Abstract
Introduction
Patients suffering from advanced heart failure may undergo left ventricular assist device (LVAD) placement as a bridge to cardiac transplantation. However, those with a BMI above 35 kg/m2 are generally not considered eligible for transplant due to their elevated cardiac risk. We review our experience with bariatric surgery in this high-risk population to assess its safety and efficacy in reducing BMI to permit cardiac transplantation.
Methods
We retrospectively reviewed all patients on durable LVAD support who underwent sleeve gastrectomy (SG) at Mount Sinai Hospital between August 2018 and December 2022. Electronic medical records were reviewed to analyze patient demographics, surgical details, and outcomes regarding weight loss and heart transplantation.
Results
We identified twelve LVAD patients who underwent SG. Three were performed laparoscopically and 9 via robotic approach. Four patients (33.3%) underwent an orthotopic heart transplant (OHTx). Half of these patients were female. For patients who underwent OHTx, mean age at LVAD placement was 41.0 (R30.6–52.2), at SG was 43.9 (R32.7–55.0) and at OHTx was 45.3 years (R33.3–56.8). Mean BMI increased from 38.8 at LVAD placement to 42.5 prior to SG. Mean time from SG to OHTx was 17.9 months (R6-7-27.5) during which BMI decreased to mean 32.8 at the time of OHTx. At most recent follow-up, mean BMI was 31.9. All patients were anticoagulated prior to surgery; one required return to the operating room on post-operative day 1 after SG for bleeding and one was re-admitted on post-operative day 7 for hematochezia treated conservatively.
Conclusion
SG is a safe and effective operation in patients with severe obesity and heart failure requiring an LVAD. 66.7% of our cohort achieved target BMI < 35 and 33.3% underwent heart transplantation. Longer term follow-up is needed to clarify full bridge-to-transplant rate and long-term survival outcomes.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The number of morbidly obese patients in the United States and the world continues to rise along with a concomitant rise in cardiovascular disease. Those with severe obesity-related comorbidities such as advanced heart failure are particularly challenging to manage. The increased metabolic demands and stroke volume of obesity cause structural and functional changes to the heart such as left ventricular dilation, muscle hypertrophy, and atrial enlargement, contributing to the development of heart failure [1]. The definitive treatment for end-stage heart failure is cardiac transplantation. However, given the scarcity of organs, increased complications, and increased mortality rates in patients with higher BMI, those with body mass index (BMI) > 35 kg/m2 are generally not considered for cardiac transplantation [2,3,4].
Mechanical circulatory support with a left ventricular assist device (LVAD) is used as a bridge to cardiac transplantation [5]. However, these patients continue to have poor functional status and exercise tolerance, making conservative weight loss management difficult [6]. Several studies show that patients gain weight after LVAD implantation [7,8,9]. Bariatric surgery has emerged as an important approach to weight loss in this population, allowing for the possibility of improved cardiac function and qualification for cardiac transplantation [7]. There is an increasing body of evidence supporting the safety and feasibility of bariatric surgery in patients on LVAD support but publications are limited to a handful of case reports [8, 10,11,12,13,14,15,16,17,18] and small series of patients [7, 19,20,21,22,23,24,25,26,27,28,29,30,31]. We review our experience with bariatric surgery, specifically minimally invasive sleeve gastrectomy (SG) in this high-risk patient population to assess its safety and efficacy in reducing BMI to permit cardiac transplantation.
Materials and methods
This is a case series done at a single academic institution, Mount Sinai Hospital, in New York City. After obtaining approval from the Mount Sinai Institutional Review Board, we reviewed patients on LVAD support who underwent bariatric surgery between August 2018 and December 2022. Electronic medical records were reviewed to analyze patient demographics, operative details, and outcomes regarding surgical details, weight loss and heart transplantation.
Patient selection
Patients followed in the Mount Sinai LVAD program who are stable on device support but remain severely obese despite usual interventions are referred to the bariatric surgical center for evaluation. The bariatric work-up process for these patients does not differ from that of other patients evaluated at the bariatric surgical center. Nutrition and psychiatric evaluation, as well as relevant bloodwork, procedures, and imaging are performed as appropriate. Cardiac evaluation and optimization are performed by the LVAD team.
Perioperative management
At our institution, patients on LVAD support are typically maintained on warfarin with a goal INR between 2 and 3. Once a surgery date is scheduled, warfarin is held 5 days prior to surgery. Patients are admitted 2 days prior to surgery for medical optimization and anticoagulation bridging as determined by the heart failure team, with a goal INR of less than 1.6 at time of surgery. Ultrasound is used to identify the subcutaneous course of the driveline and it is marked on the skin with an indelible marker. The cardiac anesthesia team assesses the patient prior to surgery and designate an appropriate anesthesia team for the operation. Additionally, a LVAD-trained provider accompanies the patient for the duration of the surgery.
Post-operatively, the patients are admitted to one of our LVAD-trained cardiovascular intensive care units. Anticoagulation is resumed when deemed appropriate by the LVAD and surgical team; in general we start warfarin at home dose on post-operative day 0 without a heparin bridge. When ICU level care is no longer needed patients are transferred to an LVAD-trained floor. Patients are started on a bariatric clear liquid diet immediately after surgery. If tolerated, they are advanced to a bariatric pureed diet on postoperative day 1. Post-operative swallow study is not done routinely. Patients are discharged once deemed appropriate by the surgical and LVAD teams with appropriate follow up.
Follow-up
The patients are followed closely in the bariatric surgery clinic at 2 weeks, 4 months, 8 months, and 12 months after surgery, then annually thereafter with appropriate weight documentation and bloodwork. The patients are followed by an interdisciplinary team of surgeons, nutritionists, and nurse practitioners.
Patients are typically seen within 1–2 weeks following surgery in the LVAD clinic. Close attention is paid to their volume status and need for medication titration, particularly antihypertensives and diuretics. Once their BMI is consistently < 35 kg/m2 and they are otherwise felt to be acceptable transplant candidates, patients are presented to the transplant listing committee.
Surgical technique
Patients receive pre-operative antibiotics and 5,000 Units of subcutaneous heparin prior to surgery. The trajectory of the subcutaneous portion of the LVAD driveline along the patient’s right abdomen has been previously marked out under ultrasound guidance, but ultrasound is kept available in the operating room if clarification of the driveline path is required. SG are performed using the minimally invasive approach. Figure 1 shows the trocar and Nathanson liver retractor placement for laparoscopic and robotic approaches; special care is taken to avoid damage to the LVAD driveline during insertion. After dividing the short gastric vessels with an advanced bipolar energy device, SG is performed using linear staples applied starting 5 cm proximal to the pylorus. We routinely use linear staple cartridges with built-in reinforcement strips with the goal of enhancing hemostasis (Medtronic Endo GIA with TRS, Medtronic, Minneapolis, MN). Robotic staple line reinforcement strips were not available at our institution during the study period. Calibration of the sleeve is obtained using a 40 French disposable orogastric tube. An upper endoscopy is routinely performed to evaluate the staple line and to perform an air leak test. The staple line is carefully inspected and 5 mm titanium clips are applied for hemostasis as needed.
Results
Twelve LVAD patients who underwent bariatric surgery were identified. All patients underwent minimally invasive SG, the first 3 performed laparoscopically and the subsequent nine via robotic approach. Patient demographics can be found in Table 1. Five (41.6%) of these patients were male. Mean age at the time of LVAD placement was 43.9 years (range 25–57.8). Mean age at time of SG was 46.8 years (range 29–61.8). The LVAD devices were Heartware HVAD (n = 1), Heartmate 2 (n = 3), or Heartmate 3 (n = 8). Four patients (33.3%) from this cohort subsequently underwent an orthotopic heart transplant (OHTx). Half of these patients were female, half were male. For patients who underwent OHTx, mean age at LVAD placement was 41.0 years (range 30.6–52.2), at SG was 43.9 years (range 32.7–55) and at OHTx was 45.3 years (range 33.3–56.8).
Table 2 shows the outcomes after LVAD placement, SG, and OHTx. Mean time from LVAD placement to SG was 35.3 months (range 5.3–72.8). Of the four patients who subsequently underwent OHTx, mean time from SG to OHTx was 17.9 months (range 6.7–27.5). Mean BMI at the time of LVAD placement was 40.2 (range 30.4–52.7). Mean BMI at time of SG was increased to 43.1 (range 35.4–52.9). Mean BMI at time of OHTx decreased to 32.8 (range 31.0–37.0). Mean follow-up time was 18.4 months (range 3–55.4). Post-operatively, one patient was readmitted on postoperative day 7 for hematochezia and hypovolemia treated conservatively. Another patient was taken back to the operating room on postoperative day 1 for bleeding from the staple line. There were no deaths within 1 year after SG.
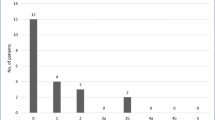
By the end of the study period, 8 (66.7%) of the patients reached target BMI for transplantation of less than 35. Target BMI was reached a mean of 6.4 months after SG (range 2 weeks–23.1 months). Four of these patients received successful heart transplants, two are currently listed and awaiting heart transplants, and the remaining two are undergoing optimization of comorbidities such as pulmonary hypertension. Figure 2 shows the BMI trends for all patients. Most LVAD patients gained weight after LVAD implantation. Mean BMI was lowest at 8 months after SG.
Discussion
Our results show that SG in patients on LVAD support is safe and feasible, and adds to the body of knowledge in support of bariatric surgery as a bridge toward cardiac transplantation in this high-risk patient population. Consistent with findings from literature, our results show that without intervention patients tend to gain weight after LVAD implantation [7,8,9], highlighting the importance of bariatric surgery. Our results showed that SG successfully aided patients in weight loss toward the goal of target BMI eligible for cardiac transplantation, with durable reduction in BMI one year after SG.
LVAD hemodynamic support is an excellent option for patients who have absolute or relative contraindications for heart transplant such as elevated pulmonary vascular resistance or an elevated BMI. These devices, while lifesaving, can cause significant complications. The MOMENTUM trial compared the latest generation of continuous flow LVAD (HeartMate 3) with an axial flow pump (Heartmate 2) [32]. Five-year outcomes of this trial showed a significantly higher incidence of the primary outcome (survival to transplant, recovery or LVAD support free of debilitating stroke or reoperation to replace the pump) in the group that received the magnetically levitated centrifugal flow pump (HeartMate 3). The most common complications of LVAD support were major infection and bleeding (mostly gastrointestinal bleeding). Other hemocompatibility-related events such as device thrombosis and stroke were very rare [33].
Patients with advanced heart disease can develop cardiac cachexia. This is often reversed after patients are placed on LVAD support. Frequently, substantial weight gain is seen in patients after their critical cardiac illness has been stabilized on LVAD support. This issue often leads to worsening of their diabetes and metabolic control and patients being unable to be listed for transplant.
There is a growing body of literature documenting the safety and feasibility of bariatric surgery in LVAD patients. Several case studies [8, 10,11,12, 14,15,16,17,18] and case series [7, 19,20,21,22,23,24,25,26,27,28,29,30,31] ranging from 3 to 22 patients show successful weight reduction after bariatric surgery and up to 45.5% of the study cohorts successfully bridged to heart transplant. Four patients (33.3%) from our cohort were successfully bridged to a cardiac transplantation. With longer follow-up times for the later patients in the study, the true potential could be higher. During our study period eight patients (66.7%) reached a target BMI of < 35.
Even for patients who have not yet received a cardiac transplant or have not reached target BMI, bariatric surgery provides benefits for LVAD patients owing to its role in weight reduction and improvement in cardiac function. Several studies looking at the ejection fractions of LVAD patients after bariatric surgery show improvement in cardiac function [14, 18, 23, 24], some even to a point of no longer requiring cardiac transplantation [21].
Several challenges were identified that are inherent to performing surgeries on LVAD patients. A dedicated multi-disciplinary team is crucial to the management of these patients from initial evaluation through postoperative follow-up. The surgery requires an experienced cardiac anesthesia team as well as having an additional LVAD-trained provider available during the case. Because these patients are on therapeutic anticoagulation, it is important to develop an anticoagulation management plan to both reduce the risk of thrombotic complications related to the LVAD and postoperative hemorrhage. Patients are also treated on dedicated cardiac LVAD-trained units and on the units typical for general surgery patients.
In our cohort, one patient was readmitted on post-operative day 7 for hematochezia and hypovolemia treated conservatively. Another patient had tachycardia and anemia on post-operative day 1 requiring return to the operating room for a bleed along the staple line. Both patients underwent SG using the robotic approach and had an appropriate INR of 1.3 at the time of surgery. For the first patient, INR at the time of readmission was 3.4, which was supratherapeutic after restarting warfarin after surgery. This highlights one of the complexities of this patient population given their anticoagulated state. The bleed in the latter patient could be related to our approach; at our institution robotic SG staple lines are not reinforced or oversewn which may potentially have contributed to the bleed. Of note, an internal review of our experience comparing bleeding rates with sleeve gastrectomy in non-LVAD patients showed no difference between the laparoscopic and robotic approaches.
The location of the LVAD driveline is an important intra-operative consideration as the subcutaneous portion of the driveline courses through the surgical field. To our knowledge, this is the first study including patients who underwent robotic SG. After adoption of the Da Vinci Xi Robot in mid-2021 at our institution, SG cases were all done using the robotic approach. The trajectory of the driveline along the right abdominal wall presents challenges to trocar placement, as close proximity of these ports could potentially damage the device or increase the risk of infection. As depicted in Fig. 1, one advantage of the robotic approach is that the trocars are placed lower along the abdomen thus providing more working space between the robotic instruments and the driveline.
SG is the bariatric procedure of choice in our study given its lower risk profile, particularly in high-risk patients who might undergo future organ transplantation. SG has been shown to have fewer complications compared to anastomotic procedures like Roux-en-Y gastric bypass or biliopancreatic diversion with duodenal switch [34, 35]. Importantly, because SG does not alter gastrointestinal continuity, absorption of immunosuppressive medications after transplant is less affected [36]. A case study by Jeng et al. showed that therapeutic levels of immunosuppression up to 36 months after SG was maintained with no evidence of rejection [8]. In a report by Ahluwalia et al. on three patients who underwent RYGB prior to heart transplant, two died due to severe allograft rejection and the third experienced cardiac arrest at home [19]. This was thought to be related to impaired medication absorption.
In our study, we did not identify a group of obese LVAD patients who did not undergo SG and thus could not draw comparisons. In a national study conducted by McElderry et al. reviewing Medicare beneficiaries who had LVAD placement from 2012 to 2019, 7.1% had bariatric surgery. They found that compared to LVAD patients who did not have bariatric surgery, there was a threefold higher probability of receiving a heart transplant as well as a reduction in heart failure hospitalizations [37]. In a study conducted by Jeng et al., the outcomes of 15 LVAD patients without bariatric surgery (LVAD alone) was compared to 14 LVAD patients who received a laparoscopic SG (LVAD + LSG). While survival outcomes did not differ greatly between the two groups, the LVAD + SG group had a significantly higher number of patients bridged to cardiac transplant and the LVAD alone group had a significant increase in average BMI during the study period [28].
Limitations of this study include its retrospective design and small sample size. Additionally, there are biases inherent to any single-center study. Furthermore, we did not have a comparison group of LVAD patients who did not undergo bariatric surgery. At present we do not have long-term outcomes data so extended survival outcomes cannot be addressed. Despite our study’s inherent limitations, we feel that it provides compelling evidence in support of minimally invasive SG as an important treatment option for severely obese LVAD patients awaiting a heart transplantation.
References
Zhai AB, Haddad H (2017) The impact of obesity on heart failure. Curr Opin Cardiol 32:196–202. https://doi.org/10.1097/HCO.0000000000000370
Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EAM, Zuckermann A (2016) The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 35:1–23. https://doi.org/10.1016/j.healun.2015.10.023
Lee SJ, Kim KH, Hong SK, Hankins S (2017) Evaluation of a heart transplant candidate. Curr Cardiol Rep 19:133. https://doi.org/10.1007/s11886-017-0934-y
Weber DJ, Didolkar P, Gracon A, Hellman Y, Hadi MA, Malik A, Caccamo M, Gradus-Pizlo I, Wozniak T, Hashmi ZA, Wang I (2014) The role of recipient BMI and age on survival after heart transplantation. J Heart Lung Trans 33:S91. https://doi.org/10.1016/j.healun.2014.01.278
John R, Pagani FD, Naka Y, Boyle A, Conte JV, Russell SD, Klodell CT, Milano CA, Rogers J, Farrar DJ, Frazier OH (2010) Post–cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg 140:174–181. https://doi.org/10.1016/j.jtcvs.2010.03.037
Carbone S, Lavie CJ, Arena R (2017) Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc 92:266–279. https://doi.org/10.1016/j.mayocp.2016.11.001
Yanagida R, Czer LSC, Mirocha J, Rafiei M, Esmailian F, Moriguchi J, Kobashigawa JA, Trento A (2014) Left ventricular assist device in patients with body mass index greater than 30 as bridge to weight loss and heart transplant candidacy. Transpl Proc 46:3575–3579. https://doi.org/10.1016/j.transproceed.2014.09.108
Jeng EI, Aranda JM, Ahmed M, Klodell CT (2016) Left ventricular assist device and bariatric surgery: a bridge to heart transplant by weight and waiting time reduction: BARIATRIC SURGERY: BRIDGE TO HEART TRANSPLANT. J Card Surg 31:120–122. https://doi.org/10.1111/jocs.12688
Clerkin KJ, Naka Y, Mancini DM, Colombo PC, Topkara VK (2016) The impact of obesity on patients bridged to transplantation with continuous-flow left ventricular assist devices. JACC Heart Failure 4:761–768. https://doi.org/10.1016/j.jchf.2016.05.010
Amro A, Murr M (2015) A video case report of LRYGB in a patient with a left ventricular assist device. Surg Obesity Related Dis 11:1406–1407. https://doi.org/10.1016/j.soard.2015.06.021
Boeker C, Hakami IA, Mall J, Reetz C, Yamac K, Koehler H (2022) Bariatric surgery as the one route to achieving donor heart transplantation in a patient with a left-ventricular assist device. Obes Facts 15:99–103. https://doi.org/10.1159/000519950
Lockard KL, Allen C, Lohmann D, Severyn DA, Schaub RD, Kauffman KE, Hodges JR, Woodhall L, Ramanathan R, Teuteberg JJ, Eckert CE, Kormos RL (2013) Bariatric surgery for a patient with a heartmate ii ventricular assist device for destination therapy. Prog Transpl 23:28–32. https://doi.org/10.7182/pit2013331
Caceres M, Czer LSC, Esmailian F, Ramzy D, Moriguchi J (2013) Bariatric surgery in severe obesity and end-stage heart failure with mechanical circulatory support as a bridge to successful heart transplantation: a case report. Transpl Proc 45:798–799. https://doi.org/10.1016/j.transproceed.2012.10.036
Leviner DB, Keidar A, Ben-Gal T, Medalion B (2014) Cardiac function recovery following LVAD implantation and bariatric surgery in a morbidly obese patient: LVAD, obesity, and bariatric surgery. J Card Surg 29:740–742. https://doi.org/10.1111/jocs.12404
Hoefnagel AL, Pasternak R, Curle AE, Eaton MP (2012) Laparoscopic gastric bypass in a patient with an implanted left ventricular assist device. J Cardiothorac Vasc Anesth 26:880–882. https://doi.org/10.1053/j.jvca.2011.02.012
Aittigrine S, Tozzi P, Hullin R, Yerly P, Regamey J, Rösner L, Rusca M, Kirsch M, Suter M, Mantziari S (2019) Laparoscopic sleeve gastrectomy for class III obesity in a patient with a left ventricular assist device (LVAD) Heartmate III. Surg Obesity Related Dis 15:1420–1421. https://doi.org/10.1016/j.soard.2019.05.008
DeNino WF, Peura JL, Toole JM (2013) Orthotopic heart transplantation after left ventricular assist device implantation and laparoscopic Roux-en-Y gastric bypass. J Heart Lung Trans 32:377–378. https://doi.org/10.1016/j.healun.2012.11.032
Nathan SS, Iranmanesh P, Gregoric ID, Akay MH, Kumar S, Akkanti BH, Salas de Armas IA, Patel M, Felinski MM, Shah SK, Bajwa KS, Kar B (2021) Regression of severe heart failure after combined left ventricular assist device placement and sleeve gastrectomy. ESC Heart Failure 8:1615–1619. https://doi.org/10.1002/ehf2.13194
Ahluwalia M, Givertz MM, Mehra MR (2020) Bariatric surgery and heart transplantation outcomes: a note of caution. J Heart Lung Trans 39:986–987. https://doi.org/10.1016/j.healun.2020.03.011
Zenilman A, Pechman D, Moran-Atkin E, Choi J, Camacho D (2019) Bariatric surgery in patients with left ventricular assist devices: a safe and effective method of weight loss as a gateway to heart transplantation. Surgery for Obesity and Related Diseases 15:1780–1784. https://doi.org/10.1016/j.soard.2019.08.003
Lim C-P, Fisher OM, Falkenback D, Boyd D, Hayward CS, Keogh A, Samaras K, MacDonald P, Lord RV (2016) Bariatric surgery provides a “Bridge to Transplant” for morbidly obese patients with advanced heart failure and may obviate the need for transplantation. OBES SURG 26:486–493. https://doi.org/10.1007/s11695-015-1789-1
Wikiel KJ, McCloskey CA, Ramanathan RC (2014) Bariatric surgery: a safe and effective conduit to cardiac transplantation. Surg Obesity Related Dis 10:479–484. https://doi.org/10.1016/j.soard.2013.11.002
Vest AR, Patel P, Schauer PR, Satava ME, Cavalcante JL, Brethauer S, Young JB (2016) Clinical and echocardiographic outcomes after bariatric surgery in obese patients with left ventricular systolic dysfunction. Circ Heart Failure 9:e002260. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002260
Punchai S, Nor Hanipah Z, Sharma G, Aminian A, Steckner K, Cywinski J, Young JB, Brethauer SA, Schauer PR (2019) Laparoscopic sleeve gastrectomy in heart failure patients with left ventricular assist device. OBES SURG 29:1122–1129. https://doi.org/10.1007/s11695-018-3570-8
Chaudhry UI, Kanji A, Sai-Sudhakar CB, Higgins RS, Needleman BJ (2015) Laparoscopic sleeve gastrectomy in morbidly obese patients with end-stage heart failure and left ventricular assist device: medium-term results. Surg Obesity Related Dis 11:88–93. https://doi.org/10.1016/j.soard.2014.04.003
daSilva-deAbreu A, Garikapati K, Alhafez BA, Desai S, Eiswirth C, Krim S, Patel H, Ventura HO, Lavie CJ, Loro-Ferrer JF, Mandras SA (2021) Laparoscopic sleeve gastrectomy in patients with obesity and ventricular assist devices: a comprehensive outcome analysis. OBES SURG 31:884–890. https://doi.org/10.1007/s11695-020-04948-9
Lin MYC, Mehdi Tavakol M, Sarin A, Amirkiai SM, Rogers SJ, Carter JT, Posselt AM (2013) Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Related Dis 9:653–658. https://doi.org/10.1016/j.soard.2013.02.013
Jeng EI, Miller AH, Friedman J, Tapia-Ruano SA, Reilly K, Parker A, Vilaro J, Aranda JM, Klodell CT, Beaver TM, Arnaoutakis GJ, Ahmed M (2021) Ventricular assist device implantation and bariatric surgery: a route to transplantation in morbidly obese patients with end-stage heart failure. ASAIO J 67:163–168. https://doi.org/10.1097/MAT.0000000000001212
Ng M, Rodgers B, Rehman S, Nathan SS, Bajwa KS, Shah SK, Akkanti BH, Jumean MF, Kumar S, Dressel JL, Radovancevic R, Felinski MM, Kar B, Gregoric ID (2022) Left ventricular assist device support and longitudinal sleeve gastrectomy combined with diet in bridge to heart transplant. Texas Heart Institute J 49:e207521. https://doi.org/10.14503/THIJ-20-7521
Shah SK, Gregoric ID, Nathan SS, Akkanti BH, Kar B, Bajwa KS (2015) Simultaneous left ventricular assist device placement and laparoscopic sleeve gastrectomy as a bridge to transplant for morbidly obese patients with severe heart failure. J Heart Lung Transplant 34:1489–1491. https://doi.org/10.1016/j.healun.2015.06.011
Greene J, Tran T, Shope T (2017) Sleeve gastrectomy and left ventricular assist device for heart transplant. JSLS 21(e2017):00049. https://doi.org/10.4293/JSLS.2017.00049
Mehra MR, Uriel N, Naka Y, Cleveland JC, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ (2019) A fully magnetically levitated left ventricular assist device — final report. N Engl J Med 380:1618–1627. https://doi.org/10.1056/NEJMoa1900486
Mehra MR, Goldstein DJ, Cleveland JC, Cowger JA, Hall S, Salerno CT, Naka Y, Horstmanshof D, Chuang J, Wang A, Uriel N (2022) Five-year outcomes in patients with fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA 328:1233. https://doi.org/10.1001/jama.2022.16197
Buchwald H (2014) The evolution of metabolic/bariatric surgery. OBES SURG 24:1126–1135. https://doi.org/10.1007/s11695-014-1354-3
Lager CJ, Esfandiari NH, Subauste AR, Kraftson AT, Brown MB, Cassidy RB, Nay CK, Lockwood AL, Varban OA, Oral EA (2017) Roux-En-Y gastric bypass vs. sleeve gastrectomy: balancing the risks of surgery with the benefits of weight loss. OBES SURG 27:154–161. https://doi.org/10.1007/s11695-016-2265-2
Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, Vinks AA (2007) Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study: pharmacokinetics of immunosuppressants in gastric bypass patients. Clin Transplant 22:281–291. https://doi.org/10.1111/j.1399-0012.2007.00783.x
McElderry B, Alvarez P, Hanna M, Chaudhury P, Bhat P, Starling RC, Desai M, Mentias A (2022) Outcomes of bariatric surgery in patients with left ventricular assist device. J Heart Lung Transplant 41:914–918. https://doi.org/10.1016/j.healun.2022.04.003
Acknowledgements
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors Catherine Tsai, Patrick Dolan, Noah Moss, Alejandro Sandoval, Julie Roldan, and Daniel Herron have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsai, C., Dolan, P., Moss, N. et al. Sleeve gastrectomy facilitates weight loss and permits cardiac transplantation in patients with severe obesity and a left ventricular assist device (LVAD). Surg Endosc 37, 8655–8662 (2023). https://doi.org/10.1007/s00464-023-10264-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10264-x