Abstract
Background
The traditional bariatric surgery guidelines issued by the National Institute of Health in 1991 did not include moderate obesity as an indication for bariatric surgery. These patients also develop risk of significant comorbidity and mortality. Nonsurgical treatment for them is not generally effective. This study compared the results of patients undergoing laparoscopic adjustable gastric banded plication (LAGBP) with laparoscopic sleeve gastrectomy (LSG) in patients with BMI between 30 and 35.
Methods
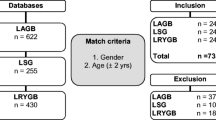
A review of data was done for patients who underwent either LAGBP or LSG in our hospital from February 2007 to October 2012. The inclusion criterion for both groups was BMI between 30 and 35 with or without comorbidity.
Results
One hundred thirty-nine patients were included in the study out of which 42 underwent LAGBP and 97 LSG. The operating time for LAGBP was significantly longer: 105.39 ± 39 vs. 59 ± 29.56 min. The postoperative hospital stay was not statistically different between the two procedures. The mean percent excess weight loss (%EWL) was significantly lower for LAGBP at 1 year but became insignificant at 2 years. Both groups had two postoperative complications, but the rate was not statistically different. The comorbidity resolution data did not show any significant difference between the two groups.
Conclusion
In the present study, both LAGBP and LSG seemed to be safe and effective bariatric procedures in moderate obesity with 2-year results. But the long-term results are still awaited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery has gained popularity in the last 20 years from its rapid but long-lasting effect on weight loss and comorbidity resolution in morbidly obese patients. The traditional bariatric surgery guidelines issued by the National Institute of Health in 1991 did not include moderate obesity (BMI ≥30 and ≤34.9) as an indication for bariatric surgery [1]. Otherwise, these patients with moderate obesity also develop risk of significant comorbidity and mortality [2].
Position statement of the American Society for Metabolic and Bariatric Surgery (ASMBS) for moderate obesity (2012) concluded that nonsurgical treatments for class I obesity are not generally effective, and bariatric surgery should be an available option to them [3]. It further added that laparoscopic adjustable gastric banding (LAGB), laparoscopic sleeve gastrectomy (LSG), and gastric bypass have been shown in randomized controlled trials to be well-tolerated and effective treatment for this group in short and medium term. The Food and Drug Administration recently voted to extend the use of Lap Band ® to people afflicted with class I obesity with >1 comorbidities [3]. Angrisani L. has recently reported long-term outcomes of gastric banding in moderate obesity with and without comorbidities and shown favorable results [4].
Though bariatric surgery definitely makes good weight loss and resolution of comorbidities, trend of surgical procedures seems to be changing in recent few years. We have seen a gradual fall in popularity of LAGB because of long-term failure of weight loss and complications [5] and a rise in the acceptance of LSG as a stand-alone bariatric procedure. Despite good weight loss after LSG, complications like hemorrhage, leaks, strictures, and gastroesophageal reflux are of concern, and its irreversible nature also make it unacceptable to some patients [6]. Few years ago, we combined adjustable gastric band and plication, laparoscopic adjustable gastric band plication (LAGBP), and reported preliminary results of weight loss comparable to LSG at 2 years [7].
The aim of the present study was to report and compare the results of patients undergoing two different restrictive bariatric procedures in patients with BMI between 30 and 35.
Methods
A retrospective review of prospectively maintained data was done for patients who underwent either LAGBP (with the approval of the E-DA Hospital institutional review board; approval numbers—LAGBP: EMRP22098N and LSG: EMRP39102N) or LSG in our hospital from February 2007 to October 2012. The inclusion criterion for both groups was BMI between 30 and 35 with or without comorbidity. Patients with type 2 diabetes of recent onset (<5 years) and C-peptide > 3 ng/ml were offered these restrictive surgeries. Patients were given choice between LAGBP and LSG after detailed discussion with them. For patients with longer duration of diabetes or lower C-peptide, we prefer gastric bypass or loop duodenojejunal bypass with sleeve gastrectomy [8, 9]. The exclusion criterion was lack of at least 1 year follow-up. All patients had failed previous attempts of losing adequate weight by diet, exercise, life style modification, or medicine. The prospectively collected data included patient demographics like age, sex, BMI, and obesity-related comorbidities. Operative time, intra- and postoperative complications, as well as the length of stay of hospitalization were also recorded. Postoperative follow-up data was recorded at 1, 3, 6, 9, 12, 18, and 24 months after surgery and analyzed.
Statistical Methods
Mean ± standard deviation (SD) was used for normally distributed continuous variables or as percentages for categorical variables. The p value was calculated using paired t test, chi-square test, or Fisher’s exact test when appropriate. All analyses were performed with SPSS statistical software (version 18.0; SPSS Inc., Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
Operative Technique
The patients were placed in the supine position with their arms abducted. Surgeon stood on the patient’s right side and assistant on the left. Five ports were used in either procedure.
In LAGBP, devascularization of the greater curvature was performed from gastroesophageal junction to 3 cm from the pylorus. Plication was started from the fundus and progressed towards the pylorus stopping 3 cm from it. Amount of plication was decided using gastric plication formula (Fig. 1). The greater curvature was inverted with five to six interrupted nonabsorbable sutures (2–0 Ethibond Excel Ethicon, St. Stevens-Woluwe, Belgium) and was then reinforced with a continuous seromuscular suture (polypropylene 2–0). Adjustable band was then placed using the pars flaccida technique.
In LSG, devascularization of greater curvature was performed from gastroesophageal junction to 4 cm from the pylorus. A 36-F bougie was inserted and marking was done along the lesser curvature. Excess stomach was resected with multiple fires of endostaplers. The stomach was then secured to the retroperitoneal tissue with a single 3–0 polyglactin suture to prevent gastric torsion.
Results
One hundred thirty-nine patients were included in the study out of which 42 underwent LAGBP and 97 LSG. Table 1 summarizes demographic and operative data. The age of LAGBP group was significantly less (mean = 31.67 years) as compared to LSG group (mean = 36.16 years). There were no significant differences in the two groups with respect to preoperative sex, BMI, or comorbidities (except fatty liver). The operating time for LAGBP was significantly longer: 105.39 ± 39 vs. 59 ± 29.56 min. The postoperative hospital stay was not statistically different between the two procedures.
The mean percent excess weight loss (%EWL) was significantly lower for LAGBP at 1 year but became insignificant at 2 years. For LAGBP, %EWL was 29.5 ± 10.04, 44.93 ± 14.93, 53.95 ± 16.47, 60.1 ± 18.68, 64.29 ± 20.62, 66.45 ± 22.9, and 70.65 ± 21.49 at 1, 3, 6, 9, 12, 18, and 24 months; whereas for LSG, it was 32.19 ± 13.1, 54.43 ± 17.99, 66.85 ± 20.16, 76.79 ± 21.81, 79.75 ± 22.58, 79.01 ± 21.86, and 75.69 ± 23.02 (Table 2).
Both groups had two postoperative complications, but the rate was not statistically different. In LAGBP group, one patient developed gastric perforation near the band. We performed de-plication and repair of gastric perforation and band removal. The total hospital stay was 10 days. The second patient had disconnection of the tube, which needed re-laparoscopy and revision of the band. Hospital stay for second procedure was 4 days.
In LSG group, two patients had complications in the form of a leak. We did a laparoscopic repair of gastric tube with drainage to one patient who recovered well with hospitalization of 44 days. The second patient required multiple surgeries and conversion to open Roux-en-Y gastric bypass. The total hospitalization was over 1 year.
In the LAGBP group, the mean gastric band adjustment rate was 3.36 ± 2.86 in 2 years. The comorbidity resolution data are shown in Table 3 and does not show any significant difference between the two groups.
Discussion
A BMI between 30 and 35 is classified as class I obesity or moderate obesity [3]. Data is increasingly showing that many of the metabolic problems that accompany obesity begin at a BMI of 30 or even earlier [10, 11]. ASMBS has recently concluded in its position statement that moderate obesity is a health problem that leads to additional serious comorbidities and a shortened life expectancy. There is no current justification on grounds of evidence of clinical effectiveness, cost-effectiveness, ethics, or equity that this group should be excluded from a life-saving treatment [3]. Therefore, bariatric surgery should be an available option for suitable individuals in this group.
It is also clear that nonsurgical therapies will not provide a durable solution to their disease of obesity. Systematic reviews and randomized controlled trials of nonsurgical therapies involving diet regimen, pharmacotherapy, behavioral therapy, and exercise have reported a mean weight loss in the range of 2–6 kg at 1 year [12, 13] with poor weight maintenance [14]. Still, there are individuals who have achieved substantial and durable weight loss with a higher initial BMI and have been able to maintain it for several years. Therefore, a trial of nonsurgical methods for weight reduction must be given before considering surgery for any obese patient.
But surgical management of patients in this category is still debated. There is currently no predictive method to match a particular patient with a particular operation to achieve the optimal outcome. A procedure which is relatively safe and gives satisfactory results can make surgery an attractive option for these patients.
Substantial comparative and long-term data have now been published in peer-reviewed studies demonstrating durable weight loss, improved medical comorbidities, long-term patient satisfaction, and improved quality of life after LSG. However, it also mentioned that LSG has a risk/benefit profile between laparoscopic adjustable gastric banding and laparoscopic Roux-en-Y gastric bypass (LRYGB) [15]. Staple line leaks and bleeding after LSG continue to be the most serious complications and occur in 1–3 % of patients in large published series [16].
In the present study, complication rate was 4.7 % in LAGBP and 2.1 % in LSG (n = 2 in both and p value = 0.584), and it revealed that the complications after LSG required a much longer stay and complex re-operation in comparison with LAGBP. This might be because of high pressure inside the gastric tube after LSG, which makes management of leak extremely challenging [17]. Sometimes, LSG might require conversion to LRYGB for managing of the leak. In case of LAGBP, after removing the band and sutures, normal anatomy is restored and management of complications becomes simpler [18]. But the larger series are needed to confirm these findings.
The mean adjustment rate in our LAGBP group was 3.56 in 2 years, which was much lower than required for LAGB patients to have a satisfactory weight loss. Dixon et al. reported that male patients who followed up less than 13 times in 2 years were not able to achieve good weight loss [19]. Since LAGBP has dual restriction (from plication and band), it needs fewer adjustments as compared to LAGB which solely depends on band for weight loss. We did not observe any complication arising out of band after starting band adjustments, and this might be because of the decreased frequency of band adjustment, as a higher number of adjustments may cause slippage, erosion, or infection. In a recently published case-matched series, Umer et al. concluded that LAGBP is a safe alternative bariatric procedure which can potentially be reversed if needed [20].
As shown in graph 1, the weight loss trend differed in the two groups. LSG group lost weight quicker than the LAGBP group in the first year, but by the end of 2 years, the EWL was similar. This may be because of more reduction in ghrelin levels after LSG as fundus of the stomach is removed and leads to higher suppression of hunger [21]. Also, it is interesting to note a stagnation of weight loss after 1 year and little regain of lost weight at 2 years after LSG. This may be explained by dilatation of the remaining gastric tube [22]. The persistent weight loss in LAGBP might be due to band adjustment.
Both the groups achieved significant resolution of comorbidities. Also, there was no significant difference between the resolutions of comorbidities in-between two groups. Although both procedures are restrictive, the substantial weight loss achieved might explain better outcomes in terms of comorbidity resolution. The number of patients with diabetes was very few in our series, and this could be explained partly by selection bias as we tend to perform malabsorptive procedures in such patients to give them a better chance of complete remission [7, 8]. Also, the patients were relatively younger (mean age 32–36 years in both groups) and diabetes was of recent onset without insulin use.
Conclusion
In the present study, both LAGBP and LSG seemed to be safe and effective bariatric procedures in moderate obesity with 2-year results. But randomized trials and long-term results are still awaited.
References
NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115(12):956–61.
Flegal M, Kit BK, OrpamaH BI, et al. Association of all cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82.
Bariatric surgery in class I obesity (body mass index 30–35 kg/m2). ASMBS Clinical Issues Committee. ASMBS statements/guidelines September 13, 2012.
Angrisani L, Cutolo PP, Formisano G, et al. Long-term outcomes of laparoscopic adjustable silicone gastric banding (LAGB) in moderately obese patients with and without co-morbidities. Obes Surg. 2013;23(7):897–902.
Suter M, Calmes JM, Paroz A, et al. A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg. 2006;16:829–35.
Dietel M, Gagner M, Erickson AL, et al. Third International Summit: current status of sleeve gastrectomy. Soard. 2011;7(6):749–59.
Huang C-K, Chhabra N, Goel R, et al. Laparoscopic adjustable gastric banded plication: a case-matched comparative study with laparoscopic sleeve gastrectomy. Obes Surg. 2013;23(8):1319–23.
Huang C-K, Shabbir A, Lo C-H, et al. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25–35. Obes Surg. 2011;21:1344–9.
Huang CK, Goel R, Tai CM, Yen YC, Gohil VD, Chen XY. Novel metabolic surgery for type II diabetes mellitus: loop duodenojejunal bypass with sleeve gastrectomy. Surg Laparosc Endosc Percutan Tech. 2013;23(6):481–5.
Collaboration PS, Whitlock G, Lewington S, et al. Body-mass index and cause- specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96.
Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and over-weight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
Dansinger ML, Gleason JA, Griffith JL, et al. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53.
Avenell A, Brown TJ, McGee MA, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J Hum Nutr Diet. 2004;17:293–316.
Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–48.
Updated position statement on sleeve gastrectomy as a bariatric procedure. soard. 2012; 02.001.
Spyropoulos C, Argentou MI, Petsas T, et al. Management of gastrointestinal leaks after surgery for clinically severe obesity. Soard. 2011;21:1650–6.
Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy—volume and pressure assessment. Obes Surg. 2008;18:1083–8.
Hussain A, Khan A, El-Hasani S. Laparoscopic management of ischemic gastric perforation after banded plication for obesity. Soard. 2014;10:745–6.
Dixon JB, Laurie CP, Anderson ML, et al. Motivation, readiness to change, and weight loss following adjustable gastric band surgery. Obesity (Silver Spring). 2009;17:698–705.
Umer I. Chaudhry, Sylvester N. Osayi, Andrew J. Suzo, BS, Sabrena F. Noria, Dean J. Mikami, Bradley J. Needleman. Laparoscopic adjustable gastric banded plication: case-matched study from a single U.S. center. Soard. 2014; 05.030
Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–9.
Langer FB, Bohdjalian A, Felberbauer FX, et al. Does gastric dilatation limit the success of sleeve gastrectomy as a sole operation for morbid obesity? Obes Surg. 2006;16:166–71.
Grant Information
None.
Conflict of Interest
Jasmeet Singh Ahluwalia, Po-Chi Chang, Chi-Ming Tai, Ching-Chung Tsai, Po-Lin Sun, and Chih-Kun Huang declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
This is a retrospective study. For this type of study, formal consent is not required.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Indentifying Information
Participants’ identifying information is not available in the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Work was performed in
E-Da Hospital, 1, E-Da Rd, Yan-Chau District, Kaohsiung City, Taiwan 824, Tel: +886-7-6150011
Rights and permissions
About this article
Cite this article
Ahluwalia, J.S., Chang, PC., Tai, CM. et al. Comparative Study Between Laparoscopic Adjustable Gastric Banded Plication and Sleeve Gastrectomy in Moderate Obesity—2 Year Results. OBES SURG 26, 552–557 (2016). https://doi.org/10.1007/s11695-015-1791-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1791-7