Abstract
Purpose
Long-term studies on the outcomes of bariatric surgery are still limited in the Middle East. The aim of this study is to compare the outcomes of laparoscopic Roux-en-Y gastric bypass (LRYGB) and sleeve gastrectomy (LSG) up to 5 years of follow-up.
Materials and Methods
A retrospective analysis of patients who underwent LRYGB and LSG was performed. The primary outcome was weight loss. Postoperative complications, operative time, and hospital length of stay were secondary outcomes.
Results
Four hundred patients underwent primary LSG and 175 patients underwent LRYGB between 2008 and 2013. Follow-up rates at 5 years were around 60%. Percent total weight loss was similar after 3, 4, and 5 years in both groups, averaging around 28%. Mean percentage of excess weight loss (%EWL) at 5 years was 72.0 ± 31.0% in the LSG group vs. 63.0 ± 21.0% in the LRYGB group (p = 0.03). Patients undergoing LRYGB had a significantly longer operative time as well as a longer hospital stay. No significant difference was found in the rates of short- and long-term complications between the two groups. However, patients undergoing LRYGB were more likely to develop small intestinal obstruction and iron-deficiency anemia.
Conclusions
Both LSG and LRYGB result in satisfactory weight loss within 5 years. Patients’ comorbidities and potential risks must be included in the choice of the appropriate bariatric procedure. LSG appears to give durable weight loss with less risk of major long-term complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become one of the most challenging threats to public health worldwide. The rates of obesity and utilization of bariatric surgery are on the rise in the Middle East [1]. In 2014, the Gulf Obesity Surgical Society highlighted a 20% annual increase in the demand for bariatric surgery [2].
Laparoscopic Roux-en-Y gastric bypass (LRYGB) dates back almost 50 years ago and is still considered by many the gold-standard operation for morbidly obese patients [3, 4]. However, it is a technically challenging procedure, requiring a long learning curve with a high incidence of long-term complications [5]. Laparoscopic sleeve gastrectomy (LSG), originally intended as a bridging first-step procedure for extremely obese patients awaiting definitive bariatric intervention, has been gaining increasing popularity and is now the leading weight loss surgery worldwide [6]. Recent studies have shown that LSG can be a single, safe, and effective treatment for morbidly obese patients with similar benefits and a lower risk of developing long-term complications compared to LRYGB [6,7,8,9].
Most published studies of bariatric surgery in our region are retrospective and mostly short-term studies with insufficient follow-up. A recent retrospective observational study on the long-term outcomes of LSG at our institution showed satisfactory percentage of excess weight loss (%EWL) and comorbidity resolution after 5 years. The results were excellent for patients with a BMI < 45 kg/m2 [7]. It was important for us to compare those outcomes to LRYGB in our center so that our patients can make informed choices on bariatric surgery options.
The aim of the present study was to reflect on the long-term experience with primary LSG and LRYGB by comparing the safety and efficacy of LSG and LRYGB in morbidly obese patients in our bariatric surgical practice.
Materials and Methods
After institutional review board approval, we conducted a retrospective review of patients who underwent bariatric surgery at the American University of Beirut Medical Center and affiliate hospitals by the senior author (BYS). Up until January 2016, around 1350 total bariatric procedures have been performed at our institution. However, we elected to narrow the time frame to achieve a minimum follow-up of 2 years and limited the age group from 18 to 65 years. We excluded patients who have already undergone any previous bariatric procedure (gastric banding, gastric bypass surgery, sleeve gastrectomy, bilio-pancreatic diversion, etc.) or presented after bariatric surgical complication from another facility. Ultimately, our study cohort included 575 patients who underwent primary LSG or LRYGB between January 2008 and December 2013.
Data
The data collected included patients’ demographics, anthropomorphic information (weight, height, and body mass index (BMI)), presence of medical comorbidities, operative information (operative time and hospital length of stay), weight loss results, and postoperative complications at different time intervals until a maximum of 5 years after the surgery.
Outcomes
The primary outcome was weight loss following LSG and LRYGB which was expressed as change in BMI, percentage of total weight loss (%TWL), and percentage of excess weight loss (%EWL). %EWL was calculated using the formula: \( \frac{\left({\mathrm{initial}}_{\mathrm{weight}}-\mathrm{Follow}\hbox{-} {\mathrm{up}}_{\mathrm{weight}}\right)}{\left({\mathrm{Initial}}_{\mathrm{weight}}-IBW\right)} \) where ideal body weight (IBW) was considered as the weight corresponding to a BMI of 25 kg/m2. Since patients present for follow-up at random time intervals postoperatively, we recorded the weights as follows: Weight at 1 year was considered that of the patient presenting between 7 and 18 months after the operation, whereas weight at 2 years was that between 19 and 30 months, at year 3 between 31 and 42 months, at year 4 between 43 and 54 months, and weight at 5 years was that between 55 and 66 months, whichever was closer to the specified time period. Postoperative complications, operative time, and hospital length of stay constituted our secondary outcomes.
Major and minor complications were defined according to the 2015 American Society for Metabolic and Bariatric Surgery (ASMBS) standardized outcomes reporting guidelines [10]. Improvement and resolution of comorbidities was not an endpoint in this study for two main reasons. First, the retrospective nature of this study and the lack of adequate laboratory testing on most follow-ups precluded the assessment of comorbidity resolution. Second, in our practice, LRYGB is the preferred bariatric procedure for morbidly obese patients with diabetes and gastroesophageal reflux disease while LSG is reserved for “healthier” patients who do not have major obesity-related comorbidities. This selection bias created a notable difference in baseline comorbidities between the two groups, and we felt that comparing comorbidity resolution will not be meaningful give these differences.
Surgical Procedure
Laparoscopic Sleeve Gastrectomy
The procedure was performed under general anesthesia in the supine position. The number of laparoscopic ports ranged from three to four with a Nathanson liver blade to retract the left lateral segment of the liver. The vessels along the greater curvature were sealed and divided with the LigaSure (Covidien, Boulder, CO) all the way to the angle of His and to 3–5 cm proximal to the pylorus. All the retrogastric adhesions were released so that the stomach was quite floppy. A 36–40-French orogastric tube was placed and oriented towards the antrum, and starting around 4 cm proximal to the pylorus, the stomach was stapled and divided along the orogastric tube with an endoscopic stapler. The staple line was then sutured with 2-0 Polydioxanone Suture (PDS) sutures serosa to serosa, and then the orogastric tube was removed. The ports were then removed under direct vision, and the stomach was retrieved through the umbilical incision. The fascia at the umbilicus was closed with PDS, and the skin was closed with absorbable sutures. When a hiatal hernia is identified on pre-operative endoscopy or upon intra-operative dissection, the phreno-esophageal membrane is incised and the gastro-hepatic ligament is opened to expose the right crus of the diaphragm. The peritoneum over the right crus of the diaphragm is incised, and with blunt dissection, a plane is developed behind the distal esophagus which is fully mobilized and encircled with a Penrose drain. The crural defect is generally closed posteriorly with non-absorbable sutures.
Laparoscopic Roux-en-Y Gastric Bypass
All procedures were done under general anesthesia with the patient supine in lithotomy position. Four laparoscopic ports were used in addition to a Nathanson liver retractor to retract the left lateral segment of the liver. The gastric pouch measuring 30 ml was fashioned in most cases using 60-mm blue or purple Endo GIA reloads (Covidien, Boulder, CO). The bilio-pancreatic limb measured 50 to 100 cm. The Roux limb measured 150 cm and was placed in an ante-colic ante-gastric orientation. The gastrojejunal anastomosis was performed in a hand-sewn fashion using two layers of 2-0 PDS over a 36-French orogastric tube. The jejunojejunostomy (JJ) was performed using a single Endo GIA stapler 60-mm white cartridge. The common enterotomy was closed by a single-layer 2-0 PDS suture. The mesenteric defect at the JJ anastomosis was closed with 2-0 Prolene sutures in a running fashion in all patients. The Petersen defect was closed in around 70% of patients, typically over the latter part of the experience.
Statistical Analysis
Analysis was conducted using the R Statistical software version 3.2.2. Continuous variables were represented as mean ± standard deviation (sd) or median (interquartile range) while categorical variables were presented as frequency (percentage). Comparison of means from continuous variables was performed using paired two-tailed Student t test or Wilcoxon rank-sum tests. Comparison of categorical variables was performed using chi-square or Fisher’s exact test as appropriate. The level of statistical significance was set at a p value < 0.05. A multivariate mixed effect model was built using the lmer() function in R (R Foundation for Statistical Computing, Vienna, Austria) [11] to estimate the chances of success (%EWL ≥ 50% at year 5) after controlling for the following fixed effects: follow-up year, type of procedure, age, gender, BMI, and major comorbidities (diabetes, hypertension, dyslipidemia, and gastroesophageal reflux disease (GERD)) and using the patient ID as the random effect. Results were presented as adjusted odds ratio with their 95% confidence intervals (CI).
Results
Five hundred seventy-five patients constituted the study cohort; 400 patients underwent LSG and 175 patients underwent LRYGB between January 2008 and December 2013. Comparison of baseline characteristics showed significant differences between the two groups (Table 1).
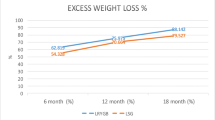
At all time intervals except year 2, the percent total weight loss was similar in both groups averaging 28% (Fig. 1). At 2 years, the %TWL and %EWL were higher in the LSG group; %TWL: 31.7 vs. 28.7%, and %EWL: 83.0 vs. 69.0%, respectively. At 5 years, the %TWL was similar in both groups; however, the mean %EWL was significantly higher in the LSG group (72.0 ± 31.0%) as compared to the LRYGB group (63.0 ± 21.0%, p = 0.03) (Fig. 2).For a BMI less than 45 kg/m2, %EWL was significantly higher in LSG (79.7 ± 30.4%) as compared to LRYGB (67.9 ± 23.6%; p = 0.03) (Fig. 3). After adjusting for the type of procedure, age, gender, BMI and major comorbidities (diabetes, hypertension, dyslipidemia, and GERD), and follow-up year, a BMI ≥ 45 kg/m2 significantly decreased the odds of favorable weight loss (aOR 0.88, 95% CI 0.84–0.92, p < 0.001). An increase of the follow-up year was found to significantly increase the odds of favorable weight loss (aOR 1.12, 95% CI 1.11–1.14, p < 0.001). The random effect variable (patient) had a relatively small variance (0.03) and standard deviation (0.17) (Table 2).
Change in percentage of unadjusted excess weight loss (%EWL) as a function of time. Change of %EWL at 1 to 5 years after LRYGB and LSG, respectively. Total number of followed up patients (T) as well as proportion of follow-up in LRYGB (RY) and LSG (SG) are indicated on the x axis. Abbreviations: SE standard error, %EWL percentage of excess weight loss, RY Roux-en-Y gastric bypass, SG sleeve gastrectomy, T total number of followed up patients
Patients undergoing LRYGB had a significantly longer operative time as compared to those undergoing LSG (154.0 ± 19.1 min. vs. 107.9 ± 20.6 min.; p < 0.0001). Median hospital length of stay was 2 days (IQR 1); however, patients undergoing LRYGB had a significantly longer hospital stay (mean (sd) 2.7 ± 1.5 days vs. 2.4 ± 1.2 days; p = 0.03). Concomitant procedures performed included laparoscopic cholecystectomies, repairs of hiatal, umbilical and incisional hernias, release of adhesions, and one splenectomy (Table 3).
Short-Term Complications
No significant difference was found in the rates of short- (30 days or less) and long-term (more than 30 days) complications between the two groups. The most common major short-term complications in both LSG and LRYGB patients were bleeding; six cases (1.5%) of bleeding were reported in the LSG groups compared to four cases (2.3%) in the LRYGB group. Other major short-term complications were splenic vein and portal vein thrombosis (~1%) observed in LSG patients and postoperative leaks (1.1%) observed among LRYGB patients. Minor short-term complications including anastomotic stricture requiring endoscopic dilation, wound infections, fluid and electrolyte imbalances, C-difficile colitis, allergic reactions, and urinary retention were observed in LSG patients (1.7%) (Table 4).
Long-Term Complications
LRYGB was associated with more major long-term complications (6.3%) as compared to LSG (0.75%, p < 0.001). Small bowel obstruction was observed mainly in LRYGB patients (6.3 vs. 0.25%, p value = 0.0001). All cases of SBO in LRYGB patients were due to internal hernias, whereas in LSG, one patient developed Small bowel obstruction (SBO) from adhesions related to a prior appendectomy. Major long-term complications in LSG included staple line leaks (0.25%) and deep vein thrombosis (0.25%). None of these complications occurred in the LRYGB group. On the other hand, symptomatic gallstones necessitating surgical intervention constituted the most common minor long-term complication. Postoperative cholecystectomy was done in 6.8% of LRYGB patients vs. 10.5% of LSG patients (p = 0.2) at a mean follow-up of 24.4 ± 18.3 months (range, 3.1–68.8 months). Hiatal hernia repair was needed in 2.0% of LSG patients. In addition, incisional hernia repair was needed in 0.5% of LSG patients vs. 1.1% of LRYGB patients (p = 0.39). One case of Wernicke-Korsakoff was reported in an LSG patient due to severe thiamine deficiency. LRYGB had more long-term complications related to nutritional deficiencies mainly iron-deficiency anemia that necessitated intravenous iron replacement (10.8%) (Table 4). The overall rate of reoperation due to major complications such as bleeding, leaks, and small bowel obstruction was 9.7% in LRYGB vs. 2.0% in LSG (p < 0.001).
Discussion
The present study showed comparable percent total weight loss (%TWL) within the first 5 years postoperatively. %EWL was higher in the LSG group at 1, 2, and 5 years, but that is likely due to patient selection bias as our LSG patients tend to be less obese at baseline (Fig. 2). At 5 years of follow-up, %EWL and %TWL were comparable in both groups (LSG 72.0 ± 31.0% and 27.6 ± 10.0% vs. LRYGB 63.0 ± 21.0% and 26.0 ± 7.6%, respectively). Those results are in line with what has been reported by several investigators. Dogan et al. showed a %EWL of 76.0 ± 23.0% in LSG compared to 71.0 ± 20.0% in LRYGB (p = 0.008) after 1-year follow-up; thereafter, no significant difference was observed [8]. Similarly, Kehagias et al. found that LSG resulted in greater %EWL compared to LRYGB up to 2 years, but this difference was not significant at the third year [12].
On the other hand, few comparative studies compared the outcomes of the two procedures in the Middle East. Satisfactory weight loss results and improvement in obesity-related comorbidity after LSG and LRYGB have been reported in many short-term (≤ 2 years) studies in Egypt, the West Bank, United Arab Emirates, Kuwait, Iran, and Saudi Arabia [13,14,15,16,17,18,19,20,21]. The longest retrospective study of 1395 Egyptian patients who underwent LSG as a sole definitive bariatric procedure demonstrated a %EWL of 57.0% at 7 years. Of those, 5.1% had postoperative complications whereas 4.0% had revisional surgeries for insufficient weight loss or severe reflux symptoms [15]. Prospective randomized clinical trials in Egypt have also shown significant weight loss and amelioration of comorbidities at 1 year after LSG done with or without antral preservation [13, 14].
In our cohort, 7 out of 87 patients (8.0%) followed up at 5 years in the LSG arm had a %EWL less than 25.0%, which is considered a failure according to Reinhold’s criteria [22]. In the LRYGB arm, 2 out of 118 patients (1.7%) had poor weight loss results at 5 years (p = 0.03). Fair weight loss results (% EWL between 26.0 and 50.0%) were noted in 10.3% of LSG patients and 11.0% of LRYGB patients followed up at 5 years (p = 0.87). At least three patients in the LSG group underwent further bariatric surgical interventions whereas none of the LRYGB did. Two patients had redo-sleeves at 3.5 and 6 years after LSG, respectively. The third patient had one single-anastomosis gastric bypass 6 years after LSG.
When we stratified the cohort according to BMI less than or greater than 45.0 kg/m2, it was evident that weight loss was better in the LSG group; however, no statistically significant difference was noted between the two procedures for a BMI ≥ 45 kg/m2 (p = 0.72). We chose the BMI cutoff of 45.0 kg/m2 because it was close to the median BMI of both groups. We had previously reported on the long-term outcomes of LSG at 5 years and noted much better weight loss in patients with BMI less than 45 kg/m2 [7]. Similarly, in the present study, increased baseline BMI (BMI ≥ 45 kg/m2) was significantly associated with lower odds of achieving favorable weight loss outcomes irrespective of the weight loss procedure (aOR 0.88, 95% CI 0.84–0.92, p < 0.001) (Table 2).
Overall, no significant difference in the rate of postoperative complications was found between LRYGB and LSG patients in our study population. However, the types of complications differed between the two. For instance, small bowel obstruction, a potentially fatal complication if not immediately treated, was mainly observed after LRYGB (6.3%). Staple line leaks, bleeding, incisional hernias necessitating surgical repair, and cholecystectomy were seen in both procedures. Micronutrient deficiency was not assessed consistently due to the lack of laboratory testing on the majority of follow-ups. However, we noted a markedly higher rate of iron-deficiency anemia in the LRYGB group, and significantly more patients had to receive intravenous iron. This is necessary after RYGB because duodenal exclusion prevents efficient iron absorption, whereas iron deficiency that occurs in LSG patients can respond to oral iron replacement.
Strengths and Limitations
The main strength of our study is that, unlike many of the studies comparing LSG to LRYGB, our study reports on the mid- to long-term outcomes and in a region where data on both procedures is limited. Second, the same surgeon evaluated all patients and performed all the procedures which prevents surgeon-related variability. However, our study has some serious limitations since it is retrospective in nature and has a significant patient selection bias creating different patient groups at baseline. It was clear from the data that LRYGB was favored for patients who were older, heavier, and more likely to have diabetes and GERD. Moreover, the follow-up at 5 years is incomplete (60%), which might provide an optimistic biased overestimation of long-term treatment outcomes. Patients lost at follow-up usually have worse outcomes, mostly related to weight regain, than patients maintaining regular follow-up [23, 24]. Due to the retrospective nature of this study, it is not feasible to verify whether the loss to follow-up at 5 years significantly biased our weight loss outcomes. Nonetheless, this study helped us get a perspective of our bariatric practice over a 5-year period and re-affirmed to us that the LSG procedure is an excellent choice especially for young patients who are less obese (BMI less than 45.0 kg/m2). We still prefer the LRYGB for patients with poorly controlled diabetes, severe GERD, and with higher BMIs (more than 45.0 kg/m2), and have been satisfied with the results so far. There is enough level I evidence in the literature showing the superiority of LRYGB in treating metabolic syndrome when compared to LSG [25, 26]. The relatively high rate of internal hernias and iron-deficiency anemia in LRYGB made us less enthusiastic offering this procedure to women in the child-bearing age since these complications are dangerous and difficult to manage. So, we favor LSG in that patient population regardless of BMI or presence of diabetes.
Conclusion
Both LSG and LRYGB result in satisfactory weight loss within 5 years, and LSG is an excellent bariatric procedure for young patients with BMI lower than 45 kg/m2. The risk of internal hernias, small bowel obstruction, and iron-deficiency anemia requiring intravenous supplementation is higher after LRYGB. Therefore, patients’ comorbidities and potential risks must be included in the choice of the appropriate bariatric procedure.
References
Fahed AC, El-Hage-Sleiman AK, Farhat TI, et al. Diet, genetics, and disease: a focus on the middle east and north Africa region. J Nutr Metab. 2012;2012:109037.
Haskins O. Obesity in the Middle East. Bariatric News. 2014;22:1–2.
Gilbert EW, Wolfe BM. Bariatric surgery for the management of obesity: state of the field. Plast Reconstr Surg. 2012;130:948–54.
Li JF, Lai DD, Lin ZH, et al. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutan Tech. 2014;24(1):1–11.
Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux-en Y gastric bypass and sleeve gastrectomy for the treatment ofmorbid obesity in China: a 5-year outcome. Obes Surg. 2014;24(10):1617–24.
Buwen JP, Kammerer MR, Beekley AC, et al. Laparoscopic sleeve gastrectomy: the rightful gold standard weight loss surgery procedure. Surg Obes Relat Dis. 2015;11(6):1383–5.
Dakour Aridi H, Alami R, Tamim H et al. Long-term outcomes of laparoscopic sleeve gastrectomy: a Lebanese center experience. Surg Obes Relat Dis. 2015.
Dogan K, Gadiot RP, Aarts EO, et al. Effectiveness and safety of sleeve gastrectomy, gastric bypass, and adjustable gastric banding in morbidly obese patients: a multicenter, retrospective. Matched Cohort Study Obes Surg. 2015;25(7):1110–8.
Arman GA, Himpens J, Dhaenens J et al. Long-term (11+years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal refluxtreatment after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016.
Brethauer SA, Kim J, el Chaar M. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11(3):489–506.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria 2016. URL https://www.R-project.org/.
Kehagias I, Karamanakos SN, Argentou M, et al. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI < 50 kg/m2. Obes Surg. 2011;21(11):1650–6.
Abdallah E, El Nakeeb A, Yousef T, et al. Impact of extent of antral resection on surgical outcomes of sleeve gastrectomy for morbid obesity (a prospective randomized study). Obes Surg. 2014;24(10):1587–94.
ElGeidie A, ElHemaly M, Hamdy E et al. The effect of residual gastric antrum size on the outcome of laparoscopic sleeve gastrectomy: a prospective randomized trial. Surg Obes Relat Dis. 2014.
Abd Ellatif ME, Abdallah E, Askar W, et al. Long term predictors of success after laparoscopic sleeve gastrectomy. Int J Surg. 2014;12(5):504–8.
Mahdy T, Atia S, Farid M, et al. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences. Obes Surg. 2008;18(12):1526–31.
Al Zabadi H, Daqour A, Hawari A, et al. Short-term outcomes of laparoscopic sleeve gastrectomy among obesity patients in the northern West Bank: a retrospective records review. BMC Res Notes. 2014;7:85.
Abusnana S, Abdi S, Tagure B, et al. Bariatric surgery outcomes: a single-center study in the United Arab Emirates. Diabetes Metab Syndr Obes. 2015;8:461–71.
Keleidari B, Mahmoudie M, Anaraki AG, et al. Six month-follow up of laparoscopic sleeve gastrectomy. Adv Biomed Res. 2016;5:49.
Toolabi K, Arefanian S, Golzarand M, et al. Effects of laparoscopic Roux-en-Y gastric bypass (LRYGB) on weight loss and biomarker parameters in morbidly obese patients: a 12-month follow-up. Obes Surg. 2011;21(12):1834–42.
Alqahtani AR, Elahmedi MO. Pediatric bariatric surgery: the clinical pathway. Obes Surg. 2015;25(5):910–21.
Reinhold RB. Critical analysis of long-term weight loss following gastric bypass. SurgGynecol Obstet. 1982;155:385–94.
Te Riele WW, Boerma D, Wiezer MJ, et al. Long-term results of laparoscopic adjustable gastric banding in patients lost to follow-up. Br J Surg. 2010;97(10):1535–40.
Puzziferri N, Roshek TB, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42.
Schauer PR, Bhatt DL, Kirwan JP, et al. STAMPEDE investigators. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13.
Shah K, Johnny Nergard B, Stray Frazier K, et al. Long-term effects of laparoscopic Roux-en-Y gastric bypass on metabolic syndrome in patients with morbid obesity. Surg Obes Relat Dis. 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Disclosure
The study was approved by the Institutional Review Board (IRB). For this type of study, the data used is de-identified and informed consents are not required.
Rights and permissions
About this article
Cite this article
Dakour Aridi, H., Khazen, G. & Safadi, B.Y. Comparison of Outcomes Between Laparoscopic Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in a Lebanese Bariatric Surgical Practice. OBES SURG 28, 396–404 (2018). https://doi.org/10.1007/s11695-017-2849-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2849-5