Abstract
Background
Use of a preoperative diet before bariatric surgery to improve postoperative complications and weight loss has been reported. However, evidence supporting this diet for laparoscopic sleeve gastrectomy (LSG) is insufficient. We aimed to investigate postoperative outcomes influenced by preoperative diet before LSG.
Methods
This study included 247 patients who underwent LSG after preoperative weight management. They were classified according to preoperative weight changes (group 1, weight gain; group 2, 0–3.0% total weight loss (TWL); group 3, 3.1–5.0% TWL; group 4, >5.1% TWL) and investigated for early postoperative complications and weight loss at 1 year.
Results
There were 37 patients in group 1, 79 in group 2, 64 in group 3, and 67 in group 4. There were no statistical differences in initial physical status among the 4 groups. The median BMI declined to 27.6 kg/m2 in the entire group. Although the average %TWL during the combined preoperative and postoperative periods showed no statistical differences (P = 0.69), the average %TWL during the postoperative period decreased gradually as the extent of preoperative weight loss increased (P = 0.01). The early postoperative complication rate for the entire group was 6.9%; it tended to be lower as the extent of preoperative weight loss increased. However, a multiple logistic regression model demonstrated that the preoperative diet was not a statistical predictor of reduced early postoperative complications (P = 0.28).
Conclusion
The extent of preoperative weight loss statistically affected postoperative weight loss. A preoperative diet might have minor advantages in reducing the risk of early postoperative complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Morbid obesity and metabolic disorders are serious problems that have grown to epidemic proportions worldwide. Therefore, laparoscopic bariatric surgery has increased over the past decade to 468,609 cases according to the latest global survey [1]. In addition, some trends and regional differences in the types of bariatric procedure exist. For example, sleeve gastrectomy (SG) is most common in North America (43%) and the Asian-Pacific region (49%); however, gastric bypass (GB) is the most common procedure in Europe (38%) [1]. However, laparoscopic sleeve gastrectomy (LSG) has rapidly gained popularity and has become the second most performed procedure in the world because of its technical simplicity and acceptable results. In our center, laparoscopic adjustable banding, gastric bypass, LSG, and LSG with duodeno-jejunal bypass have been performed. Prior to all types of bariatric surgery, all patients are managed with a strict, multidisciplinary, preoperative weight loss program following the risk-reducing preoperative strategies previously reported regarding GB surgery. However, preoperative preparation regarding LSG remains unclear in terms of evidence of surgical benefits and postoperative clinical outcomes. Because LSG is generally considered to be a simpler surgical procedure than GB, the postoperative effects of weight loss are obviously different from those of GB. Therefore, the aim of this retrospective cohort study was to investigate the clinical outcomes after a preoperative diet by investigating body weight loss at 1 year and early postoperative complications.
Methods
This study included all patients who underwent LSG as a standalone procedure at our center during October 2005 through October 2014. All patients were ordered to achieve nearly 3 to 5% preoperative total weight loss (TWL) by participating in a multidisciplinary program that involved nutritional, medical, and physical evaluations. Regarding physical exercise, we encouraged them to increase their physical activities. An expert dietician supervised all patients regarding their energy-restricted diets; the caloric intake was set to 1500 to 1600 kcal/day during the 4 weeks prior to surgery. This was mainly performed in the outpatient clinic. One week before surgery, their progress was evaluated. If we thought that patients could not achieve their goals, then we recommended hospitalization for calorie restriction or a very-low-caloric diet (VLCD) that included 2 to 3 meals per day for 1 week.
Our surgical technique for performing LSG has been described previously [2]. Although some techniques varied during different periods, the staple line was routinely imbricated with a non-absorbable suture for reinforcement to prevent bleeding from the staple line on the remnant stomach.
After surgery, patients were usually discharged on postoperative day 3 if there were no perioperative complications. Patients were followed up by visiting the outpatient clinic at 1 month, 3 months, 6 months, and 1 year, and then annually thereafter. Physical exercise recommended by a physical therapist and detailed dietary counseling by a bariatric dietician were also provided at every visit.
We classified patients according to the extent of their preoperative %TWL achieved: group 1, those who gained weight; group 2, those who lost only 0.0 to 3.0% of their initial weight and failed to achieve what was recommended; group 3, those who lost between 3.1 and 5.0% of their initial weight; and group 4, those who lost 5.1% of their initial weight more than what we recommended. We investigated patient characteristics, visceral and subcutaneous fat, comorbidity frequency, preoperative weight loss, operative complications, and weight loss and %TWL during the period from initial surgery until the last check-up at 1 year, which was defined as the postoperative period (postoperative %TWL), and during the period from the initial physical examination before preoperative weight management until the last check-up at 1 year, which was defined as the overall period (overall %TWL) in the prospectively maintained database. We defined postoperative complications according to Standardized Outcomes Reporting in Metabolic and Bariatric Surgery [3]. Moreover, to subanalyze %TWL at 1 year, we extracted patients who achieved >10% preoperative %TWL from group 4 and investigated the difference in %TWL between them and patients in group 2 during the same periods.
Statistical Analysis
Descriptive results regarding continuous variables were reported as median and interquartile range (IQR). The Kruskal-Wallis test was used to compare demographics and weight loss. Differences in %TWL during the overall and postoperative periods were also investigated using analysis of variance. The χ2 test was used to compare frequency distributions. All tests were two-sided and P < 0.05 indicated a statistically significant difference. The Cochran-Armitage trend test and multiple logistic regression were used to evaluate whether weight changes before LSG were related to early postoperative complications.
Results
Two hundred forty-seven patients underwent LSG; their characteristics are described in Table 1. There were 116 men and 131 women with a median age of 40 years (IQR, 33–48). The median initial body weight and BMI were, respectively, 112 kg (IQR, 97.0–133) and 40.1 kg/m2 (IQR, 37–47). The median preoperative %TWL was 3.8% (IQR, 2.2–5.6) for the entire group. The median weight gain for group 1 (n = 37) was 1.2 kg (IQR, 0.6–2.6). The median preoperative %TWL values were 1.8% (IQR, 1.1–2.3) for group 2 (n = 79), 3.9% (IQR, 3.5–4.4) for group 3 (n = 64), and 6.9% (IQR, 5.7–8.7) for group 4 (n = 67). No statistically significant differences were found among the four groups regarding age, height, preoperative body weight, preoperative BMI, visceral and subcutaneous fat, and comorbidity frequency, except for dyslipidemia. All patients who tested positive for liver damage according to the blood test were found to have fatty liver on abdominal ultrasound examination.
Weight Loss
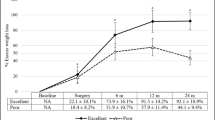
Weight loss amounts for all groups after 1 year are shown in Table 2. The median body weight and BMI declined to 77.2 kg (IQR, 64.6–89.5) and 27.6 kg/m2 (IQR, 24.5–31.7), respectively, without any statistical differences among the four groups. Although %TWL calculated during the overall period showed no statistical differences among the four groups (P = 0.69), the average %TWL during the postoperative period tended to decrease with the increase in the extent of preoperative %TWL (P = 0.01), as shown in Fig. 1. Subgroup analysis regarding the differences in %TWL between group 2 and the group with >10% preoperative %TWL (in which the number of the patients was 10) indicated median initial body weight and BMI of 117 kg (IQR, 106–185) and 46.4 kg/m2 (IQR, 37–68), respectively, with no statistical differences compared to the other groups, thereby revealing a remarkable decrease in %TWL during the postoperative period for the group with >10% preoperative TWL (P < 0.01). However, no statistical difference was recognized during the overall period, as shown in Fig. 2 (P = 0.98).
Early Complications
There was no conversion to an open procedure and no surgical mortality occurred in the study groups. The median intraoperative bleeding and time were 16 g (IQR, 0–50) and 131 min (IQR, 112–154), respectively, without any statistical differences among the four groups. Morbidities within 30 days are summarized in Table 3. Early complications occurred in 17 patients (6.9%); of these, 10 patients required surgical treatments. Postoperative hemorrhage, which was the most common complication in this study, occurred in 10 patients. All patients were required to undergo blood transfusion, except for those with intraluminal and subcutaneous hemorrhages, and five patients with intra-abdominal bleeding required reoperation. Intraluminal hemorrhage was treated with endoscopic clipping, and both instances of subcutaneous bleeding were conservatively treated. Sleeve stenosis occurred in one patient who underwent laparoscopic strictureplasty on postoperative day 19. Leakage occurred in four patients; three of these four required surgical treatment (drainage and re-suturing with omental patch). Pulmonary infection was treated with antibiotics and acute renal failure required additional intravenous fluids. Although the frequency of any surgical complications tended to decrease as the extent of preoperative %TWL increased (group 2, 8.9%; group 3, 6.3%; group 4, 4.5%), a multiple logistic regression model controlled for age, sex, and preoperative BMI for the prediction of early postoperative complications demonstrated that there was no significant difference in the frequency of early postoperative complications with preoperative weight management (P = 0.28; Table 4).
Discussion
To our knowledge, this is the first report to investigate whether preoperative weight management contributes to the extent of weight loss achieved in the short term and to the reduction of surgical complications limited to LSG. Fris et al. reported that preoperative weight loss during the course of 2 weeks had a mean 5.1% impact on the significant decrease in liver size for 50 obese patients [4]. For 32 morbidly obese patients who were managed using a very-low-calorie diet (VLCD), Colles et al. demonstrated that there was a highly significant decrease in visceral and subcutaneous adipose tissue and that liver volume decreased nearly 25% per 5%TWL over the course of 2 weeks [5]. They suggested that the reduction in liver volume was likely to reduce surgical difficulty and blood loss, but no clinical analysis was performed during their study. During SG, visceral fat and hepatomegaly in the left lobe create a limited view and narrow working space, thereby possibly obscuring the view around the His angle and spleen, causing incomplete hemostasis and the inability to identify a hiatal hernia or some gastric folds. One of the causes of poor weight loss after LSG is considered to be the dilation of the gastric tube and consequent increase in the gastric capacity due to incomplete removal of the gastric fundus because of overlooking an obscured hiatal hernia, which could transform into the pseudo-fornix; these technical errors of initial LSG do not decrease the secretion of fasting ghrelin [6, 7]. Moreover, the fact that the high leak point is in the uppermost part of the staple line in the cardial region is commonly known [8]. Therefore, we had thought that preoperative weight management had influenced overall weight loss and the rate of operative complications because we believed that the liver volume and visceral fat must have been different as a result of preoperative weight management. Unfortunately, because we did not measure the liver size just before surgery, we could not prove the statistical advantage of preoperative weight management with the rate of early postoperative complications. Consequently, these results proved that careful maneuvering could technically prevent bleeding due to carelessness and inadequate resection of the fornix during LSG regardless of the narrow view and working space. Regarding weight loss, we thought that long-term observation over 3 years must be more important because secondary dilation of the gastric tube occurs by mechanical stretching due to failure of the patients to follow proper dietary regimens [9, 10].
Alvarado et al. reported a positive correlation of preoperative loss of 1% of initial weight with an increase of 1.8% in postoperative excess weight loss (EWL) after GB surgery [11]. However, in our series, the statistical relationship between preoperative weight loss and postoperative %TWL was not positive in the entire group and there was an inverse relationship regarding the extent of preoperative %TWL increase. In addition, a subgroup analysis also indicated a similar relationship for weight loss during two periods between group 2 and the group with >10% preoperative TWL. Consequently, these results indicated that excessive preoperative weight loss led to less postoperative %TWL, and the extent of preoperative weight loss did not contribute to better weight loss during the overall period. It was unclear in this study whether the causes depended on patients’ compliance, tolerance, or physical status; however, we think that our findings clearly presented that the %TWL with LSG during the combined preoperative and postoperative periods was unvarying and unaffected by preoperative weight loss.
Brethauer et al. reported in their systematic review that the major postoperative complication rates ranged from 0 to 23.8% in all included studies and that the complication rates ranged from 0 to 15.3% in studies with more than 100 patients [12]. Another systematic review by Shi et al. reported that the mean leakage rate and bleeding rate were 1.17% (range, 0 to 5.5%) and 3.57% (range, 0 to 15.8%), respectively [13]. In our series, complication rates (leakage, 1.6%; bleeding, 4.0%) were acceptable compared with these reports.
According to the largest retrospective review of 881 patients who were treated with preoperative weight management [14], increased preoperative weight loss was associated with reduced complication frequencies in the open and laparoscopic GB group. Still et al. also reported that patients who lost 5 to 10% EWL during the preoperative period had a statistically higher probability of shorter hospital stay lengths and more rapid postoperative weight loss with open and laparoscopic GB [15]. Alvarado et al. reported that limited preoperative weight loss with laparoscopic GB contributed to shortening the operative time by 36 min, but no statistical difference was found regarding perioperative complication rates [11]. Nieuwenhove et al. performed a randomized, multicenter study and reported that the preoperative VLCD reduced postoperative complications and surgical difficulty compared with no preoperative dietary restrictions for patients who underwent laparoscopic GB [16]. In our analysis, the frequency of complications indicated a minor advantageous effect of preoperative diet in regard to any surgical complication, but the adjusted odds ratio demonstrated that preoperative weight management was not a statistical predictor of reduced early postoperative complications. There were few negative findings regarding the complication rates for LSG, thus proving that LSG is relatively safer than other procedures.
Our analysis was limited to LSG and demonstrated results contrary to those of previous reports. Should we recommend preoperative weight loss for all LSG candidates? We think that the answer remains controversial because the adjusted odds ratio for the frequency of early postoperative complications showed a tendency for better effects regarding preoperative weight loss. In addition, we believe that the decreasing visceral fat and liver size allows for easier dissection of the stomach around the His angle and sealing of short gastric vessels. Moreover, the current major complication rate of bariatric surgery is less than 3%; complications tend to occur in older patients, those with BMI >50 kg/m2, men, and those undergoing advanced bariatric surgery [17, 18]. Similar to previous reports, our study showed that perioperative complications occurred more often in men. Therefore, the necessity for preoperative weight loss for LSG should be assessed not only for body weight and BMI but also for liver volume, visceral fat, and sex.
Conclusion
The extent of preoperative weight loss statistically affected postoperative %TWL at 1 year, but the overall %TWL including the initial weight-reducing management before surgery was not statistically different. Preoperative weight loss may have a minor advantageous effect on reducing early postoperative complications. Therefore, the necessity of preoperative weight loss for LSG should be investigated in more patients with a randomized, prospective trial and multivariate analysis.
Change history
11 February 2019
In Table 2 on p. 2517 the heading for Group 3 should read as follows:
11 February 2019
In Table 2 on p. 2517 the heading for Group 3 should read as follows:
11 February 2019
In Table 2 on p. 2517 the heading for Group 3 should read as follows:
11 February 2019
In Table 2 on p. 2517 the heading for Group 3 should read as follows:
11 February 2019
In Table 2 on p. 2517 the heading for Group 3 should read as follows:
11 February 2019
In Table 2 on p. 2517 the heading for Group 3 should read as follows:
References
Angrisani L, Satonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg. 2015;25:1822–32.
Seki Y, Kasama K, Hashimoto K. Long-term outcome of laparoscopic sleeve gastrectomy in morbidly obese Japanese patients. Obes Surg. 2016;26(1):138–45.
Brethauer SA, Kim J, el Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;11(3):489–506.
Fris RJ. Preoperative low energy diet diminishes liver size. Obes Surg. 2004;14(9):1165–70.
Colles SL, Dixon JB, Marks P, et al. Preoperative weight loss with a very-low-energy diet: quantitation of changes in liver and abdominal fat by serial imaging. Am J Clin Nutr. 2006;84(2):301–11.
Kalinowski P, Paluszkiewicz R, Wróblewski T. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus roux-en-Y gastric bypass-results of a randomized clinical trial. Surg Obes Relat Dis. 2016;13(2):181–8.
Noel P. Marius Nedelcu David, NoccaRevised, et al. sleeve gastrectomy: another option for weight loss failure after sleeve gastrectomy. Surg Endosc. 2014;28:1096–102.
Gagner M, Deitel M, Kalberer TL, et al. The second international consensus summit for sleeve gastrectomy, march 19-21, 2009. Surg Obes Relat Dis. 2009;5:476–85.
Braghetto I, Cort C, Herquiñigo D, et al. Evaluation of the radiological gastric Capacityand evolution of the BMI 2–3 years after sleeve gastrectomy. Obes Surg. 2009;19(9):1262–9.
Lauti M, Kularatna M, Hill G, et al. Weight gain following sleeve gastrectomy-a systematic review. Obes Surg. 2016;26:1326–34.
Alvarado R, Alami RS, Hsu G, et al. The impact of preoperative weight loss in patients undergoing laparoscopic roux-en-Y gastric bypass. Obes Surg. 2005;15(9):1282–6.
Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5(4):469–75.
Shi X, Karmali S, Sharma AM, et al. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20(8):1171–7.
Benotti PN, Still CD, Wood GC, et al. Preoperative weight loss before bariatric surgery. Arch Surg. 2009;144(12):1150–5.
Still CD, Benotti P, Wood GC, et al. Outcome of preoperative weight loss in high-risk patients undergoing gastric bypass surgery. Arch Surg. 2007;142(10):994–8.
Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, et al. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: a randomized multicenter study. Arch Surg. 2011;146(11):1300–5.
DeMaria EJ, Portenier D. Wolfe l. obesity surgery mortality risk score: proposal for a clinically useful score to predict mortality risk in patients undergoing gastric bypass. Surg Obes Relat Dis. 2007;3(2):134–40.
Sarela AI, Dexter SP, McMahon MJ. Use of the obesity surgery mortality risk score to predict complications of laparoscopic bariatric surgery. Obes Surg. 2011;21(11):1698–703.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in this study.
Grant Information and an Acknowledgment of Grant Support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Watanabe, A., Seki, Y., Haruta, H. et al. Preoperative Weight Loss and Operative Outcome After Laparoscopic Sleeve Gastrectomy. OBES SURG 27, 2515–2521 (2017). https://doi.org/10.1007/s11695-017-2697-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2697-3