Abstract
Background
Gastrointestinal bypass changes the gut microbiota and decreases systemic endotoxemia in obese subjects. Epithelial barrier integrity is crucial for confining enteric bacteria in the lumen and preventing gut-derived endotoxemia. The effect of bypass surgery on intestinal barrier functions remains poorly understood. This study aimed to evaluate the changes in intestinal permeability and gut barrier between rats receiving Roux-en-Y duodenojejunal bypass (DJB) or sham operation (SO).
Methods
Eighteen Sprague–Dawley rats were assigned to DJB or SO groups. Tissues of the alimentary, biliopancreatic, and common limbs in the small intestine, and the colon, were collected 2 weeks after operation. Mucosa-associated bacteria were quantified by colony forming units. Intestinal permeability was determined by mucosal-to-serosal dextran flux measured in Ussing chambers. Expression of occludin and proliferating cell nuclear antigen (PCNA) in the intestinal mucosa was examined by western blots.
Results
Enteric bacterial numbers were increased in the alimentary and common limbs after DJB. Reduced dextran permeability was found in the alimentary limb, common limb, and colon after DJB. Moreover, increased villus height and crypt depth were found to be associated with higher mucosal levels of occludin and PCNA levels in the alimentary and common limbs after DJB.
Conclusions
DJB in rats altered gut microbiota and reduced intestinal permeability due to increased epithelial proliferation and tight junctional protein expression. Our results show that bypass surgery led to fortification of the intestinal barrier functions, which may provide an explanation for the decreased risk of systemic endotoxemia in postoperative patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gastrointestinal tract is the largest reservoir of microorganisms in the human body. Physical, chemical, and immune barriers of the gastrointestinal tract prevent bacterial dissemination and invasion of the systemic bloodstream and other viscera [1]. The intestinal epithelial barrier is formed by intercellular tight junctions that determine gut permeability and is maintained by dynamic turnover of the crypt-villus axis. Abnormal intestinal permeability may predispose the host to gut-derived endotoxemia.
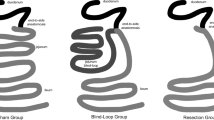
Recent studies have demonstrated increased intestinal permeability, systemic endotoxemia, and inflammation in obese subjects and diabetic patients [2–6]. Bypass surgeries are clinically applied in these patients, showing numerous benefits postoperatively. Roux-en-Y gastric bypass (RYGB), one of the most popular bariatric surgeries, sustains long-term weight loss in severely obese subjects. Following changes of the anatomical structures of the gastrointestinal tract, RYGB alters the gut microbiota [7–9] and reduces systemic endotoxemia [10, 11]. Duodenojejunal bypass (DJB) (Figure S1) and duodenojejunal bypass with sleeve gastrectomy are novel metabolic surgeries for type II diabetes mellitus [12–14]. Low-grade inflammation is a common feature in diabetic patients [15]. Although there are still relatively few clinical reports, improvement of diabetes after DJB or DJB with sleeve gastrectomy may also be accompanied by reduced systemic inflammation. These findings suggest that bypass surgery might change intestinal permeability. To date, limited reports have focused on the relationship between bypass surgery and intestinal permeability, and the results of these reports are still controversial [16–18].
To explore the effect of bypass on intestinal permeability, we chose a surgery model of DJB over RYGB for the following reason. RYGB restricts the volume of food intake by limiting gastric accommodation, but it also impacts on gastric secretion of enzymes, hormones, and acid. Hence, changes in pathophysiology after RYGB might be due to the combined effects of volume restriction, malsecretion, and malabsorption. Because DJB represents a pure intestinal bypass procedure without reduction of gastric volume and secretion, we used a DJB model to investigate the effects of bypass surgery on intestinal permeability and to explore possible mechanisms underlying the changes in epithelial barrier function.
Materials and Methods
Experimental Animals
Eighteen male Sprague–Dawley rats received water and standard chow (5001, LabDiet, St. Louis, MO, USA) ad libitum after birth. At 10 weeks of age, rats were randomly allocated to DJB or sham operation (SO) groups. For healing of the surgical anastomoses, rats were not allowed to eat and had access only to water, for 24 h after surgery. Thereafter, standard chow and water were available ad libitum for 2 weeks after the operation. All rats were housed under a 12/12-h light–dark cycle at room temperature (21 ± 2 °C). The investigation was approved by the Institutional Animal Care and Use Committee in National Taiwan University. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Surgery
After overnight fasting, rats were anesthetized with isoflurane (5 % for induction and 2–3 % for maintenance). Under sterile conditions, an upper midline laparotomy of approximately 4 cm was made. For the DJB group, the proximal duodenum was divided, and the distal duodenal stump was closed with 5-0 vicryl (Ethicon, Somerville, NJ, USA) by hand-sewn suture. The small bowel was divided to create a 30-cm biliopancreatic limb, a 35-cm alimentary limb, and then a common limb. The duodenojejunostomies and jejunojejunostomies were performed with interrupted sutures, followed by abdominal closure. For SO rats, a duodenostomy over the proximal duodenum was made and then re-closed. A transection of the jejunum at 35 cm and a jejunostomy at 65 cm below the ligament of Treitz were also made, and re-anastomoses and re-closures were completed. All rats received similar preoperative and postoperative care.
Euthanasia was performed at 12 weeks of age. Fasting blood samples were collected after euthanasia. Dipeptidyl peptidase-4 inhibitor was added to the blood samples to prevent the degradation of glucagon-like peptide-2 (GLP-2). Intestines, including the proximal alimentary limb, proximal biliopancreatic limb, distal common limb, and proximal colon in DJB animals, and anatomically similar regions in SO animals were dissected for analyses.
Detection of Serum LPS, LBP, and GLP-2 Concentration
Lipopolysaccharide (LPS) is one of the major components in the gram-negative bacterial cell wall [19]. LPS binds with lipopolysaccharide binding protein (LBP) in blood and then induces systemic immune responses [20]. Recent studies show that LPS and LBP could both serve as effective clinical markers of endotoxemia [10, 20–22]. Serum LPS levels were measured using the limulus amebocyte lysate (LAL) test (Associates of Cape Cod, East Falmouth, MA, USA), and serum LBP and GLP-2 concentrations were measured using the relevant enzyme-linked immunosorbent assay kits (Biometec, Greifswald, Germany for LBP; BioVendor, Brno, Czech Republic for GLP-2) for rats according to the manufacturers’ instructions.
Quantification of Mucosa-Associated Bacteria in Intestines
Intestinal segments of the alimentary limb, biliopancreatic limb, common limb, and colon were excised using aseptic techniques. Tissues were prepared as previously described [23, 24]. The tissue homogenates were cultured at 37 °C overnight on fresh blood agar (Scientific Biotech, Taiwan) to examine the growth of total bacteria. The bacterial colony-forming units (CFUs) were calculated and normalized to log10 of CFUs per gram of the intestinal tissue (log CFU/g).
Ussing Chamber Studies and Intestinal Permeability Assay
Fresh intestinal segments of the alimentary limb, biliopancreatic limb, common limb, and colon were excised. The external muscle layers were stripped off, leaving the submucosal plexus and mucosa intact. The muscle-stripped tissues were mounted and managed in Ussing chambers (WPI Instruments, Sarasota, FL, USA) according to the Ussing chamber and macromolecular flux assay protocol previously reported [23, 25, 26]. The opening area (2 cm2) of the chamber exposed the tissue to 5 ml of circulating oxygenated Krebs buffer (115 mmol/L NaCl, 8 mmol/L KCl, 1.25 mmol/L CaCl2, 1.2 mmol/L MgCl2, 2.0 mmol/L KHPO4, 25 mmol/L NaCO3, pH 7.33–7.37). The serosal buffer contained 10 mmol/L of glucose that was osmotically balanced with 10 mmol/L of mannitol in the mucosal buffer. A circulating water bath maintained the temperature of the buffer at 37 °C. The tissues were clamped at 0 V using a voltage clamp. The potential difference (PD, mV) and the short-circuit current (Isc, μA/cm2) of the tissues were determined on line to verify tissue viability. Intestinal permeability was determined by the level of mucosal-to-serosal flux of dextran conjugated to fluorescein isothiocyanate (dextran-FITC, molecular weight = 4 kDa; Sigma, St. Louis, MO, USA). The dextran probe was added to the mucosal buffer at a final concentration of 500 μM. Samples (250 μL) of serosal buffer were collected at 0, 30, 60, 90, and 120 min after addition of dextran probe and were replaced with Krebs buffer/glucose. The fluorescence units of dextran-FITC in serosal buffer were determined at ex/em = 490/530 nm using a multi-mode plate reader (Beckman Coulter Paradigm, USA), and the concentration (nM) was calculated according to a standard curve.
Histopathology
Intestinal segments of the alimentary limb, biliopancreatic limb, and common limb were collected, fixed in 4 % paraformaldehyde, and embedded in paraffin wax with proper orientation of the crypt-villus axis. Sections of 4 μm thickness were deparaffinized and stained with hematoxylin and eosin. Histologic structures were observed by a light microscope.
Western Blotting
Scraped intestinal mucosa of the alimentary limb, biliopancreatic limb, and common limb were prepared as previously described [25, 27]. Western blotting with anti-occludin (1:3000; Invitrogen, Carlsbad, CA, USA), anti-proliferating cell nuclear antigen (PCNA; 1:2000; Cell Signaling Technology, Danvers, MA, USA), and anti-β-actin (1:50000; Genetex, Irvine, CA, USA) were performed. Band density was quantified by photoimage analysis.
Statistical Analyses
All results were expressed as mean ± SEM, except that bacterial CFU numbers were presented as median values. The means were compared by Student’s t test. When comparing the bacterial CFU values that failed to satisfy the normality assumption, the data was compared by nonparametric Mann–Whitney U test. A p value <0.05 was considered significant.
Results
The preoperative and postoperative body weights, and postoperatively circulating biological characteristics, are shown in Table 1. The preoperative body weight, postoperative circulating LPS, and LBP levels did not differ between rats in the DJB and SO groups. The postoperative body weight of DJB rats appeared to be lower than that of SO rats, although this was not statistically significant. In contrast, the postoperative circulating GLP-2 concentrations of DJB rats were significantly higher than those of SO rats.
Enteric bacterial numbers were significantly increased in the alimentary limb and common limb after DJB compared with SO rats. In contrast, there was no difference of total bacterial contents in the biliopancreatic limb and common limb between DJB and SO groups (Fig. 1).
Enteric bacterial numbers were increased in the alimentary limb and common limb after DJB compared with SO. The CFU results are plotted as box and whisker graphs. The boxes (containing 50 % of all values) show the median (horizontal line across the middle of the box) and interquartile range, while the whiskers represent the 10th and 90th percentiles. The extreme data are indicated by circles. DJB duodenojejunal bypass, SO sham operation, CFU colony-forming unit. *p < 0.05
Concerning intestinal permeability, the mucosal-to-serosal dextran-FITC fluxes were lower in the alimentary limb, common limb, and colon after DJB compared with SO rats (Fig. 2). Furthermore, the expression levels of epithelial tight junctional occludin in the mucosa of alimentary limb and common limb of DJB were higher than in those of SO (Fig. 3).
Intestinal permeability was decreased in the alimentary limb, common limb, and colon after DJB compared with SO. The mucosal-to-serosal dextran flux in Ussing chambers was measured at 0, 30, 60, 90, and 120 min in the alimentary limb (a), biliopancreatic limb (b), common limb (c), and colon (d). Values are expressed as mean ± SEM. DJB duodenojejunal bypass, SO sham operation. *p < 0.05; **p < 0.01
With respect to microscopic morphology, the villus height and crypt depth were both increased in the alimentary limb and common limb after DJB compared with SO (Fig. 4). DJB rats also had higher mucosal levels of PCNA in the alimentary limb and common limb (Fig. 5).
Villus height and crypt depth were both increased in the alimentary limb and common limb after DJB compared with SO. (a) Micrographs of intestinal section (hematoxylin and eosin staining, ×100). (b) Villus height. (c) Crypt depth. Values are expressed as means ± SEM. Bar indicates 100 μm. DJB duodenojejunal bypass, SO sham operation. *p < 0.05; **p < 0.01
Increased levels of PCNA in the intestinal mucosa of the alimentary limb and common limb of rats which received DJB compared with SO. (a) Western blotting images. (b) Results of densitometric analysis. Values are expressed as means ± SEM. PCNA proliferating cell nuclear antigen, DJB duodenojejunal bypass, SO sham operation. *p <0.05; **p <0.01
Discussion
In this study, we have shown that DJB in rats alters gut microbiota, reduces intestinal permeability, and induces mucosal hypertrophy (Table S2). In rats receiving DJB, the small intestine was surgically manipulated into three discrete sections which may each contribute to distinct local responses of gut microbiota, intestinal permeability, and intestinal epithelial turnover. One of the differences among the alimentary limb, biliopancreatic limb, and common limb between the DJB and SO treatments is the exposure to undigested nutrients and biliopancreatic enzymes. The macronutrients, bile, and pancreatic juice may therefore play an important role in the pathophysiologic changes of gut bacteria, intestinal permeability, and intestinal adaption.
Recent studies describe changes of intestinal microbiota in obese humans, and in patients with diabetes mellitus. Obesity is associated with higher Firmicutes and lower Bacteroides in comparison with lean subjects [28–30]. Furthermore, a metagenome-wide association study showed type II diabetic patients have gut microbial dysbiosis with a decrease in butyrate-producing bacteria and an increase in various opportunistic pathogens [31]. Fecal studies of the gut microbiota in humans and rats after RYGB also reveal alterations of intestinal bacteria [7–9]. Furthermore, Osto et al. confirmed that RYGB in rats induces changes in the microbiota of the alimentary limb and common limb [32]. Although bacterial species were not analyzed in the current study, the total bacteria numbers increased in the alimentary limb and common limb of rats after DJB. The alteration of microbiota after DJB might be due to the effects of relatively lower body weight and intestinal bypass, but the detailed mechanism remains obscure.
Evidence that supports reduced intestinal permeability after DJB is scant. Our data show that intestinal permeability decreased in the alimentary limb, common limb, and colon after DJB, but not in the biliopancreatic limb. Gut permeability is mainly determined by the integrity of paracellular tight junctions, which include transmembrane junctional proteins such as occludin and claudins, junction-associated molecules linked to intracellular zonula occludens, and bridges to cytoskeletal actin and myosin filaments [33]. We found increased expression of occludin in the mucosa of the alimentary and common limbs after DJB, which is consistent with the observation of decreased gut permeability.
DJB rats exhibited significant adaptive intestinal changes with increased villus height and crypt depth in the alimentary and common limbs, indicative of heightened epithelial proliferation. The increased expression levels of PCNA in the common limb after DJB confirmed the increased intestinal proliferation and crypt hyperplasia. Fully differentiated epithelial cells often exhibit high levels of tight junction proteins compared to newly proliferated cells, to fortify barrier functions at the interface with enteric microbes. These findings are similar to the results from a short bowel syndrome rat model receiving RYGB, which also showed increased bowel width, villus height, and crypt depth in the alimentary and common limbs [34]. Strengthening of epithelial barrier function and tight junctions may therefore be an adaptive mechanism attributed to the increase of crypt proliferation and villus growth after DJB.
The different intestinal segments after DJB are exposed to different stimulants. The alimentary limb is exposed to undigested nutrients; the biliopancreatic limb is exposed to bile and pancreatic juice; and the common limb is exposed to a mixture of nutrients and digestive enzymes. Previous studies have shown that macronutrients, including carbohydrate, protein, and fat, stimulate intestinal adaptation [35–39]. Furthermore, the gut microbiota can regulate the proliferation and apoptosis of the intestinal epithelium [1]. In addition to macronutrients and hormones, alteration of the gut microbiota after DJB may therefore be one of the mechanisms inducing intestinal adaptation.
GLP-2, a gut hormone released from enteroendocrine L cells, directly affects the gut mucosa to increase the absorptive surface area. GLP-2 stimulates intestinal mucosal cell proliferation, and then either induces expansion of the normal mucosal epithelium or attenuates intestinal injury [40, 41]. The increased circulating level of GLP-2 after DJB might reflect the increased local function of L cells to modulate hypertrophy of the small intestine.
It is well known that the gram-negative bacterial cell wall product LPS is the main component underlying the pathology of septicemia [19]. A high circulating LPS level can trigger systemic inflammation and thus induce the development of obesity and insulin resistance [42]. LBP is an acute-phase protein, mainly derived from the liver, and is easily detectable in the blood for binding to LPS [20]. Compared with the short half-life of LPS and the technical limitations of its measurement, LBP is relatively stable and easy to measure. Recent reports reveal that LBP could serve as a clinical marker of effective endotoxemia in chronic low-grade inflammation [10, 20–22]. Although the bacteria counts increased in the alimentary and common limbs after DJB, the circulating levels of LPS and LBP were not different between DJB and SO treatments, possibly due to the reduced intestinal permeability. These results indicate that the adaptive mechanism of the gut following DJB may decrease the risk of systemic endotoxemia.
The strengths of current study are to disclose DJB in rats altered gut microbiota and fortified the intestinal barrier, which were due to increased epithelial proliferation and tight junctional protein expression. Table S2 summarizes the changes after DJB compared with those after SO. Nevertheless, our study may have limitations. First, recent studies indicate that obesity is a factor that increases the intestinal permeability via unknown mechanisms [4]. To focus on the modulatory effect of DJB surgery per se on gut barrier, we chose standard rats instead of an obese and/or diabetic rat model. The present results could not simulate the postoperative change in obese subjects. Although there is no significantly statistical difference, the postoperative body weights in DJB rats are relatively lower than those in SO rats. The finding might be due to the short postoperative period and/or the compensatory effect of the increased villus height and crypt depth in the intestine after DJB. As obesity increases endotoxemia [10], the similar weight might be one of the possible mechanisms that there are no differences of the postoperative serum levels of LPS and LBP between DJB and SO. An ongoing project for the change of intestinal barriers in a high-fat diet-induced obese rat model is currently in progress to clarify the postoperative pathophysiologic change in obese subjects. Second, Taqi et al. reported significant adaptive changes with increased villus height and crypt depth 2 weeks after RYGB in a short bowel syndrome rat model [34]. Therefore, we analyze the physiological changes 2 weeks after DJB. The postoperative changes are dynamic and complex. Further works with different postoperative time points would be helpful to elucidate the longitudinal changes after operation.
Conclusions
In summary, decreased intestinal permeability and increased tight junction protein expression were found after DJB in rats. Although bacterial overgrowth was observed in the alimentary limb and common limb after DJB, there was no increase in the circulating LPS levels, most likely due to the intact barrier function of the intestinal epithelium. The increased villus height and crypt depth served as an adaptive mechanism to maintain epithelial barrier integrity after DJB. Our findings suggested that intestinal barrier function was strengthened following bypass surgery, which may play a critical role in the decreased risk of systemic endotoxemia in postoperative patients.
References
Yu LC, Wang JT, Wei SC, et al. Host-microbial interactions and regulation of intestinal epithelial barrier function: from physiology to pathology. World J Gastrointest Pathophysiol. 2012;3(1):27–43.
Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103.
Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35(5 Suppl):14S–20.
Teixeira TF, Collado MC, Ferreira CL, et al. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32(9):637–47.
Jayashree B, Bibin YS, Prabhu D, et al. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388(1-2):203–10.
Horton F, Wright J, Smith L, et al. Increased intestinal permeability to oral chromium (51 Cr)-EDTA in human Type 2 diabetes. Diabet Med. 2014;31(5):559–63.
Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60(9):1214–23.
Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57.
Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–70.
Yang PJ, Lee WJ, Tseng PH, et al. Bariatric surgery decreased the serum level of an endotoxin-associated marker: lipopolysaccharide-binding protein. Surg Obes Relat Dis. 2014;10(6):1182–7.
Monte SV, Caruana JA, Ghanim H, et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after Roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery. 2012;151(4):587–93.
Ramos AC, Galvao Neto MP, de Souza YM, et al. Laparoscopic duodenal-jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI < 30 kg/m2 (LBMI). Obes Surg. 2009;19(3):307–12.
Ferzli GS, Dominique E, Ciaglia M, et al. Clinical improvement after duodenojejunal bypass for nonobese type 2 diabetes despite minimal improvement in glycemic homeostasis. World J Surg. 2009;33(5):972–9.
Lee WJ, Lee KT, Kasama K, et al. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): short-term result and comparison with gastric bypass. Obes Surg. 2014;24(1):109–13.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107.
Carswell KA, Vincent RP, Belgaumkar AP, et al. The effect of bariatric surgery on intestinal absorption and transit time. Obes Surg. 2014;24(5):796–805.
Savassi-Rocha AL, Diniz MT, Vilela EG, et al. Changes in intestinal permeability after Roux-en-Y gastric bypass. Obes Surg. 2014;24(2):184–90.
Casselbrant A, Elias E, Fandriks L, et al. Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2015;11(1):45–53.
Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115(6):457–69.
Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans. 2011;39(4):989–93.
Gonzalez-Quintela A, Alonso M, Campos J, et al. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One. 2013;8(1):e54600.
Sun L, Yu Z, Ye X, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33(9):1925–32.
Lu YZ, Wu CC, Huang YC, et al. Neutrophil priming by hypoxic preconditioning protects against epithelial barrier damage and enteric bacterial translocation in intestinal ischemia/reperfusion. Lab Investig. 2012;92(5):783–96.
Wu LL, Chiu HD, Peng WH, et al. Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta. Crit Care Med. 2011;39(9):2087–98.
Huang CY, Hsiao JK, Lu YZ, et al. Anti-apoptotic PI3K/Akt signaling by sodium/glucose transporter 1 reduces epithelial barrier damage and bacterial translocation in intestinal ischemia. Lab Investig. 2011;91(2):294–309.
Yu LC, Shih YA, Wu LL, et al. Enteric dysbiosis promotes antibiotic-resistant bacterial infection: systemic dissemination of resistant and commensal bacteria through epithelial transcytosis. Am J Physiol Gastrointest Liver Physiol. 2014;307(8):G824–35.
Hsiao JK, Huang CY, Lu YZ, et al. Magnetic resonance imaging detects intestinal barrier dysfunction in a rat model of acute mesenteric ischemia/reperfusion injury. Investig Radiol. 2009;44(6):329–35.
Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31(6):817–44.
Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4.
Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3.
Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60.
Osto M, Abegg K, Bueter M, et al. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav. 2013;119:92–6.
Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177(2):512–24.
Taqi E, Wallace LE, de Heuvel E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45(5):987–95.
Tappenden KA. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology. 2006;130(2 Suppl 1):S93–9.
Sukhotnik I, Mor-Vaknin N, Drongowski RA, et al. Effect of dietary fat on fat absorption and concomitant plasma and tissue fat composition in a rat model of short bowel syndrome. Pediatr Surg Int. 2004;20(3):185–91.
Bines J, Francis D, Hill D. Reducing parenteral requirement in children with short bowel syndrome: impact of an amino acid-based complete infant formula. J Pediatr Gastroenterol Nutr. 1998;26(2):123–8.
Weser E, Babbitt J, Hoban M, et al. Intestinal adaptation. Different growth responses to disaccharides compared with monosaccharides in rat small bowel. Gastroenterology. 1986;91(6):1521–7.
Kollman KA, Lien EL, Vanderhoof JA. Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastroenterol Nutr. 1999;28(1):41–5.
Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17(2):161–71.
Estall JL, Drucker DJ. Dual regulation of cell proliferation and survival via activation of glucagon-like peptide-2 receptor signaling. J Nutr. 2003;133(11):3708–11.
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72.
Acknowledgments
This project is in part supported by the grants from the Ministry of Science and Technology of Taiwan (MOST 103-2314-B -002-122-MY3) and the National Taiwan University Hospital (NTUH 102-M2275 and 103-M2542).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Informed Consent
Does not apply.
Additional information
Linda Chia-Hui Yu and Ming-Tsan Lin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, PJ., Yang, WS., Nien, HC. et al. Duodenojejunal Bypass Leads to Altered Gut Microbiota and Strengthened Epithelial Barriers in Rats. OBES SURG 26, 1576–1583 (2016). https://doi.org/10.1007/s11695-015-1968-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1968-0