlations were similar with the exception of GLP-1 and LSBMD that was only observed in the mRYGB group. No correlation was found between bone remodeling markers and gastrointestinal hormones.
Changes in BMD Regarding T2D Outcomes After Surgery
Taking into account the probable influence of metabolic outcomes on skeleton metabolism, we compared BMD changes in patients achieving T2D remission vs. non-remitters. FN BMD was similar between both groups. On the contrary, LS BMD reduction was greater in patients with T2D remission compared with patients with partial remission and non-remitters − 4.26 (5.13) vs. − 7.17 (5.57) vs. 0.12 (2.37) p = 0.012. Higher osteocalcin values were also observed in patients with T2D remission 38.4 (20.7) vs. 29.1 (22.2) vs. 21.3 (10.2), p = 0.045.
Predicting Factors of BMD Decrease After Bariatric Surgery
We performed a bivariate analysis to explore variables associated with FN and LS BMD after surgery (Table 3). In the multiple regression analysis after adjusting for age, sex and BMI, the type of surgery (mRYGB) remained the only factor associated with BMD decrease at LS and FN. No association was found with gastrointestinal hormones nor with phospho-calcium parameters. The association of BMD decrease with diabetes remission was also explained by the surgical technique (mRYGB) in the multivariate analysis.
Discussion
To our knowledge, this is the first study that has compared bone outcomes between three different bariatric procedures (mRYGB, SG, and GCP) in the setting of a RCT. We found similar BMD loss at the FN but greatest descent at LS a year after mRYGB. Also, our study assessed the contribution of gastrointestinal hormones on bone metabolism. In spite of a few observed correlations of these hormones with BMD parameters, our findings indicate that they might influence but do not play a major role in bone health in the short term after bariatric surgery.
The effect of bariatric surgery on bone might differ among techniques but only a few studies have compared BMD outcomes after different surgical procedures [2,3,4,5,6,7], most of them comparing RYGB with SG in a non-randomized manner [2,3,4,5,6]. The Stampede study is the most important RCT comparing RYGB and SG to intensive medical treatment in T2D with grade I obesity [7]. At 2 years, in this trial, BMD changes and bone fractures were similar between groups, but at 5 years, RYGB showed a greater increase in bone metabolism markers compared with SG [22]. Our findings are in this line, observing greater BMD loss at LS and increases in bone remodeling markers after mRYGB. One explanation might be that mRYGB is characterized by a more malabsorptive component compared with standard gastric bypass, and even though vitamin D and PTH were similar between groups, we cannot rule out higher deficiencies of other minerals important for the bone matrix after this procedure. Another factor that might have an influence is the superior amount of weight loss after mRYGB, since the mechanical unloading reduces sclerostin that stimulates osteoclastogenesis [23]. Moreover, as the weight descends, there is usually a decrease not only in fat mass but also in lean mass that can lead to sarcopenia further impairing bone health [24]. Nevertheless, in our study, lean mass was similar after the three techniques, probably related to a protein-rich diet with protein powder supplementation and exercise counseling provided to patients.
The role of gastrointestinal hormones in the regulation of bone metabolism has been elucidated, but in humans, there is still scarce information of this gut-bone axis after bariatric surgery [11, 25]. In vitro, the orexigenic gut peptide ghrelin suppresses osteoclastogenesis and enhances osteoblast proliferation and differentiation in rats [26]. In humans, Carrasco et al. [2] described a reduction in ghrelin that was associated with BMD loss after RYGB and SG. In our study, fasting total ghrelin after surgery had an inverse correlation with BMC at the LS. The rise in ghrelin concentrations after mRYGB as a compensatory response to the negative energy balance at the time of maximal bone loss might explain this negative association. Studies performed in anorexic patients have shown a negative association between PYY and bone formation markers and lumbar BMD [27]. As bariatric surgery is related to increases in PYY levels [28], a potential negative effect would be expected in the bone. A small study performed after RYGB and adjustable gastric banding found that changes in fasting peptide YY positively correlated with C-terminal telopeptide (CTX) and procollagen type 1 N-terminal propeptide (P1NP) [29]. However, in our study, fasting PYY correlated positively with BMD at the LS before surgery and showed no association with bone remodeling markers. These differences could be explained by the fact that most studies previously performed in humans come from anorexic patients that have opposed phenotypes to our morbidly obese patients.
In our study, fasting glucagon before surgery also correlated positively with BMC at the LS. In agreement with these findings, a previous study performed in rodents embryonic bone showed a direct inhibitory effect of glucagon on bone resorption [30]. GLP-1 receptors have been described in osteoblasts [31] and their agonist has shown an anabolic effect on bone in rodents [14] but not in humans [32]. GLP-2 receptors are also expressed in osteoclasts and inhibit bone resorption [33]. In a previous study after RYGB and lap band, no correlations were found between GLP-1 and CTX or P1NP levels [29]. Our study is the first to analyze the relationship of these hormones with bone metabolism after bariatric surgery. The increase in the AUC for GLP-1 during the first year correlated negatively with BMD at the LS at the same time period. Probably, the fact that the most important increase in GLP-1 was observed after mRYGB that also showed the greatest BMD loss might explain this association. No correlations were observed between BMD and GLP-2. It is important to highlight that all the associations observed between gastrointestinal hormones and bone parameters lost significance after adjusting for weight loss and type of surgery. This suggest that they may be surrogates of the relationship between weight, BMD reduction, and hormonal secretion changes after surgery, rather than having a strong direct effect on bone loss.
In T2D, a reduction in bone turnover [34] and an alteration of bone matrix mechanical properties by advanced glycation end products have been described [35]. As bariatric surgery can induce diabetes improvement and remission, some authors have proposed that bone turnover markers might be influenced by glycamia changes [36, 37]. Ivaska et al. [38] described similar trends in osteocalcin in patients who had diabetes remission after bariatric surgery compared with those without T2D previous to intervention. However, in other papers, increases in CTX were significantly associated with diabetes remission [39] and an inverse correlation was observed between HbA1c and fasting CTX [29]. Our results showed greater LS BMD descent and higher osteocalcin values in patients with T2D remission, probably explained by a greater weight loss decline observed in these patients.
The overall analysis of factors influencing BMD showed that the strongest predictor of BMD changes at the FN and LS was the type of surgery (mRYGB) employed. The mRYGB procedure being the one that occasioned the greatest changes at all levels (in weight, body composition, metabolism, and hormonal environment). This therefore should be taken into account when choosing the surgical option in a patient with osteoporosis and with risk of fracture. Moreover, those patients undergoing mRYGB should have their BMD closely monitored.
Our study has several limitations, the number of patients was small and the follow-up was short, being only 12 months after surgery. Also, we used DEXA scans that are of limited accuracy after surgery due to changing fat-lean tissue ratios in the region of interest. We only determined a few gut hormones but others might be implicated in bone metabolism. Our observations nevertheless warrant further studies in larger cohorts that could provide higher statistical power with longer term follow-up.
In conclusion, our study has shown that mRYGB induces greater short-term deleterious effects on bone mainly at the LS compared with SG and GCP. Gastrointestinal hormones correlate with some BMD parameters but do not play a major role in bone changes in the short term after bariatric surgery. Bone metabolism of patients undergoing this type of surgery should be closely followed.
References
Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14(3):160–9.
Carrasco F, Basfi-Fer K, Rojas P, et al. Changes in bone mineral density after sleeve gastrectomy or gastric bypass: relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes Surg. 2014;24(6):877–84.
Muschitz C, Kocijan R, Haschka J, et al. The impact of vitamin D, calcium, protein supplementation, and physical exercise on Bone metabolism after bariatric surgery: the BABS study. J Bone Miner Res. 2016;31(3):672–82.
Vilarrasa N, de Gordejuela AG, Gómez-Vaquero C, et al. Effect of bariatric surgery on bone mineral density: comparison of gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23(12):2086–91.
Hsin MC, Huang CK, Tai CM, et al. A case-matched study of the differences in bone mineral density 1 year after 3 different bariatric procedures. Surg Obes Relat Dis. 2015;11(1):181–5.
Nogués X, Goday A, Peña MJ, et al. Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass. Cir Esp. 2010;88(2):103–9.
Maghrabi AH, Wolski K, Abood B, et al. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity (Silver Spring). 2015;23(12):2344–8.
Napoli N, Chandran M, Pierroz DD, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–19.
Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2(2):165–74.
Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int. 2014;25(2):423–39.
Gagnon C, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2(3):121–33.
Wei JH, Lee WJ, Chong K, et al. High incidence of secondary hyperparathyroidism in bariatric patients: comparing different procedures. Obes Surg. 2018;28(3):798–804.
Mieczkowska A, Irwin N, Flatt PR, et al. Glucose-dependent insulinotropic polypeptide (GIP) receptor deletion leads to reduced bone strength and quality. Bone. 2013;56(2):337–42.
Nuche-Berenguer B, Moreno P, Esbrit P, et al. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int. 2009;84(6):453–61.
Henriksen DB, Alexandersen P, Byrjalsen I, et al. Reduction of nocturnal rise in bone resorption by subcutaneous GLP-2. Bone. 2004;34(1):140–7.
Fukushima N, Hanada R, Teranishi H, et al. Ghrelin directly regulates bone formation. J Bone Miner Res. 2005;20(5):790–8.
Wong IP, Driessler F, Khor EC, et al. Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PLoS One. 2012;7(7):e40038.
Casajoana A, Pujol J, Garcia A, et al. Predictive value of gut peptides in T2D remission: randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes Surg. 2017;27(9):2235–45.
Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–5.
Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–81.
Wolever TM, Jenkins DJ, Jenkins AL, et al. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54(5):846–54.
Crawford MR, Pham N, Khan L, et al. Increased bone turnover in type 2 diabetes patients randomized to bariatric surgery versus medical therapy at 5 years. Endocr Pract. 2018;24(3):256–64.
Muschitz C, Kocijan R, Marterer C, et al. Sclerotin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100(3):891–901.
Voican CS, Lebrun A, Maitre S, et al. Predictive score of sarcopenia occurrence one year after bariatric surgery in severely obese patients. PLoS One. 2018;13(5):e0197248.
Brzozowska MM, Sainsbury A, Eisman JA, et al. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev. 2013;14(1):52–67.
Maccarinelli G, Sibilia V, Torsello A, et al. Ghrelin regulates proliferation and differentiation of osteoblastic cells. J Endocrinol. 2005;184(1):249–56.
Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91(3):1027–33.
Chan JL, Mun EC, Stoyneva V, et al. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring). 2006;14(2):194–8.
Yu EW, Wewalka M, Ding SA, et al. Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J Clin Endocrinol Metab. 2016;101(2):714–22.
Stern PH, Bell NH. Effects of glucagon on serum calcium in the rat and on bone resorption in tissue culture. Endocrinology. 1970;87(1):111–7.
Nuche-Berenguer B, Portal-Núñez S, Moreno P, et al. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010;225(2):585–92.
Bunck MC, Eliasson B, Cornér A, et al. Exenatide treatment did not affect bone mineral density despite body weight reduction in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(4):374–7.
Karsdal MA, Holst JJ, Henriksen D. GLP-2 reduces bone resorption in vitro via the osteoclast GLP-2 receptor. J Bone Miner Res. 2004;19(S1):S416.
Vestergaard P. Diabetes and bone fracture: risk factors for old and young. Diabetologia. 2014;57(10):2007–8.
Walsh JS, Vilaca T. Obesity, type 2 diabetes and bone in adults. Calcif Tissue Int. 2017;100(5):528–35.
Pittas AG, Harris SS, Eliades M, et al. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94(3):827–32.
Kanazawa I, Yamaguchi T, Yamauchi M, et al. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22(1):187–94.
Ivaska KK, Huovinen V, Soinio M, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95:47–54.
Madsen LR, Espersen R, Ornstrup MJ, et al. Bone health in patients with type 2 diabetes treated by Roux-En-Y gastric bypass and the role of diabetes remission. Obes Surg. 2019;29(6):1823–31.
Acknowledgments
The authors thank Dr. Jonathan Rogerson for helpful discussions on the manuscript and Bernat Miguel Huguet for statistical analysis. We thank CERCA Programme Generalitat de Catalunya for institutional support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest. NV is the recipient of grants “Ajuts per a projectes de recerca clínica de l’Hospital Universitari de Bellvitge (2011-PR143/11)” and of the project “PI11/01960; PI14/01997 and PI17/01556” funded by the Instituto de Salud Carlos III and co-funded by the European Union (ERDF, “A way to build Europe”). JV has funding from the Instituto de Salud Carlos III through the project PI14/00228 and PI17/01503 co-funded by the European Union (ERDF, “A way to build Europe”). SFV has funding from the Spanish Ministry of Economy and Competitiveness and the European Regional Development Fund (ERDF) (SAF2015–65019-R). The Spanish Biomedical Research Center in Diabetes and Associated Metabolic Disorders (CIBERDEM) (CB07708/0012) is an initiative of the Instituto de Salud Carlos III. SFV acknowledges support from the Miguel Servet tenure-track program (CP10/00438 and CPII16/0008) from the Fondo de Investigación Sanitaria (FIS) co-financed by the ERDF.
Ethical Approval
The study was conducted according to the principles of the Declaration of Helsinki.
Informed Consent
All patients included in the study signed an informed consent form approved by the institutional ethics committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nuria Vilarrasa and Joan Vendrell are co-senior and co-corresponding authors
Rights and permissions
About this article
Cite this article
Guerrero-Pérez, F., Casajoana, A., Gómez-Vaquero, C. et al. Changes in Bone Mineral Density in Patients with Type 2 Diabetes After Different Bariatric Surgery Procedures and the Role of Gastrointestinal Hormones. OBES SURG 30, 180–188 (2020). https://doi.org/10.1007/s11695-019-04127-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04127-5