Abstract
Purpose
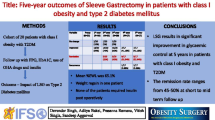
The objective of the study is to evaluate the effects of laparoscopic sleeve gastrectomy (LSG) on mid- to long-term regulation of blood glucose in patients with obesity and type 2 diabetes mellitus (T2DM)
Materials and Methods
In this prospective and observational single-center study, a total of 234 patients with obesity and a diagnosis of T2DM who underwent LSG between 2015 and 2020 were evaluated. The demographics and laboratory data, consisting of body mass index (BMI), glycosylated hemoglobin (HbA1c%), and fasting plasma glucose (FPG) and total weight loss (TWL%), were compared preoperative and postoperative at 12th and 18th months and annual follow-up for seven consecutive years.
Results
The mean age of 234 patients (female(n)/male(n):191/43) included in the study was 44.69±9.72 years, while the preoperative mean BMI, FPG, and HbA1c values were 47.9±6.82, 132.09±42.84 mg/dl, and 7.02±1.35% respectively. The mean rate of weight loss (TWL%), which was 34.7 in the 18 months, decreased to 23.15 in the 7th year. While the HbA1c % value was 7.02±1.35 in the preoperative, it was found 5.71 ± 0.75 (p<0.001) and 6.30 ± 1.77 (p<0.05) at the 18th month and 7th year after the operation, respectively. While the DM remission rate was 71.1% at the postoperative 18th month, it was 45.4% at the 7th year, despite the patients regaining weight in the follow-ups.
Conclusions
Our study revealed that LSG resulted in high remission rates that continued for 7 years after the surgery, although sustained improvement or remission of diabetes despite some weight regain after the first 18 months.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, a global health problem, is long been associated with common comorbid conditions, such as hypertension (HT), type 2 diabetes mellitus (T2DM), and dyslipidemia [1, 2]. The number of bariatric procedures is increasing rapidly worldwide for several years, as they provide significant and long-term weight loss [3]. Laparoscopic sleeve gastrectomy (LSG), which has become the most common bariatric procedure in recent times, is a mainly restrictive surgery. Due to the inherently restrictive nature of the procedure, the body mass index (BMI) is affected by the highly limited calorie intake. The remission of diabetes is not only caused by restrictive properties but also due to several metabolic mechanisms after LSG. The optimal homeostasis of glucose metabolism after LSG is provided by the combined effects of durable weight loss, gut microbiota alterations, reduction in insulin resistance, and variation in the hormone secretions, such as leptin, glucagon-like peptide 1 (GLP-1), ghrelin, glucose-dependent insulinotropic peptide (GIP), and peptide-YY (PYY) [4, 5]. Metabolic surgery benefits from the insulinomimetic effect, mainly GLP-1 and GIP, that are secreted from the epithelium of the small intestine in response to circulating glucose concentrations and cause pancreatic beta-cells to secrete glucose-dependent insulin. Moreover, glucagon production in pancreatic alpha cells is inhibited by GLP-1. It is observed that both the basal and the stimulated GLP-1 levels were increased following LSG. It is shown that GIP levels do not change after LSG. The early phase insulin response, which is impaired in patients with diabetes, improves after LSG [6, 7].
Ghrelin, produced by neuroendocrine P/D1 cells in oxyntic glands in the fundus and body of the stomach, is a molecule associated with metabolic syndrome and obesity. Additionally, ghrelin not only boosts appetite but also stimulates the hypothalamic neuropeptite Y that leads to orexigenic effects. LSG reduces effect on the fasting ghrelin levels of post-prandial suppression, even in case of weight loss [8]. Follow-up studies of LSG demonstrate a durable decrease in serum ghrelin concentrations [9].
In this study, we aimed to evaluate the mid- to long-term effects of LSG on the regulation of blood glucose in patients with obesity and T2DM.
Materials and Methods
Study Population
In this prospective and observational study, the medical records of patients with T2DM (DM), who had LSG for obesity management in the University of Health Sciences, Fatih Sultan Mehmet Training and Research Hospital, between January 2015 and January 2020, were evaluated. The study, which was in accordance with the Declaration of Helsinki, was approved by the institutional ethics committee. Informed consent was obtained from the patients.
Study Protocol
Inclusion criteria were age ≥ 18 years and preoperative diagnosis of T2DM. Qualification criteria for the bariatric procedure were body mass index (BMI) equal or greater than 35 kg/m2. DM was considered in patients with HbA1c and FPG levels of ≥6.5% and ≥126 mg/dl. LSG operation was performed on all patients included in the study [10, 11]. The patients whose follow-up period was less than a year were excluded. The study protocol is shown in the flow chart (Fig. 1).
The demographical and clinical data consisting of age, basal height, and weight were noted for patients. We contacted the patients whose contact data we had and who did not come for regular follow-up by phone, and invited them to the hospital to come for the examination. Laboratory test results that included fasting plasma glucose (FPG) and HbA1c at preoperative and postoperative at 12th month and 18th month and then annual follow-up visits up to 7 years were retrieved from the medical records. FPG and HbA1c levels were measured in all postoperative controls of patients who were diagnosed with diabetes before the operation. The response of patients who were questioned for the medication use for diabetes (oral antidiabetics [OAD] or insulin) at each annual follow-up visit was recorded.
The achievement of complete remission of diabetes was accepted when the levels of HbA1c and FPG levels were below 5.7% and 100 mg/dl, respectively, in addition to no antidiabetic therapy by the American Diabetes Association (ADA) [12].
The efficiency of LSG intervention was assessed using the BMI, postoperative weight, and the ratio of total weight loss (TWL%) as previously described [13]. BMI was calculated using the formula: BMI=kg/m2. TWL% was calculated using the formula: (surgery weight − follow-up weight/surgery weight) × 100.
Surgical Procedure
LSG was performed using the five-trocar technique. The whole greater curvature from approximately 5 cm proximal to the pylorus to the angle of His was dissected near the stomach. The left crus was used as a landmark for dissection. A 36-Fr gastric calibration bougie was inserted for calibration. The gastric resection was performed with an endoscopic staple device.
Statistical Analysis
The statistical analyses were conducted using SPSS Statistics 22.0 (SPSS IBM, Turkey). The Shapiro-Wilks test was used to control the normal distribution of data. Descriptive statistics were expressed with mean, median, standard deviation, and frequency values. Spearman’s correlation analysis was used for correlation analysis. A p-value below 0.05 was accepted for the significance threshold.
Results
The prospective review of patients who met the defined criteria resulted in the inclusion of 234 patients in the study. The preoperative characteristics of the patients are shown in Table 1.
During the 7-year follow-up of diabetic patients, the mean TWL% values that were higher in the first 18 months started to decrease as the reduction in weight loss ratio after the 18th month ultimately resulted in weight gain in patients (18th month–7th year mean TWL% 34.7–23.15) (Table 2).
While the HbA1c% value was 7.02±1.35 in the preoperative period, at the 1st year, 18th month, 2nd, 3rd, 4th, 5th, 6th, and 7th years, respectively, it was determined as 5.84 ± 0.6, 5.71 ± 0.75, 5.78 ± 0.85, 5.98 ± 0.72, 6.03 ± 0.77, 6.01 ± 0.77, 6.19 ± 1.05, and 6.30 ± 1.77. The HbA1c% value obtained in all follow-up periods in the first 5 years postoperatively was found to be p<0.001 when compared with the preoperative period. It was determined that the statistical significance decreased as p<0.01 at the postoperative 6th year and p<0.05 at the 7th year (Table 2).
In the postoperative follow-ups, after the 18th month, with the decrease in the rate of weight loss and the patients starting to gain weight again, HbA1c% values started to increase, and a strong negative correlation was found between weight loss and HbA1c% levels in the correlation analysis (p: 0.001 and r : −929).
While the rate of complete remission was 71.1% in the first 18 months when weight loss was highest, this rate decreased to 45.4% in the 7th year with the weight gain of the patients. The rate of patients who received treatment for DM in the preoperative period was 91.5%. While 18.4% of these patients received insulin-based therapy, the rate of patients using single OAD was 50.9% and the rate of patients receiving 2 or more OAD was 22.2%. At the postoperative 7th year, the rate of patients receiving diabetes treatment was 36.4%, the rates of patients receiving insulin therapy, using single OAD and using 2 or more OAD, were 6.1%, 24.2%, and 6.1% respectively. In the 7th year, it was determined that the rates of insulin use and the rates of patients using 2 or more OAD decreased, and the rates of patients receiving single OAD increased (Fig. 2).
The follow-up rates of the patients are shown in Table 3. The lowest follow-up rates were 19% at the 18th month, and the highest was 54% at the 5th year. At 7 years, 38% of the patients were followed up (Table 3).
Discussion
The treatment of obesity viaLSG provides additional improvements for the many endocrinologic comorbidities observed in obesity, such as diabetes. There are few studies showing the long-term effects of LSG on diabetes. Our study of a single center shows 7-year results of LSG on glucose metabolism related to diabetes. In our study, we observed the highest rate of DM remission/weight loss (median) (kg) (%71.1/41) in diabetic patients in the 18th month after LSG. It was observed that the rate of weight loss and the remission rates of diabetes decreased in the subsequent years. In the 2nd, 3rd, 4th, 5th, 6th, and 7th years, the remission rate/weight loss (median) (kg) were determined as %60.49/39, %47.12/35.5, %41.25/32, %40.86/32, %42/27, and %45.45/19.63, respectively. In the literature, diabetes remission rates after LSG differ in the short and mid term. The DM remission rates in the 1st, 3rd, and 5th years after LSG were 78.5%, 71.4%, and 66.6%, respectively, in the study of Misra et al. [14]. In a study by Gissey et al. on 182 diabetic patients with obesity who were followed for 10 years after LSG, the DM remission rate was found to be 64.7% [15]. In another study on 88 diabetes patients, who had LSG and 1-year follow-up, the complete remission rate was 87% in patients who initially under insulin therapy and 98% in patients who were initially using OAD [16]. In a multicenter study by Mizera et al. [17], the remission rates in the 5th after LSG was found %46. We also observed that the patients started to gain weight after the first 18 months after the operation. We hypothesize that the decrease in diabetes remission rates was associated with weight gain. Factors such as behavioral problems related to nutrition, refusing to follow-up, orientation to high-calorie foods, impaired motivation, and the pathophysiological mechanism underlying obesity and its chronic nature may cause the patient to regain weight in the postoperative long term. The decrease in the effect of late incretin may also decrease the beneficial effects of LSG on diabetes. Lemanu et al. [18] observed that the rate of weight loss compared to baseline after LSG decreased as the duration increased during the 5-year follow-up period. However, the improvement in comorbid conditions continued in their study with a reported DM remission rate of 79%.
Although an important goal is the achievement of permanent weight loss via LSG, the success of the operation is additionally determined by its positive effects on the metabolic parameters. In our study, weight loss ratios in diabetic patients were similar to the previous study results in the literature [19,20,21,22]. In the studies in the literature, it has been shown that as the postoperative follow-up period increases, the patients begin to gain weight [20, 21]. Our study, in diabetic patients, the TWL% was 33.50 ± 8.05, 34.31 ± 8.44, 30.71 ± 10.54, 29.69 ± 9.85, 26.74 ± 10.49, 25.32 ± 9.46, 21.28 ± 11.14, and 23.75 ± 14.51 at 1st year, 18th month, 2nd, 3rd, 4th, 5th, 6th, and 7th years, respectively. In a study of 218 diabetic patients with obesity, the TWL% was evaluated for 5 years after LSG, and it was shown that weight gain started again after 2 years. The mean TWL% ratios at 1st, 2nd, 3rd, 4th, and 5th years were 33.8, 28.8, 26.6, 18.0, and 15.0, respectively [23].
In our study, the rate of patients using diabetes treatment in the 7th year after the operation was significantly lower than in the preoperative period (91.5–37%). Although the number of drugs used was less at 7 years, HbA1c values were lower than at baseline. We observed that the rates of single drug use increased while the rates of multiple drug use decreased in the postoperative follow-up. The fact that patients have better HbA1c results with less medication indicates that bariatric surgery facilitates glycoregulation. Our results support previous literature in which the effects of physiological changes developing apart from weight loss via LSG were considered to be continuous on achieving control over metabolism.
In addition to the weight loss in the early postoperative period, which occurs as a result of reduced caloric intake due to the restrictive nature of the operation, LSG can be considered an effective therapeutic option for blood glucose regulation independent of the weight loss in patients with diabetes. LSG reduces, sometimes eliminates the need for medical treatment in patients with diabetes due to the decreased ghrelin along with the increased basal and stimulated GLP-1/GIP levels that all together provide an increase in insulin secretion and sensitivity [4,5,6,7,8,9, 24]. In a study by Yang et al. [25], despite similar preoperative characteristics and postoperative weight loss, LSG patients experienced significantly higher rates of medication discontinuation for diabetes than LAGB. These results suggest that LSG may have weight-independent effects on metabolic disease. Schauer et al. [26] reported a randomized prospective trial of 150 patients with obesity and T2DM who received intensive medical therapy with or without LSG. The researchers observed a complete remission in 5% of patients under medical therapy compared to 24% of patients who also had LSG in addition to the medical therapy after a 36-month follow-up period in the 91% patients. It has been determined that patients in the LSG group use glucose-lowering therapeutics, including insulin, less than patients without LSG, and who only receive medical treatment [27]. Schauer et al. [28] reported a randomized prospective trial the 5th postoperative year follow-up results demonstrated the superiority of surgery to medical treatment in the achievement and long-term persistence of diabetes remission.
Our study has certain limitations. First, the patients assessed at each follow-up visit were not always the same patients. Second, some patients could not be followed because they moved to another city. There were patients who left the follow-up because the patient’s contact details could not be reached. Therefore, our follow-up rates were variable. It was thought that admissions to the hospital increased due to the fact that the patients needed support in terms of treatment, with weight regain in the postoperative period. We are aware that this may increase diabetes rates.
Conclusion
In our study with LSG, which is a mainly restrictive procedure, it was observed that patients started to gain weight again after 18 months in the postoperative period. Our study found that LSG results in resolution or improvement in diabetes mellitus with results persisting to 7 years despite weight regain. However, the magnitude of the improvement/resolution decreased with weight regain. Our results support the notion that physiological changes resulting from LSG, independent of weight loss, have a continuous impact on achieving control over metabolism and contribute to diabetes improvement.
References
Grundy SM. Multifactorial causation of obesity: implications for prevention. Am J Clin Nutr. 1998;67(Suppl):563S–72S.
Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–4.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg. 2017;27(9):2279–89. https://doi.org/10.1007/s11695-017-2666-x. Erratum in: Obes Surg. 2017
Thaler JP, Cummings DE. Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150(6):2518–25.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244(5):741.
Melissas J, Daskalakis M. Gastric emptying after sleeve gastrectomy. Obes Surg. 2011;21(11):1810–1.
Melissas J, Koukouraki S, Askoxylakis J, et al. Sleeve gastrectomy-a restrictive procedure? Obes Surg. 2007;17(1):57–62.
Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and post-prandial Ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–7.
Bohdjalian A, Langer F, Shakeri-Leidenmühler S, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and Ghrelin. Obes Surg. 2010;20(5):535–40.
Seeras K, Sankararaman S, Lopez PP. Sleeve gastrectomy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
Benaiges D, Flores Le-Roux JA, Pedro-Botet J, Chillarón JJ, Renard M, Parri A, Ramón JM, Pera M, Goday A. Sleeve gastrectomy and Roux-en-Y gastric bypass are equally effective in correcting insulin resistance. Int J Surg. 2013;11(4):309–13.
Riddle MC, Cefalu WT, Evans PH, Gerstein HC, Nauck MA, Oh WK, Rothberg AE, le Roux CW, Rubino F, Schauer P, Taylor R, Twenefour D. Consensus report: Definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438–44. https://doi.org/10.2337/dci21-0034.
Bray GA, Bouchard C, Church TS, et al. Is it time to change the way we report and discuss weight loss? Obesity (Silver Spring). 2009;17(4):619–21.
Misra S, Bhattacharya S, Saravana Kumar S, Nandhini BD, Saminathan SC, Praveen RP. Long-term outcomes of laparoscopic sleeve gastrectomy from the Indian subcontinent. Obes Surg. 2019;29(12):4043–55.
Gissey LC, Casella JR, Mariolo AG, et al. 10-year follow-up after laparoscopic sleeve gastrectomy: outcomes in a monocentric series. Surg Obes Relat Dis. 2018;14(10):1480–7.
Mihmanli M, Isil RG, Bozkurt E, Demir U, Kaya C, Bostanci O, Isil CT, Sayin P, Oba S, Ozturk FY, Altuntas Y. Post-operative effects of laparoscopic sleeve gastrectomy in patients with extremeobesity with type 2 diabetes. Springerplus. 2016;22(5):497.
Mizera M, Wysocki M, Bartosiak K, Franczak P, Hady HR, Kalinowski P, Myśliwiec P, Orłowski M, Paluszkiewicz R, Piecuch J, Szeliga J, Walędziak M, Major P, Pędziwiatr M. Type 2 diabetes remission 5 years after laparoscopic sleeve gastrectomy: multicenter cohort study. Obes Surg. 2021;31(3):980–6. https://doi.org/10.1007/s11695-020-05088-w.
Lemanu DP, Singh PP, Rahman H, Hill AG, Babor R, MacCormick AD. Five-year results after laparoscopic sleeve gastrectomy: a prospective study. Surg Obes Relat Dis. 2015;11(3):518–24.
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–24.
Juodeikis Ž, Brimas G. Long-term results after sleeve gastrectomy: a systematic review. Surg Obes Relat Dis. 2017;13(4):693–9.
Diamantis T, Apostolou KG, Alexandrou A, et al. Review of long-term weight loss results after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(1):177–83.
Neagoe R, Muresan M, Timofte D, Darie R, Razvan I, Voidazan S, Muresan S, Sala D. Long-term outcomes of laparoscopic sleeve gastrectomy - a single-center prospective observational study. Wideochir Inne Tech Maloinwazyjne. 2019;14(2):242–8.
Hans PK, Guan W, Lin S, Liang H. Long-term outcome of laparoscopic sleeve gastrectomy from a single center in mainland China. Asian J Surg. 2018;41(3):285–90.
Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24(5):1005–10.
Yang P, Bonham AJ, Ghaferi AA, Varhan OA. Comparing diabetes outcomes: weight-independent effects of sleeve gastrectomy versus matched patients with similar weight loss. Ann Surg. 2022;275(5):924–7. https://doi.org/10.1097/SLA.0000000000004298.
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in patients with obesity with diabetes. N Engl J Med. 2012;366(17):1567–76.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370:2002–13.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR, Investigators STAMPEDE. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Acknowledgements
Seda Sancak, as principal investigator, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
To conduct the study, institutional review board approval was obtained. The study was carried out in accordance with the Declaration of Helsinki (2013) of the World Medical Association. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points:
• Obesity and diabetes mellitus are a global health problem.
• Metabolic surgery may be an option for non-responsive diabetic obese patients.
• Metabolic surgery in diabetic obese patients aids weight loss and metabolic control.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çeler, Ö., Er, H.C., Sancak, S. et al. The Effects of Laparoscopic Sleeve Gastrectomy (LSG) on Obesity-Related Type 2 Diabetes Mellitus: a Prospective Observational Study from a Single Center. OBES SURG 33, 2695–2701 (2023). https://doi.org/10.1007/s11695-023-06707-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06707-y