Abstract
Background
There is limited data in the literature evaluating outcomes of bariatric surgery in severely obese patients with left ventricular assist device (LVAD) as a bridge to make them acceptable candidates for heart transplantation. This study aims to assess the safety and effectiveness of laparoscopic sleeve gastrectomy (LSG) in patients with previously implanted LVAD at our institution.
Methods
All the patients with end-stage heart failure (ESHF) and implanted LVAD who underwent LSG from2013 to January 2017 were studied.
Results
Seven patients with end stage heart failure (ESHF) and implanted LVAD were included. The median age and median preoperative BMI were 39 years (range: 26–62) and 43.6 kg/m2 (range 36.7–56.7), respectively. The median interval between LVAD implantation and LSG was 38 months (range 15–48). The median length of hospital stay was 9 days (rang: 6–23) out of which 4 patients had planned postoperative ICU admission. Thirty-day complications were noted in 5 patients (3 major and 2 minor) without any perioperative mortality. The median duration of follow-up was 24 months (range 2–30).
At the last available follow-up, the median BMI, %EWL, and %TWL were 37 kg/m2, 47%, and 16%, respectively. The median LVEF before LSG and at the last follow-up point (before heart transplant) was 19% (range 15–20) and 22% (range, 16–35), respectively. In addition, the median NYHA class improved from 3 to 2 after LSG. Three patients underwent successful heart transplantations.
Conclusion
Patients with morbid obesity, ESHF, and implanted LVAD constitute a high-risk cohort. Our results with 7 patients and result from other studies (19 patients) suggested that bariatric surgery may be a reasonable option for LVAD patients with severe obesity. Bariatric surgery appears to provide significant weight loss in these patients and may improve candidacy for heart transplantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Left ventricular assist device (LVAD) is an electromechanical circulatory support device for end-stage heart failure (ESHF). LVAD is used as a bridge to heart transplantation or destination therapy for ESHF [1, 2]. It is a mechanical pump that is implanted in the patient’s left ventricle to improve cardiac output. The LVAD receives blood from the left ventricle and delivers it to the aorta. It consists of an implanted pump, driveline that connects the pump to the controller (not shown), and an energy supply (not shown) (Fig. 1). The driveline exits the lower chest and passes subcutaneously across the upper abdomen exiting on the right side. Pulsatile pumps have largely been replaced by continuous flow pumps. All LVAD patients are anticoagulated to prevent clot formation in the pump.
Patients with a LVAD and severe obesity present a very challenging management problem. Not only does severe obesity exacerbate ESHF but it is a relative contraindication for heart transplant due to increased perioperative complications [3, 4]. Bariatric surgery in patients with LVAD and severe obesity may potentially improve ESHF and enable qualification for heart transplantation; however, the risks of bariatric surgery in these patients may be high. This study reviews the outcomes of laparoscopic sleeve gastrectomy (LSG) in LVAD patients with severe obesity at our institution. The impact of LSG on weight loss, left ventricular ejection function (LVEF), and the New York Heart Association (NYHA) class was assessed.
Materials and Methods
Study Cohort
After approval by our institutional review board, a retrospective chart review was completed to identify patients who underwent LVAD implantation and subsequently underwent a bariatric procedure at our institution from 2013 to 2017. All patients who had LVAD implantation prior to LSG were included. Patients without a previous history of LVAD implantation, with LVAD explanation prior to the bariatric procedure, with a previous history of heart transplantation and those in whom no data were available were excluded from the study.
Study Endpoints and Data Collection
Preoperative data collected included patient demographics, preoperative weight, body mass index (BMI), NYHA class and LVEF, etiology of ESHF, interval time between LVAD placement and LSG, and comorbidities (diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, atrial fibrillation, sleep apnea). The number of heart failure medications was also collected. Perioperative variables included the preoperative American Society of Anesthesiologist (ASA) classification, heart rate, blood pressure, operative time, estimated blood loss, length of hospital stay, and postoperative complications (≤ 30 days and 1 year). Postoperative weight, BMI, percentage excess weight loss (%EWL), percentage total weight loss (%TWL), improvement in weight-related comorbidities, NYHA class, LVEF, and status of heart transplantation candidacy were collected at the last available follow up visit.
Perioperative Management
All patients were assessed by a multidisciplinary team before surgery and deemed a safe candidate for bariatric surgery. Preoperative optimization by medical, cardiology, and anesthesiology teams was performed. All patients had continuous arterial blood pressure monitored intraoperatively. The LVAD parameters including pump speed, pump flow, pump power, and pulse index were monitored throughout the operation. No cardiac surgeon was involved intraoperative, but they were available in emergency cases if needed. After surgery, all patients were co-managed by cardiologist and bariatric surgeon in the cardiac intensive care unit and nursing floor. Patients were discharged home on the liquid diet and seen in the clinic 1 week after discharge. Further follow up visits were at 1, 3, 6, 9, 12, 18, 24, and 36 months including visits with dietician, psychologist, bariatric surgeon, physician, cardiologist, and transplant team.
Surgical Technique
Laparoscopic sleeve gastrectomy for patients with an LVAD is performed using the 6-port technique (shown in Fig. 1). Caution is warranted during the port placement to avoid injury to the driveline and the pump. Before the operation, the subcutaneous driveline is marked with an absorbable skin marker as shown. Veres needle should be placed below the left costal margin away from the driveline and pump. All the incisions for the ports are made 1–2 cm away from the driveline. We generally use same port sites as shown in Fig. 1. The pump itself sometimes extends slightly below the left costal margin and it is palpable. The left subcostal port sometimes needs to be placed slightly more caudal to avoid injury to the driveline.
Statistical Analysis
Continuous variables were summarized by the median with range and categorical variables by counts and percentages.
Results
Patient Characteristics
Between October 2013 and January 2017, a total of 7 patients with ESHF and implanted LVAD underwent LSG. Four patients were female, with a median age of 39 years (range 26–62) and median preoperative BMI was 43.6 kg/m2 (range 36.7–56.7). Etiology for ESHF included ischemic cardiomyopathy (n = 4, 57%), dilated non-ischemic cardiomyopathy (n = 2, 29%), and peripartum cardiomyopathy (n = 1, 14%). Associated pertinent comorbidities included hypertension (n = 7, 100%), sleep apnea requiring CPAP (n = 5, 71%), atrial fibrillation (n = 4, 57%), dyslipidemia (n = 4, 57%), coronary artery disease (n = 4, 57%), and diabetes mellitus (n = 3, 43%). Preoperatively, patients were categorized by NYHA as class 2 (n = 1, 14%), class 3 (n = 3, 43%), and class 4 (n = 3, 43%) and according to ASA as class 4 (n = 7, 100%). The median preoperative LVEF with LVAD was 19% (range 15–20). Median heart rate was 74 beats per minute (range 54–79). Preoperatively, all patients had no heart failure symptoms with median of four medications (range 4–5) used to control heart failure. All the patients were on anticoagulation therapy after LVAD. Two patients were on anti-arrhythmic medications but none of them were on inotropes prior to LSG. Pertinent patient characteristics are summarized in Table 1.
Perioperative Details
The median interval time between LVAD implantation and LSG was 38 months (range 15–48). The median operative time and intraoperative blood loss were 168 min (range 70–230) and 100 ml (range 15–200), respectively. The median length of hospital stay was 9 days (range 6–23). Four patients had a planned 1-day intensive care unit (ICU) admission after surgery. There were five 30-day complications, including two major and three minor complications and no perioperative deaths. One patient experienced gastrointestinal bleeding on postoperative day 2 secondary to anticoagulation which was managed with vitamin K, and another patient developed acute cholecystitis with septic shock requiring intubation and percutaneous cholecystotomy tube drainage. There was no 30-day or 1-year mortality. Table 2 summarizes the perioperative data and the complications.
Postoperative Outcomes
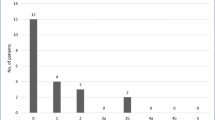
The median duration of follow-up was 24 months (range, 2–30). At the last available follow-up, the median BMI, %EWL, and %TWL were 37.2 kg/m2 (range 24.3–46.3), 47% (range 11–108%), and 16% (range 11–35%). At 1 year follow-up, all six patients experienced improvement of diabetes, dyslipidemia, and sleep apnea. Hypertension improved in two out of five patients at 1 year. The NYHA class at last follow-up point (before heart transplant) included class 1 (n = 1), class 2 (n = 3), and class 3 (n = 3). Three patients underwent heart transplantation after LSG at median of 16 months. Two patients died during the follow up period due to their pre-existing ESHF related complications. One patient died 22 months after LSG due to sepsis secondary to device-related infection, and another patient had sudden death 9 months post heart transplant.
Changes in LVEF and NYHA classification after LSG are depicted in Figs. 2 and 3, respectively. There was an improvement of LVEF after LSG. The median LVEF before LSG and after LSG (before heart transplant) were 19% (range 15–20) and 22% (range 16–35), respectively. In addition, the median NYHA class improved from preoperative class 3 (range 2–4) to class 2 (range 1–3) after LSG.
Discussion
This series, consisting of seven ESHF patients with implanted LVAD who underwent LSG, represents one of the largest series to date evaluating the feasibility of bariatric surgery in LVAD patients. LSG appears to be reasonably safe and feasible in this high-risk group. LSG also showed effective weight loss outcome and improvement in the cardiac function at a median follow up of 24 months. At the last follow-up, the median BMI decreased from 43.6 to 37.2 kg/m2, achieving a median EWL and TWL of 47 and 16%, respectively; overall improvement of the LVEF and cardiac function classified by NYHA class. Three of the patients underwent heart transplantation after the targeted BMI was achieved.
Obesity is a known risk factor for heart failure [3, 5]. Heart failure limits functional status of the patients and precludes them from exercising [6, 7]. Dietary modification alone is rarely sufficient to overcome the severe obesity in these patients. Bariatric surgery provides an alternative option in the ESHF patient with severe obesity. Besides providing sustainable weight loss outcome, bariatric surgery also showed improvement and resolution cardiovascular risk factors. The Swedish Obese Subjects (SOS) trial [8] showed significant decrease in cardiovascular risk and mortality after bariatric surgery. Vest et al. [9] in a systematic review showed evidence of left ventricular hypertrophy regression and improvement in diastolic function post bariatric surgery. There was improvement or resolution of hypertension (63%) and hyperlipidemia (65%) and reduction in all-cause mortality compared to non-operative controls.
Perioperative management of these high-risk patients is challenging. There are a number of concerns during bariatric surgery including (i) preventing injury of LVAD components during trocars placement by using ultrasound imaging; (ii) preload augmentation with intravenous fluid to counter adverse effect of drug-induced vasodilatation, pneumoperitoneum, and reverse Trendelenburg position; (iii) invasive monitoring such as arterial catheter and pulmonary artery catheter to monitor hemodynamic changes; and (iv) requirement of perfusionists and cardiac anesthesiologist [10, 11]. A multidisciplinary team approach involving cardiac anesthesiologist, dietician, psychologist, bariatric surgeon and physician, cardiologist, and transplant team is essential for optimal outcomes.
Complications in this high-risk group should be anticipated. There were five patients with early postoperative complications in our series including two major and two minor complications. One patient on long-term anticoagulation therapy experienced upper gastrointestinal bleeding on postoperative day 2, while another patient was readmitted for arrhythmias secondary to hypokalemia. There was no 30-day or 1-year postoperative mortality. There were two mortalities in our series; device-related infection at 22 months and post-transplant cardiac arrest at 24 months. Of note, device-related infection is one of the long-term complications of LVAD with an overall incidence of 32% [12].
To date, 11 studies (19 patients) have reported the outcome of bariatric surgery in LVAD patient with obesity [7, 10,11,12,13,14,15,16,17,18,19]. The majority of them were single case reports and two were case series. Three studies reported LVAD placement at the time of LSG [7, 13] and gastric band (14). Five studies including ours were on LVAD patients who underwent LSG [7, 12, 13, 15], and six studies reported outcome after laparoscopic Roux-en-Y gastric bypass (LRYGB) [10, 11, 16,17,18,19]. Laparoscopic adjustable gastric band (LAGB), LSG, and LRYGB resulted in significant short-term weight loss in the LVAD patients; the median excess weight loss was 61% (range, 23–80%) [7, 11,12,13,14,15,16,17,18,19]. A summary of 12 studies is provided in Table 3.
Improvements of cardiac function in LVAD patients, including LVEF and NYHA classification and candidacy for heart transplantation, have been reported after bariatric surgery [7, 11, 12, 14,15,16,17,18]. Chaudhry et al. [7] reported three LVAD patients with severe obesity that underwent LSG to become eligible for transplantation. Two patients successfully underwent heart transplantation with the mean interval time to transplant post-LSG of 13 months. Jeng et al. [15] described a heart failure patient with LVAD and BMI 40 kg/m2, who underwent LSG. At 7 months, the BMI decreased to 29 kg/m2 and the patient underwent heart transplantation successfully.
Leviner et al. [12] reported a LVAD patient with a BMI of 45 kg/m2 who underwent LSG. At 6 weeks, the patient lost 17 kg but developed LVAD thrombosis. The patient was hemodynamically stable, and echocardiography revealed significant improvement of LVEF from 25 to 40%. Subsequently, the LVAD was explanted, and the heart failure was controlled with medical treatment. Morrow et al. [17] showed in a case report an LVAD patient who underwent LRYGB resulting in LVEF improvement from 30 to 71%. The LVAD was subsequently explanted at 13 months of the follow up as it was no longer required. In our series, we also demonstrated improvement in LVEF and NYHA classification after LSG (Figs. 2 and 3).
This study has its limitations. It is a small retrospective study with a median follow up of 24 months (ranging from 2 to 30). There is one patient in this study with only 2 months follow up, therefore data on the post-LSG LVEF was not available.
Conclusion
Patients with morbid obesity, ESHF, and implanted LVAD constitute a high-risk cohort. Our results with 7 patients and result from other studies (19 patients) suggested that bariatric surgery may be a reasonable option for LVAD patients with severe obesity. Bariatric surgery appears to provide significant weight loss in these patients and may improve candidacy for heart transplantation. These preliminary results warrant further investigation.
References
Prinzing A, Herold U, Berkefeld A, et al. Left ventricular assist devices—current state and perspectives. J Thorac Dis. 2016;8(8):E660–6.
Derose JJ, Argenziano M, Sun BC, et al. Implantable left ventricular assist devices. Ann Surg. 1997;226:461–8.
McCloskey CA, Ramani GV, Mathier MA, et al. Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surg Obes Relat Dis. 2007;3:503–7.
Wikiel KJ, McCloskey CA, Ramanathan RC, et al. Bariatric surgery: a safe and effective to cardiac transplantation. Surg Obes Relat Dis. 2014;10:479–84.
Zhai AB, Haddad H. The impact of obesity on heart failure. Curr Opin Cardiol. 2017;32:196–202.
Ramani GV, McCloskey C, Ramanathan RC, et al. Safety and efficacy of bariatric surgery in morbidly obese patients with severe systolic heart failure. Clin Cardiol. 2008;31:516–20.
Chaudhry UI, Kanji A, Sai-Sudhakar CB, et al. Laparoscopic sleeve gastrectomy in morbidly obese patients with end-stage heart failure and left ventricular assist device: medium-term results. Surg Obes Relat Dis. 2015;11:88–93.
Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65.
Vest AR, Heneghan HM, Agarwal S, et al. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763–77.
Hoefnagel AL, Pasternak R, Curle AE, et al. Laparoscopic gastric bypass in a patients with an implanted left ventricular assist device. J Cardiothorac Vasc Anesth. 2012;26:880–2.
DeNino WF, Peura JL, Toole JM, et al. Orthotopic heart transplantation after left ventricular assist device implantation and laparoscopic Roux-en-Y gastric bypass. J Heart Lung Transplant. 2013;32:377–8.
Leviner DB, Keider A, Ben-Gal T, et al. Cardiac function recovery following LVAD implantation and bariatric surgery in morbidly obese patients. J Card Surg. 2014;29:740–2.
Shah SK, Gregoric ID, Nathan SS, et al. Simultaneous left ventricular assist device placement and laparoscopic sleeve gastrectomy as a bridge to transplant for morbidly obese patients with severe heart failure. J Heart Lung Transplant. 2015;34:1489–91.
Saeed D, Meehan K, McGee EC, et al. Bariatric surgery at the time of ventricular assist device implantation for morbidly obese patients prior to heart transplantation. Artif Organs. 2012;36:450–1.
Jeng EI, Aranda Jr JM, Ahmed M, et al. Left ventricular assist device and bariatric surgery: a bridge to heart transplantation by weight and waiting time reduction. J Card Surg. 2016;31:120–2.
Caceres M, Czer LS, Esmailian F, et al. Bariatric surgery in severe obesity and end-stage heart failure with mechanical circulatory support as a bridge to successful heart transplantation: a case report. Transplant Proc. 2013;45:798–9.
Morrow EH, Pellegrini CA, Mokadam NA, et al. Laparoscopic gastric bypass during left ventricular assist device support and ventricular recovery. J Heart Lung Transplant. 2014;33:870–1.
Lockard KL, Allen C, Lohmann D, et al. Bariatric surgery for a patient with a HeartMate II ventricular assist device for destination therapy. Prog Transplant. 2013;23:28–32.
Amro A, Murr M. A video case report of LRYGB in a patient with a left ventricular assist device. Surg Obes Relat Dis. 2015;11:1406–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and /or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
This study does not require informed consent.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Punchai, S., Nor Hanipah, Z., Sharma, G. et al. Laparoscopic Sleeve Gastrectomy in Heart Failure Patients with Left Ventricular Assist Device. OBES SURG 29, 1122–1129 (2019). https://doi.org/10.1007/s11695-018-3570-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3570-8