Abstract
Background
Laparoscopic Roux-en-Y gastric bypass (LRYGB) has been proven to be effective on treating type 2 diabetes mellitus (T2DM) in severely obese patients, but whether LRYGB surgery should be performed in obese class I patients is controversial.
Materials and Methods
A retrospective study of 3-year bariatric and metabolic outcomes in different obese class T2DM patients who underwent LRYGB was conducted to compare the effectiveness of LRYGB in obese class I patients with that in obese class II/III patients in a Chinese T2DM population.
Results
Totally, 58 patients with class I obesity and 45 patients with class II/III obesity were enrolled in this study. Major complications included two cases of incomplete intestinal obstructions and one anastomotic leak. The remission rates of T2DM were 70.6% in obese class I group and 77.8% in obese class II/III group at 1 year after surgery and 55.6 versus 64.3% at 3 years (all P > 0.05). Logistic regression analysis showed that higher waist circumference, lower fasting plasma glucose, and higher FCP at 2 h of OGTT were independently associated with diabetes remission at 1 year after surgery. At 1 year and thereafter, the percentage of excess weight loss was significantly greater in obese class II/III patients. At 3 years, body mass index was not significantly different between the two groups, and the obese class I patients had high recurrence rates of hypertension and hyperuricemia.
Conclusions
LRYGB surgery is feasible, safe, and effective in Chinese obese class I patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is presently accepted to be the most effective approach for achieving weight loss in morbidly obese patients. Laparoscopic Roux-en-Y gastric bypass (LRYGB), considered the gold standard procedure, has been shown to be capable of rapidly reducing blood sugar levels in selected patients with type 2 diabetes mellitus (T2DM). Body mass index (BMI), one of the most commonly used parameters to reflect the severity of obesity, is also a very useful tool for identifying patients who can be expected to benefit from bariatric surgery. According to the World Health Organization (WHO) criteria, obesity in Asian populations can be classified into three grades: (1) obese class I (BMI 27.5–32.5 kg/m2), (2) obese class II (BMI 32.5–37.5 kg/m2), and (3) obese class III (BMI ≥ 37.5 kg/m2) [1]. Many guidelines or statements, mostly from Western countries, have demonstrated that bariatric surgery should only be recommended to T2DM patients with BMI ≥ 35 kg/m2; since the BMI action point decreases by 2.5 for Asian populations, this translates to ≥ 32.5 kg/m2 in Asians. Whether surgery should be applied in obese class I patients with T2DM is still controversial.
Bariatric surgery, although popular worldwide, is still a new concept in China. It has only been applied in China for about 15 years, and most (89.2%) of the surgeries have been conducted in the last 5 years [2]. China has now become the country with the largest T2DM population. A study conducted in 2010 showed that China had nearly 92.4 million adults with diabetes and another 148.2 million adults with prediabetes [3]. China is also home to the largest number of obese individuals [4]. However, most Chinese T2DM patients with obesity are either only mildly overweight or have class I obesity; they also commonly manifest a type of abdominal visceral obesity [5]. Whether this large population is eligible for bariatric surgery, and whether outcomes in this group differ from those in patients with higher obesity levels, is still unclear.
Our department has been performing laparoscopic bariatric surgeries since 2006. LRYGB is our first choice for diabetes patients. This retrospective study was conducted to assess the metabolic efficacy of LRYGB on Chinese T2DM patients of different obese classes and to verify the feasibility of performing bariatric surgery on nonseverely obese T2DM patients.
Materials and Methods
Patient Population
Patients who received LRYGB at West China Hospital, Sichuan University, China, between January 1, 2011, and July 31, 2013, and had completed at least 1 year of follow-up, were eligible for inclusion in this study. Patients were included if they had (1) age ≥ 18 years; (2) BMI ≥ 27.5 kg/m2; (3) a diagnosis of T2DM based on American Diabetes Association (ADA) criteria [6], with no serious hyperglycemia-associated complications; and (4) fasting C peptide (FCP) ≥ 50% of normal or a twofold increase in FCP during an oral glucose tolerance test (OGTT) at 2 h (2h-CP). This study was approved by the Research and Ethics Committee of West China Hospital, and informed consent was obtained from all patients.
Preoperatively, all patients underwent gastroscopy, Helicobacter pylori detection, electrocardiography, pulmonary function testing, abdominal computed tomography scan, and laboratory tests (including fasting plasma glucose [FPG], FCP, glycated hemoglobin [HbA1c], fasting insulin [FINS], OGTT, and blood lipids). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to the formula: FINS (mU/L) × FPG (mmol/L)/22.5. Waist circumference was recorded to the nearest centimeter. Standard BMI was defined as 25 kg/m2 for males and 24 kg/m2 for females. The operative time, postoperative complications, and length of hospitalization were also recorded. All surgeries were performed by the same surgeon (C.Z.; who has performed over 300 bariatric surgeries). All the LRYGB procedures were performed in the standard manner with a 100-cm biliopancreatic limb and a 100-cm alimentary limb [7].
Follow-Up
A barium swallow was routinely performed on the first postoperative day. If there was no evidence of leakage, patients were allowed to drink clear liquids, which were gradually increased to a full liquid diet over 2 days and discharged on the third postoperative day. All patients were requested to receive a semiliquid diet during the first four postoperative weeks as well as long-term oral supplements (multivitamins, iron, and calcium) after surgery. A guide list of postoperative diet and nutrition was available for every patient. Follow-ups were requested to be attended 3 monthly for the first year and 6 monthly thereafter at outpatient clinics. At each follow-up visit, body weight, the percentage of excess weight loss (%EWL), current BMI and waist circumference, and any comorbid conditions were recorded. Routine laboratory tests and OGTT were performed. A nutritionist and an endocrinologist monitored patients for malnutrition and hyperglycemia. Patients were questioned about major and minor surgical complications; a major complication was defined as any condition necessitating rehospitalization for medical or surgical interventions or surgery-related death.
The primary endpoint of this study was T2DM control. Secondary endpoints were the effect of weight loss and remission or improvement (R/I) of obesity-related comorbidities. Complete remission of T2DM was defined as FPG < 5.6 mmol/L and HbA1c < 6.0%; improvement was defined as FPG 5.6–6.9 mmol/L and HbA1c < 6.5%, without antidiabetes medication [6]. With regard to weight loss, the bariatric procedure was considered inadequate if, at the end of 1 year after surgery, the %EWL was 30–50%, and a failure if %EWL was < 30%. Underweight was defined as BMI < 20 kg/m2.
The criteria for R/I of other comorbidities were as follows: (1) With respect to hypertension, remission was defined as blood pressure ≤ 120/80 mmHg without medication, and improvement was defined as any reduction in antihypertensive medication; (2) for hyperlipidemia and hyperuricemia, remissions were defined as serum cholesterol and triglycerides, or serum uric acid, maintained below the cutoff point without the use of medication; any reduction in medication was considered as improvement.
Statistical Analysis
Continuous variables were presented as means ± standard deviation. The independent samples t test was used to compare continuous variables, and either the chi-square test or Fisher’s exact test (two-sided) was used for categorical variables. Logistic regression analysis was used to identify associated parameters of T2DM remission at 1-year follow-up. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was employed for all analyses. GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA) was used for generating the graphics. Statistical significance was set at P < 0.05.
Results
General Characteristics
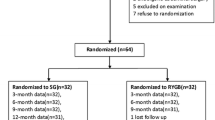
Totally, 103 consecutive patients who met the inclusion criteria were enrolled in this study. Among these, 58 patients were obese class I, and 45 patients were obese class II/III obese (26 patients class II and 19 patients class III). Two patients from the obese class I group and one patient from the obese class II/III group were lost to follow-up at 2 years; a further two patients from the obese class I group and two patients from the obese class II/III group failed to attend follow-up at the end of 3 years.
As shown in Table 1, both groups were comparable in terms of gender distribution, age, duration of T2DM, family history of T2DM, and preoperative treatment. The mean values of FPG, plasma glucose at 2 h of OGTT (2h-PG), HbA1c, FCP, 2h-CP, FINS, and HOMA-IR were also comparable between the two groups. The mean values of BMI, waist circumference, diastolic blood pressure, total cholesterol, high-density lipoprotein, uric acid, as well as the prevalence rates of hypertension and hyperuricemia were significantly higher in the obese class II/III group. Mean operation time, length of hospitalization, and major complications were comparable in the two groups. Major complications were seen in three patients: One patient in each group had rehospitalization for treatment of incomplete intestinal obstruction, both recovered with conservative treatment; another patient in the obese class II/III group was readmitted for gastrointestinal anastomotic leak and needed emergency laparoscopic drainage. Minor complications were seen in both groups: marginal ulcers occurred in two obese class I patients and three obese class II/III patients, mild dumping in two patients in each group, iron-deficiency anemia in one patient in the obese class I group, gastroesophageal reflux in one obese class I patient and two obese class II/III patients, and mild diarrhea in two obese class I patients. All of these patients recovered either spontaneously or with medication.
Primary Outcomes
Remission and improvement rates at 1- and 3-year follow-ups were higher in the obese class II/III group, but the difference from the obese class I group was not statistically significant (Table 2, Fig. 1a). In this study population, overall R/I were achieved in 73.8% (76/103) patients at 1 year and in 59.4% (57/96) patients at 3 years. The mean values of 2h-PG at 12, 24, and 36 months; FCP at 24 and 36 months; HbA1c at 36 months; and HOMA-IR at 36 months were significantly higher in the obese class I group than in the obese class II/III group (Fig. 2b, c, e, f). There were no significant differences between the two groups in the postoperative values of FPG and 2h-CP (Fig. 2a, d).
Remissions or improvements of obesity-related comorbidities in the two groups at 1 and 3 years after LRYGB. a Type 2 diabetes mellitus. b Hypertension. c Dyslipidemia. d Hyperuricemia. R/I remissions or improvement, T2DM type 2 diabetes mellitus. * Comparison between obese class I and obese class II/III groups; P < 0.05
Changes in mean values of a fasting plasma glucose, b plasma glucose at 2 h of OGTT, c fasting C peptide, d C peptide at 2 h of OGTT, e glycated hemoglobin, and f homeostatic model of assessment-insulin resistance after LRYGB in the two groups. FPG fasting plasma glucose, 2h-PG plasma glucose at 2 h of OGTT, FCP fasting C peptide, 2h-CP C peptide at 2 h of OGTT, HbA1c glycated hemoglobin, HOMA-IR homeostatic model of assessment-insulin resistance. * Compared with preoperative value; P < 0.05. # Comparison between obese class I and obese class II/III groups; P < 0.05
Patients who achieved R/I of T2DM at 1 year had higher waist circumference, lower FPG, higher FCP, higher 2h-CP, lower HbA1c, and lower HOMA-IR at baseline than the other patients (Table 3). Age, preoperative BMI, duration of T2DM, and %EWL at 1 year were not significantly different between remission/improvement patients and other patients. Logistic regression analysis showed that higher waist circumference, lower FPG, and higher 2h-CP were independently associated with diabetes remission at 1 year after bariatric surgery in this population (Table 3).
Secondary Outcomes
The weight losses after bariatric surgery in the two groups are listed in Table 4, and the mean %EWL reached highest values at 12 months after surgery in both groups, after which it began decreasing. At 12 months and thereafter, the %EWL was significantly greater in the obese class II/III group than in the obese class I group (80.4 ± 12.3 vs. 70.4 ± 12.9% at 12 months, 78.4 ± 10.9 vs. 66.3 ± 9.8% at 24 months, 77.8 ± 10.9 vs. 62.3 ± 11.1% at 36 months, respectively; Table 4, Fig. 3a). Mean BMI was significantly lower than preoperative BMI at every follow-up visit in both groups. Obese class II/III patients had significantly higher mean BMI than obese class I patients at follow-up visits during the first 2 years (29.1 ± 2.8 vs. 27.3 ± 2.9 kg/m2 at 6 months, 27.6 ± 2.4 vs. 26.5 ± 1.9 kg/m2 at 12 months, 27.9 ± 3.1 vs. 26.8 ± 2.0 kg/m2 at 24 months, respectively; Fig. 3b). At 36 months, however, the BMI in the two groups were comparable (28.0 ± 3.2 vs. 27.1 ± 2.0 kg/m2; P > 0.05; Fig. 3b). No patient’s BMI dropped below 20 kg/m2. As with BMI, in both groups, the mean waist circumference at follow-up was significantly lower than that at baseline. Obese class II/III patients had significantly higher values than the obese class I patients at all four time points (102.2 ± 10.6 vs. 94.6 ± 5.8 cm at 6 months, 94.3 ± 8.3 vs. 90.7 ± 5.3 cm at 12 months, 96.5 ± 8.3 vs. 92.0 ± 5.0 cm at 24 months, 97.2 ± 9.6 vs. 93.2 ± 6.7 cm at 36 months, respectively; Fig. 3c).
Changes in mean values of a the percentage of excess weight loss, b body mass index, and c waist circumference after LRYGB in the two groups. %EWL the percentage of excess weight loss, Pre-OP preoperation, BMI body mass index. * Compared with preoperative value; P < 0.05. # Comparison between obese class I and obese class II/III groups; P < 0.05
At 1-year follow-up, both groups showed good R/I of hypertension, dyslipidemia, and hyperuricemia, with R/I rates being more than or close to 50% (Table 2, Fig. 1b–d). However, at 3-year follow-up, the R/I of hypertension and hyperuricemia were unsatisfactory in obese class I patients, with the rates being ≤ 20%. At 3 years, 60% of patients who had R/I of hypertension at 1 year (3/5, lost one patient), as well as 66.7% of patients who had R/I of hyperuricemia at 1 year (2/3, lost one patient), in obese class I group, had relapsed. At 3 years, the remission rates of hypertension and dyslipidemia were similar in the two groups; however, the remission rate of hyperuricemia was significantly higher in obese class II/III patients (Table 2).
Discussion
Our results, with a mid-term follow-up, showed a satisfactory overall remission rate of diabetes of 73.8% at 1 year and 59.4% at 3 years, which proved the effectiveness of LRYGB as a long-standing standard bariatric procedure for treating diabetes. Early normalization of glucose levels—even before significant weight loss occurred [8]—may indicate the existence of weight loss-independent mechanisms of glycemic control by the surgery. Lines of evidence from studies on nonobese rodent models of T2DM also support the existence of such mechanisms [9, 10]. The 5-year outcome of the STAMPEDE study, which included 36% patients with a BMI of 27 to 34 kg/m2, presented that there was no significant difference of diabetes control between BMI < 35 and of 35 or more, which is similar to our finding. But the study did not show whether the baselines of pancreatic function were comparable between the different BMI groups [11]. Another randomized controlled trial with 5-year follow-up demonstrated that gastric banding (GB) in patients with BMI of 25–30 kg/m2 was significantly superior to medication treatment for T2DM [12]. However, it should be noticed that the final diabetes remission rate at 5 years was only 23%, indicating that GB might be not so effective compared to other bariatric surgical approaches.
In this study, we found comparable T2DM remission between obese class I and obese class II/III groups at both 1- and 3-year follow-ups, confirming the metabolic efficacy of LRYGB in Chinese T2DM patients with BMI of 27.5–32.5 kg/m2. However, despite this similarity, the two groups differed significantly with respect to some important variables associated with diabetes. There was obvious decrease of FCP, 2h-CP, and HOMA-IR levels from the baseline in both groups, indicating reduction of hyperinsulinemia and recovery of insulin sensitivity; however, the 2h-PG (at 12, 24, 36 months) and HbA1c (at 36 months) were significantly lower in the obese class II/III group, showing that patients with higher degree of obesity respond better to the surgery. It is known that the pathogenesis of T2DM involves an initial insulin resistance, followed by a compensatory increase in insulin secretion, with progressive beta cell dysfunction occurring due to chronic insulin resistance. Thus, in the early stage of T2DM, the major cause of hyperglycemia is insulin resistance. Bariatric surgery affects the whole endocrine regulation network and one important outcome is enhancing insulin sensitivity. Another observation from clinical practice is that normal BMI T2DM patients show less satisfactory response to bariatric surgery. Therefore, it looks like T2DM patients with severe obesity might present more insult on insulin sensitivity, while patients with nonsevere obesity might experience islet cell function impairment in the early stages of diabetes, which is also a characteristic of eastern diabetes populations who were generally with normal weight or only mildly overweight [13]. This theory that less obesity leads to relatively faster impairment of pancreatic function, and thus results in poorer response to bariatric surgery, needs to be investigated.
Identification of the predictors of response to surgery will be useful for selecting patients for surgery and for improving the risk/benefit profile of the procedure. In this study, log-rank analysis showed higher waist circumference, lower FPG, and higher 2h-CP to be independent predictors of T2DM remission at 1 year after surgery; however, the small sample size and the lack of randomization of patients leave these results open to question. Various clinical predictors of poor response of T2DM to LRYGB have been reported, including age, duration of T2DM, HbA1c level, history of insulin use, and so on [14, 15]. These studies, however, agree that pancreatic beta cell function is one of the most important indicators [16, 17], as our results too showed. Other finding in this study needed to be discussed was that the waist circumference, not BMI, was an independent influencing factor of success of surgery. Obesity phenotypes in the East are very different from those in the West. In the Orient, obese T2DM people often have severe intra-abdominal fat accumulation, which may have a great influence on the functioning of abdominal organs, including the pancreas, and lead to islet function deficiency at an early stage of diabetes [13]. An official survey in China also found that, compared with that in 1993, Chinese adults in 2009 might have a higher metabolic risk due to their significantly increased waist circumference, even if the BMI remained constant [18]. Thus, it seems that waist circumference is a more valuable index than BMI in Chinese patients with metabolic syndrome.
In this cohort, LRYGB had comparable and satisfactory effects on hypertension, dyslipidemia, and hyperuricemia in both groups at the end of 1 year after surgery. At 3 years after surgery, however, a significant difference was seen between the groups; the obese class I group had high recurrence rates of hypertension and hyperuricemia, but the outcomes in obese class II/III patients were still acceptable. In fact, although there is worldwide consensus about the positive effects of bariatric surgery on both weight loss and T2DM, no guideline or statement has recommended bariatric surgery as a primary treatment for other obesity-related comorbidities. In this study, we found that LRYGB has unsatisfactory effects on hypertension and hyperuricemia in mildly obese patients. Other authors have also reported high relapse rates for hypertension [19]. Many studies have reported significant decrease in serum uric acid level after bariatric surgery, but all of these studies were on patients with BMI > 35 kg/m2 [20].
A secondary aim of this study was to assess the weight loss effect of LRYGB in nonseverely obese patients. In this study, the weight loss following surgery was significantly higher in obese class II/III patients. The %EWL was significantly higher in the more obese patients at 12 months after surgery; thus, the difference of BMI between the groups decreased over time, and at the end of 3 years, the mean BMI was comparable between the two groups. This result confirms that bariatric surgery is more effective in heavier patients, which is also the reason why many associations, including the Chinese Society for Metabolic and Bariatric Surgery (CSMBS), recommend bariatric surgery only for patients with BMI ≥ 35 kg/m2 (≥ 32.5 for Chinese patients) and no comorbidities. Nevertheless, in this study, we found that bariatric surgery is safe and acceptable in those with BMI of 27.5–32.5 kg/m2. Excessive weight loss did not occur in our study. The lowest postoperative BMI was 21.9 kg/m2 and no patient developed malnutrition. We did not observe significant differences between the two groups in terms of operation time, hospital days, and complications, indicating that with experience and adequate operation skills, the severity of obesity does not influence surgical safety. There were no deaths or serious surgical morbidity in this study, which is evidence of the safety and efficacy of LRYGB in patients with obesity of any grade.
Our results have broad implications for health policy. Bariatric surgery is currently restricted to patients with BMI ≥ 40 kg/m2 or those with BMI ≥ 35 kg/m2 plus obesity-related comorbidities such as T2DM. Many guidelines or statements do not recommend bariatric surgery for T2DM patients with BMI < 35 kg/m2 [21,22,23]. Others are only in favor of bariatric surgery in nonseverely obese patients when diabetes is poorly controlled [24,25,26,27,28]. Because RYGB typically promotes complete remission of T2DM in severely obese patients [11, 29], and mounting evidence indicates that this results from hormonal and metabolic mechanisms and is not just the consequence of weight loss [30], it is logical to evaluate the use of LRYGB to treat diabetes in less obese patients [31]. Some preliminary studies have reported excellent results in terms of comorbidity resolution after surgery in patients with mild obesity [32, 33]. Bariatric surgery, with a one-time cost of US $7000–9000 in China, is also the more economic option over the long term when compared to medical treatment with its recurring annual costs. Effective surgical treatment does away with the need for patient compliance with regular medication, dietary restrictions, and daily exercise, all of which are very difficult to achieve in most patients [34]. It is time, therefore, to reassess the BMI threshold for bariatric surgery. A decrease in the threshold for LRYGB, to include obese class I patients, would have major implications because a large number of diabetic patients, both in the USA and in China, have BMI in this range [5, 35].
Limitations
Our study has several limitations that have to be considered. The retrospective nature of the study, the lack of random allocation of patients, the small sample size, and the relatively short follow-up all reduce the strength of the evidence. Second, some postoperative variables that could modify metabolic outcomes, such as dietary habits and exercise, have not been taken into account; these factors should be included in future studies. Third, we did not use a standard complication reporting table, such as the US Accordion Classification, to record complications; in addition, complications were not graded according to severity. Fourth, we used FCP, 2h-CP, and HOMA-IR to estimate beta cell function and insulin resistance; future studies should consider using insulin clamp, the gold standard measure, to assess insulin sensitivity more precisely.
Conclusions
In conclusion, the LRYGB procedure is feasible, safe, and effective in this Chinese obese class I population with T2DM. Waist circumference, not BMI, was found to be an independent influencing factor of diabetes remission after surgery. The recurrence rates of hypertension and hyperuricemia at 3 years after surgery were higher in obese class I patients. Further high-quality studies are needed to confirm these preliminary findings.
References
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63.
Du X, Dai R, Zhou HX, et al. Bariatric surgery in China: how is this new concept going? Obes Surg. 2016;26:2906–12.
Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101.
NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–96.
Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59.
Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–5.
Du X, Zhou HX, Zhang SQ, et al. A comparative study of the metabolic effects of LSG and LRYGB in Chinese diabetes patients with BMI<35 kg/m2. Surg Obes Relat Dis. 2017;13:189–97.
Boza C, Munoz R, Salinas J, et al. Safety and efficacy of Roux-en-Y gastric bypass to treat type 2 diabetes mellitus in non-severely obese patients. Obes Surg. 2011;21:1330–6.
Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11.
Wang TT, Hu SY, Gao HD, et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg. 2008;247:968–75.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641–51.
Wentworth JM, Burton P, Laurie C, et al. Five-year outcomes of a randomized trial of gastric band surgery in overweight but not obese people with type 2 diabetes. Diabetes Care. 2017;40:e44–e5.
Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40.
Bruno G, Gruden G, Barutta F, et al. What is the impact of sleeve gastrectomy and gastric bypass on metabolic control of diabetes? A clinic-based cohort of Mediterranean diabetic patients. Surg Obes Relat Dis. 2015;11:1014–9.
Boza C, Valderas P, Daroch DA, et al. Metabolic surgery: roux-en-Y gastric bypass and variables associated with diabetes remission in patients with BMI <35. Obes Surg. 2014;24:1391–7.
Lee WJ, Almulaifi A, Tsou JJ, et al. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surg Obes Relat Dis. 2015;11:991–6.
Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36:20–6.
Stern D, Smith LP, Zhang B, et al. Changes in waist circumference relative to body mass index in Chinese adults, 1993–2009. Int J Obes. 2014;38:1503–10.
Wilhelm SM, Young J, Kale-Pradhan PB. Effect of bariatric surgery on hypertension: a meta-analysis. Ann Pharmacother. 2014;48:674–82.
Peterli R, Borbely Y, Kern B, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258:690–4.
Health. UNIo. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55:615S–9S.
Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24:42–55.
American Diabetes Association. Standards of medical care in diabetes—2016: summary of revisions. Diabetes Care. 2016;39(Suppl 1):S4–5.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Surg Obes Relat Dis. 2011;7:433–47.
Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis. 2013;9:159–91.
National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. Melbourne: NHMRC; 2013.
National Institute for Health and Care Excellence. Obesity: identification, assessment and management. London: NICE; 2014.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861–77.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes. 2009;33(Suppl 1):S33–40.
Rubino F, Kaplan LM, Schauer PR, et al. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251:399–405.
Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420–8.
Lakdawala M, Shaikh S, Bandukwala S, et al. Roux-en-Y gastric bypass stands the test of time: 5-year results in low body mass index (30–35 kg/m(2)) Indian patients with type 2 diabetes mellitus. Surg Obes Relat Dis. 2013;9:370–8.
Paes AH, Bakker A, Soe-Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care. 1997;20:1512–7.
Leibson CL, Williamson DF, Melton LJ, et al. Temporal trends in BMI among adults with diabetes. Diabetes Care. 2001;24:1584–9.
Acknowledgments
The study is an internal teamwork from the Multi-disciplinary Treatment Group of Bariatric and Metabolic Surgery (BMS-MDT) and Gastrointestinal Tract Reconstruction and Metabolic Surgery Association (GIRMSA), West China Hospital, Sichuan University.
Funding
The study is supported by the National Natural Science Foundation of China (81502613), Sichuan Province Science and Technology Support Program (2014SZ0002-5), and Xindu Social Science and Technology Development Project (20160740401).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflicts of interest.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human and Animal Rights
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Research and Ethics Committee of West China Hospital.
Rights and permissions
About this article
Cite this article
Du, X., Fu, Xh., Shi, L. et al. Effects of Laparoscopic Roux-en-Y Gastric Bypass on Chinese Type 2 Diabetes Mellitus Patients with Different Levels of Obesity: Outcomes After 3 Years’ Follow-Up. OBES SURG 28, 702–711 (2018). https://doi.org/10.1007/s11695-017-2903-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2903-3