Abstract
Frying, a prevalent cooking method, subjects oils to high temperatures, causing chemical changes and the formation of harmful by-products. Repeated oil use poses health risks, necessitating effective restoration methods. Agricultural waste ashes, including rice, barley, and oak hulls, were investigated for their adsorption capabilities in comparison to conventional adsorbents. Refined sunflower oil was used, and five filter aids, including Magnesol XL, diatomaceous earth, and agricultural waste ashes, were employed. Rice, barley, and oak hull ashes were prepared and characterized for metal content. Scanning electron microscopy (SEM) and Fourier Transform Infrared (FTIR) spectroscopy assessed the surface morphology and chemical structure of ashes. The frying process involved heating oil daily for five consecutive days, with each session lasting 4 h, followed by treatment with filter aids. Comparing the agricultural waste ashes, oak hull ash (OHA) exhibited the highest content of silicate which was almost equal to the conventional adsorbents. SEM revealed the surface properties and superior performance of OHA in adsorbing degradation products. FTIR analysis showed similarities in chemical structure between pure silica and oak hull ash. Initial sunflower oil quality was confirmed through various physico-chemical properties. Frying led to quality deterioration, evidenced by color changes, increased refractive index, viscosity, acidity, peroxide levels, and decreased iodine value. This study underscores the potential of agricultural waste ashes, particularly oak hull ash, as effective adsorbents for enhancing the quality of fried sunflower oil. Their comparable performance to conventional adsorbents, cost-effectiveness, and lack of harmful effects position them as viable alternatives in restoring used frying oils for sustainable culinary practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Frying is a widely embraced cooking method across the globe for its convenience. However, this technique involves subjecting cooking oils to high temperatures, typically between 150 and 200˚C, which triggers chemical changes in the oil. These alterations result in the formation of harmful by-products, posing health risks when oils are reused excessively. Moreover, there’s a financial aspect to consider as you must either reuse the used oil or dispose of it. Repeated use of the same oil manifests noticeable transformations such as increased viscosity, altered scent, darkening, foaming, and a reduced smoke point [1]. These changes occur due to a series of chemical reactions encompassing oxidation, hydrolysis, cyclization, polymerization, and degradation, leading to the generation of volatile compounds, free fatty acids, lipid peroxides, hydrocarbons, and polymers [2]. These substances are toxic and pose significant health hazards. Consistent consumption of heated oil elevates the risk of conditions like high blood pressure, cardiovascular diseases, and atherosclerosis [3–4]. While used oils can potentially serve as a resource for biodiesel production, it’s imperative to eliminate free fatty acids (FFAs) to prevent engine corrosion and undesirable reactions during combustion [5–6].
Numerous approaches have been explored to enhance the longevity of cooking oil or restore its quality for reuse. These methods encompass the incorporation of antioxidants and esterification to prevent deterioration, as well as the utilization of filter aids and advanced membrane technologies for purification. Another promising technique involves adsorption, where both naturally occurring and modified materials serve as adsorbents. These materials span a wide range, including silica gel, magnesium silicate, zeolite, clay, alumina, charcoal, chitosan, and activated carbon [7,8,9]. Additionally, agricultural waste presents an economically viable and readily available source of adsorbents. The effectiveness of using plant-derived waste as adsorbents has been substantiated through the utilization of materials such as sugarcane bagasse, olive waste, rice hulls, and Salacca zalacca [10,11,12,13].
Agricultural waste products such as rice, barley, wheat, oats, and oak hull ashes represent abundant biological sources of silicate minerals and residual carbon. The amorphous silica in these materials can be extracted easily using low-temperature. These inherent characteristics render them pivotal in serving as key materials for the adsorption of Free Fatty Acids (FFA). Notably, silica-containing agricultural by-products or waste materials have garnered substantial attention among potential adsorbents aimed at prolonging the shelf life of frying oils. This interest is primarily driven by their cost-effectiveness, well-defined framework structures, and impressive adsorption capacities. Biogenic silica forms can be harnessed from both agricultural residues and commercially available adsorbents boasting significant silica content. Recent investigations have indicated that treatment with all-natural adsorbent materials may result in reductions in parameters such as red coloration, viscosity, acidity, peroxide content, Thiobarbituric Acid (TBA) values, as well as levels of conjugated diene/triene and polar compounds, when compared to untreated frying oils. In contrast to commercial options like Magnesol XL, agro-industrial-based adsorbents offer various advantages including diminished free fatty acid content and p-anisidine values, reduced formation of trans-fatty acids, and cost-effectiveness owing to simplified combustion processes. Moreover, oils treated with synthetic adsorbents may exhibit heightened refractive index and smoke point [14].
In the world of vegetable oil processing, common tools of the trade include substances like activated carbon, silica, and acid clay. These materials work wonders in removing minor oil components such as free fatty acids (FFA), peroxides, carotenoids, and phospholipids [15,16,17]. However, there’s a growing curiosity about finding alternatives to these tried-and-true adsorbents. Enter rice hull silica (RHS) [18–19] and soy hull carbon [20–21], which have piqued the interest of those in the field as potential game-changers.
Iran is a country actively involved in agriculture, with a wealth of agricultural byproducts such as wheat hulls, rice hulls, barley hulls, and more. These materials have various industrial applications, including their use in fuel production and as animal feeds [15]. Of particular interest is the potential of agricultural waste ashes as effective adsorbents for removing oxidation products from oil in the field of vegetable oil processing. The primary objective of this study was to investigate the suitability of agricultural waste ashes and compare their adsorption capabilities with the synthetic adsorbents typically used to restore the quality of used frying oil. To achieve this, we conducted a comprehensive analysis of the metal content in agricultural waste ashes obtained from rice, barley, and oak hulls (RHA, BHA & OHA respectively). Additionally, we performed rigorous quality assurance assessments to evaluate the quality of non-fried, fried, and regenerated sunflower oil.
Materials and methods
Source of sunflower oil
We sourced our refined sunflower oil from a reputable local supermarket in Shiraz, Fars, Iran. The oil exhibited excellent quality with peroxide level at 0.59 active oxygen peroxides per kilogram of oil and acid value at just 0.08 mg of KOH per gram of oil.
Filter aids
Five different filter aids were used: Magnesol XL (hydrous, white, amorphous and odourless) obtained from Magnesol Product Division (Reagent Chemical and Research, Inc., Houston, TX, USA). Diatomaceous earth clay (white, amorphous and odourless) was obtained from Qasr El-Sagha Deposit (Fayoum Governorate, Egypt). Rice, barley and oak hulls were obtained from the field crops (Yasuj, Kohgiloye, Iran).
Preparation of hull ashes
The ashes of rice, barley, and oak hulls were prepared following the procedure outlined in AOAC [22]. Initially, the hulls were finely ground to achieve a particle size of approximately 180 nm using a grinder (Perten, USA). Subsequently, they were placed in a crucible, subjected to carbonization in an air environment within a muffle furnace (Furnace 5000; Thermolyne, Iowa, USA) at 550 °C for a duration of 12 h. Afterward, the ashes were allowed to cool to room temperature within a desiccator and were then stored in glass screw-capped bottles for future use.
Determination of metals
The metal compositions, including Si, Mg, Ca, Fe, Mn, and Cu, within different filter aids were analyzed using a Perkin-Elmer Model 3300 atomic absorption spectrophotometer (The Perkin-Elmer Corporation, Norwalk, USA). To prepare the samples for analysis, they were digested using fluoro-boric acid, following the procedure outlined by Tan [23].
Scanning electron microscopy (SEM)
We examined the microstructures of RHA, BHA and OHA using a scanning electron microscope, SEM, (Hitachi Model S-2380 N, Tokyo, Japan). The SEM was operated at an accelerated voltage of 18 kV and a beam angle of 90 degrees. To prevent surface charges, all samples underwent coating with an 80:20 wt% gold-palladium layer under a vacuum of 8 × 10− 1 mbar/Pa, with an applied voltage of 10 mA. SEM images were captured at an acceleration voltage of 15 kV.
Chemical structure by FTIR spectroscopy
We employed a Fourier Transform Infrared (FTIR) Spectrometer, (Alpha FTIR spectrometer, Bruker, Karlsruhe, Germany) to examine the chemical structure of OHA, as a silica-containing agricultural by-product, in comparison to pure silica. The FTIR analysis utilized a versatile high-throughput ZnSe ATR crystal, covering a wavelength range from 4000 to 750 cm− 1 at room temperature. Prior to analysis, the samples underwent oven drying, and a background spectrum was collected to eliminate signals not associated with the sample.
Frying process
To commence the experiment, we placed a measured quantity (approximately 2 kg) of refined sunflower oil into a stainless steel pan fryer, which measured 60 cm in diameter and 30 cm in height. The oil was then heated to a consistent temperature of 180 ± 5 °C. Subsequently, we fried potato chips, each measuring 2 mm in thickness, after they had been pre-soaked in a 10% (w/v) NaCl solution. This frying process was repeated daily for five consecutive days, with each session lasting 4 h, resulting in a total continuous frying duration of 20 h. Afterwards, the oil samples were allowed to cool and were subsequently stored at a temperature of 5 °C to facilitate further analysis [12].
Treatment of fried oils with filter aids
Each filter aid was introduced individually into the fried oil at a concentration of 2% (w/v). Subsequently, mechanical stirring was applied for a duration of 15 min at a temperature of 105 °C. The mixture was then subjected to filtration through Whatman No. 1 filter paper. This adsorption procedure was repeated three times, with fresh portions of fried sunflower oil treated with the specific filter aid (including Magnesol XL, diatomaceous earth, and hull ashes from rice, barley, and oak) at the end of the oil heating period (20 h) [12].
Quality assurance tests for non-fried, fried and fried-treated sunflower oil
We conducted various tests on the sunflower oil samples, including refractive index, acid value, peroxide value, and iodine value, following AOAC Official Methods , numbered 969.18, 969.17, 965.33, and 965.158, respectively. The smoke point, indicating when the oil starts to smoke, was recorded following Nielsen’s method [23]. To assess color in non-fried, fried, and fried-treated sunflower oil, we used a Lovibond tintometer from The Tintometer Ltd. (Salisbury, England), with yellow glass slides set at 35 and red glasses matched to the oil samples as per Nielsen’s protocol [24]. Relative flow times of the sunflower oil samples were measured using an Ostwald viscometer from Brookfield Engineering Laboratories, Inc. (Stoughton, MA, USA), as per Joslyin’s instructions [25]. All analyses were performed in triplicate for each sunflower oil sample with different filter aids, and the results are presented as averages.
Statistical analysis
Statistical analysis was conducted on the results, considering three distinct samples each for non-fried, fried, and fried-treated sunflower oil at the end of the heating period. For each of these independent samples, we carried out three determinations of the physical and chemical properties of the oil. Consequently, all the data presented in this study are expressed as means (n = 3) ± SD (n = 3). To enable comparisons between the mean values of the factors under investigation, we employed analysis of variance and the least significant differences (LSD) test (p < 0.05), following the methodology outlined by Cochran and Cox [26].
Results and discussion
Mineral pattern of various adsorbents
Table 1 presents the mineral composition of various filter aids, including Magnesol XL (a synthetic filter aid), diatomaceous earth (a natural filter aid), and ashes from rice, barley, and oak hulls (derived from agricultural waste). Each of these filter aids exhibited elevated levels of silicate (Si) and varying concentrations of other minerals. Their distinct mineral profiles set them apart from each other. For instance, oak hull ash showed the highest copper (Cu) content, while Magnesol XL had the highest silicate (Si) content, followed by diatomaceous earth and oak hull ash. In general, the primary mineral combinations in Magnesol XL, diatomaceous earth, rice hull ash, barley hull ash, and oak hull ash were Si + Mg + Mn, Si + Mn + Ca, Si + Mn, Si + Mn and Si + Mn + Cu, respectively.
The data (values ± SE) are the mean values of three measurements for the same sample. Superscript lowercase letters indicate significant differences between rows (p < 0.05).
Adding value to agricultural wastes, produced on a large scale, is a challenge for sustainable and green chemistry. Among these by-products, oak hull ash can be highlighted because of its high Si content, as the main chemical element, more similar to the Magnesol XL (a synthetic filter aid). It is well known that adsorbent mineral pattern is a key factor determining the adsorbing characterization. Minerals such as silica and manganese are widely distributed in the ashes of the agricultural plant wastes and can be considered as good adsorbents to get rid of the oxidation products of oil [12].
Scanning electron microscopy (SEM)
The surface morphology of adsorbent materials derived from agro-waste, employed in the regeneration of frying oils, was examined using scanning electron microscopy (SEM) to elucidate their crystal structure, framework, and surface properties. These characteristics play a crucial role in determining their adsorption capacity and effectiveness in removing impurities from frying oil (Fig. 1).
The SEM images presented in Fig. 1 (a, b) depict the heterogeneous nature of rice hull ash (RHA) and barley hull ash (BHA), exhibiting irregular shapes alongside notable size discrepancies and pronounced surface roughness. These observations corroborate previous findings by [14, 27]. In contrast, the SEM analysis of oak hull ash (OHA) reveals a distinct morphology characterized by a more defined structure, displaying homogenous sponge-like tissues and clearly visible pores. These unique structural attributes of OHA suggest an elevated absorption capacity compared to its RHA and BHA counterparts.
Observations revealed that oak hull ash exhibited superior performance in adsorbing degradation products from oil, attributed to its surface properties, elevated adsorption capacity and SiO2 content. Meanwhile, RHA and BHA garnered attention for their expansive surface area and ability to reduce the peroxide value of oil. These findings underscore the significance of SEM in comprehending the surface morphology and structure of agro-waste-based adsorbent materials utilized in oil regeneration.
Chemical structure by FTIR spectroscopy
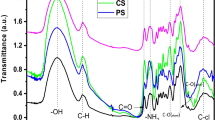
FTIR analysis was employed to to identify the main functional groups and to confirm the presence of OHA components such as lignin, cellulose and hemicellulose (Fig. 2). Both samples exhibited a broad absorption band in the range of 3200–3500 cm− 1, attributed to the stretching of hydroxyl groups in cellulose, hemicelluloses, and lignin [28]. Vibrations at 2950 and 2850 cm− 1 were observed in both pure silica and OHA, likely associated with C–H bond stretching in cellulose and hemicelluloses [29]. The intensity of the peak at 2950 cm− 1 exceeded that at 2850 cm− 1. Neitzel et al. noted similar absorption bands in oak husks (OH) and linked them to the stretch of asymmetric alkyl groups’ C–H bonding [30].
Peaks at 1738 cm− 1 and 1642 cm− 1 were indicative of the C–C linkage in the aromatic ring of lignin. The presence of aryl-alkyl ether bonds (C-O-C) in cellulose and lignin was suggested by a peak at 1260 cm− 1 [31]. Additionally, absorption bands at 1022 and 1149 cm− 1 were attributed to C-O stretching vibrations. The FTIR spectra of the examined materials displayed similarities, primarily consisting of the three main components: cellulose, hemicelluloses, and lignin. Moreover, the spectra revealed the presence of oxygenated functional groups (e.g., carboxyl, carbonyl, hydroxyl, etc.) within the OHA sample. These functional groups are anticipated to engage in interactions with adsorbates, potentially amplifying its adsorption efficacy [32].
Quality of initial sunflower oil
3- 9 illustrate various physico-chemical properties of the initial sunflower oil. Overall, the physical and chemical characteristics assessed for the sunflower oil quality in this study, including color, refractive index, viscosity, smoke point, acid value, peroxide value, and iodine value, align with values reported in numerous prior studies [12, 33–34]. The lowercase letters indicate significant differences between filter aids (p < 0.05). These findings affirm the high quality of the fresh sunflower oil utilized in our study
Quality loss of fried sunflower oil
The transitions in the quality attributes of sunflower oil during intermittent frying are graphically depicted in Figs. 3, 4, 5, 6, 7, 8 and 9.
We evaluated the color of the fried sunflower oil samples using a consistent yellow glass slide set at a value of 35, in conjunction with variable red glass slides. The evolution of the sunflower oil’s color was striking, transitioning from its initial clear yellow appearance to a progressively deeper, dark brown shade. This transformation was most pronounced during the later stages of the frying process. Notably, these distinct color shifts exhibited significant variation (p < 0.05) across the various heating periods and demonstrated a direct correlation with the duration of the deep-fat frying process, as visually represented in Fig. 3.
The refractive index serves as an effective indicator of oil unsaturation, particularly in relation to the quantity of conjugated double bonds present. Figure 4 data reveals a marginal yet consistent rise in the refractive index of sunflower oil across different frying durations.
As described by Kress-Rogers et al. [35], the progressive rise in refractive index observed during frying is concurrent with the amplification of polymerization reactions, saturation of carbon double bonds, and escalating turbidity of the oil [35]. The alterations in the viscosity of sunflower oil, subjected to frying at 180 ± 5 °C for four hours daily over five days, are detailed in Fig. 5. A gradual and substantial increase in viscosity was observed during the frying process. It is important to highlight that by the end of the frying duration; the viscosity had escalated approximately 4 times higher than the initial viscosity at the experiment’s commencement.
The elevation in viscosity observed in frying oils can be attributed to the formation of high molecular weight compounds, which predominantly arise from the polymerization of carbon-carbon or carbon-oxygen-carbon bonds [36]. Notably, intermittent frying of sunflower oil resulted in a steady and noteworthy reduction in smoke point values, from 235 to 200 °C (Fig. 6). The smoke point, indicative of the chemical transformation of fats into glycerol and Free Fatty Acids (FFAs), serves as a pivotal metric. Following thermal treatments, a universal decline in smoke point values across all oils was observed, a phenomenon inherently associated with the concomitant escalation in FFA content [2]. The acid levels in sunflower oil shot up notably during frying, showing a strong correlation with the duration of heating (Fig. 7). According to this study’s findings, by the end of the frying process, the acidity in the sunflower oil was roughly 18 times higher than that in non-fried sunflower oil. When it comes to lipid oxidation, hydroperoxides take the lead as the primary products. Therefore, measuring peroxides serves as an indicator for the initial stages of sunflower oil oxidation. Figure 8 data illustrates a gradual increase in the peroxide levels of sunflower oil over the frying period, reaching unacceptable levels after the second day of frying.
The iodine value is a marker of oil unsaturation. In our study, the iodine values of fried sunflower oil samples notably dropped as the frying progressed. This could be attributed to the creation of conjugated dienes in sunflower oil while frying (Fig. 9).
Effect of filter aids on fried sunflower oil quality
The color assessment of fried sunflower oil revealed a yellow shade at 32.5 and a red shade at 17. Introducing Magnesol XL at a 2% concentration led to a significant decrease in the red coloration (p < 0.05). Similar results were observed with diatomaceous earth treatment, but among waste hull ashes, OHA exhibited the most pronounced effect in reducing red coloring (Fig. 3).
The refractive index of the fried sunflower oil stood at 2.3 at 25 °C. Employing various filter aids at a 2% level resulted in significant reductions in the refractive index values (p < 0.05). The efficacy of agricultural waste hull ashes in removing unsaturated compounds during oil frying mirrored that of Magnesol XL and diatomaceous earth (Fig. 5).
When heated at 180 °C for 20 h, the viscosity of sunflower oil reached 10.20 min. Treatment with OHA significantly reduced the viscosity, similar to the effect of Magnesol XL (p < 0.05). Meanwhile, diatomaceous earth and hull ashes of rice and barley exhibited comparable impacts on the viscosity of the fried sunflower oil (Fig. 5).
The smoke point of the fried sunflower oil was determined to be 200 °C. Treating the oil with Magnesol XL and OHA at a 2% level notably lowered the smoke point. Similar effects were observed with diatomaceous earth and hull ashes of rice and barley (Fig. 6).
The findings highlight the considerable decrease in free fatty acid levels in fried sunflower oil treated with OHA at a 2% concentration. Results from samples treated with Magnesol XL, diatomaceous earth, and hull ashes of rice and barley aligned closely with the efficacy seen in OHA treatment (Fig. 7). Proctor et al. [16] and Farag et al. [9] obtained comparable findings while employing agricultural waste hull ashes, specifically from rice, wheat, and barley, to rejuvenate soy oil and fried sunflower oil, respectively [12, 18].
The peroxide value of the fried sunflower oil initially measured 34.60. OHA treatment significantly reduced this value from 34.60 to 2.6, surpassing the effect of Magnesol XL treatment (p < 0.05). The varied filter aids demonstrated nearly equal effectiveness in reducing peroxide values of the fried sunflower oil (Fig. 8). Overall, filter aids, especially OHA, displayed promising potential in decreasing peroxide values in fried or oxidized oils. This finding aligns with the standardization and regulation of oil quality, as documented by Firestone et al. [37] and Farag et al. [9] [12, 37]. The analysis indicated minimal changes in the iodine value between fried and filter aid-treated oils, with exceptions noted for Magnesol XL and OHA (Fig. 9). In parallel investigations, Farag & El-Anany (2006) and Farag et al. [9] observed a slight reduction in the iodine value of fried oil following treatment with various adsorbing materials [12, 38].
In general, the data implies that the filter aids investigated hold valuable scavenging properties, particularly OHA, which displays impressive effects in eliminating oxidation products from fried sunflower oil, allowing for the oil’s potential reuse in the food industry. While hull ashes from rice, and barley differed in mineral levels, their adsorption efficiencies for secondary oxidation products showed no significant distinctions (p < 0.05). Overall, this study offers insights into enhancing the quality of fried sunflower oil using Magnesol XL, diatomaceous earth, and hull ashes from rice, barley, and oak at a 2% concentration. Among these, Magnesol XL and OHA displayed the most significant effects compared to others. However, the application of Magnesol XL as a filter aid for removing oxidation products from frying oils is limited due to its irritating effects on the skin and eyes [12], making OHA a potential substitute. Natural filter aids like diatomaceous earth and agricultural hull ashes from rice, barley, and oak pose no harmful effects on humans and exhibit comparable efficacy in removing oil oxidation products. Furthermore, they are cost-effective and valuable for restoring the quality of fried oils.
Conclusion
Our study found distinct mineral compositions in various filter aids, with OHA showing high mineral content such as silica and manganese make it a promise adsorbent. SEM analysis highlighted structural differences, indicating oak hull ash’s distinct morphology enhanced adsorption capacity. FTIR spectroscopy confirmed functional groups conducive to adsorption interactions in OHA. Treatment of fried sunflower oil with filter aids resulted in significant quality improvements, particularly notable reductions in color, viscosity, acid and peroxide values with OHA. These findings underscore the potential of natural filter aids, including OHA, for enhancing fried oil quality sustainably and cost-effectively in the food industry.
Data availability
The datasets generated and/or analyzed during the current study are those included in the submitted manuscript.
Refrences
K. Bordin, M.T. Kunitake, K.K. Aracava, C.S.F. Trindade, Arch. Latinoam. Nutr. 63(1), 5 (2013)
M.A.R. Gao, T. Song, L. Zang, L. Jiang, Y. Li, J. Zhang, X.F. Gao, G. Zhou, Food Sci. Technol. Campinas. 36(2), 329 (2016)
T.V. Gadiraju, Y. Patel, J.M. Gaziano, L. Djousseé, Profitable Visit Nutrients. 7, 8424 (2015)
R.P. Venkata, R. Subramanyam, Toxicol. Rep. 3, 636 (2016)
M. Azhari, R. Faiz, T.I. Yunus, G. Mohd, T.C.S. Yaw, Int. J. Sci. Eng. Technol. 5(2), 92 (2008)
G.G. Kombe, A.K. Temu, H.M. Rajabu, G.D. Mrema, J. Kansedo, K.T. Lee, Adv. Chem. Eng. Sci. 3, 242 (2013)
A. Miyagia, M. Nakajimab, JAOCS. 80(1), 91 (2003)
A. Dülger, E. Yilmaz, Eur. J. Lipid Sci. Technol. 115, 668 (2013)
F. Yemiscioglu, O. Ozdikicierler, O. Onder, J. Food Nutr. Res. 3(3), 176 (2015)
R. Wannahari, M.F.N. Nordin, Am. J. Engg Appl. Sci. 5(1), 59 (2012)
A.M.M. Basuny, S.M. Arafat, H.M. Soliman, Curr. Sci. Int. 3(4), 311 (2014)
R.S. Farag, A.M.M. Basuny, S.M. Arafat, S.A. Arafa, Int. J. Food Sci. Tech. 44, 1850 (2009)
G. Ermi, A.K. Agung, I. Refi, A. Hermansyah, C. Zulkarnain, Z. Rahmiana, J. Chem. Pharm. Res. 7(9S), 59 (2015)
S. Özcan, B. Aydeniz-Guneser, Rivista Italiana Delle Sostanze Grasse. 99(1), 37 (2022)
S. Lin, C.C. Akoh, A.E. Reynolds, Food Res. Int. 34(2–3), 159 (2001)
A. Proctor, S. Palaniappan, JAOCS. 67(1), 15 (1990)
R.A. Yates, J.D. Caldwell, JAOCS. 70(5), 507 (1993)
A. Proctor, P.K. Clark, C.A. Parker, JAOCS. 72(4), 459 (1995)
A. Farook, S. Ravendran, JAOCS. 77, 437 (2000)
A. Proctor, C.D. Harris, JAOCS. 73, 527 (1996)
R. Gnanasambandam, A. Proctor, JAOCS. 74(6), 685 (1997)
AOAC, Official Methods of Association of Agricultural Chemicals, 17th edn, (Washington, DC, 2000)
K.H. Tan, S. Sampling, Preparation and Analysis (New York, Marcel Dekker,, 1996), pp. 160–163
S.S. Nielsen, Food Analysis, 2nd edn. (Gaithersburg, Maryland, Aspen Inc.,, 1998), pp. 222–223
M.A. Joslyin, Methods in Food Analysis (Academic Press, Inc., New York, 1950)
W.G. Cochran, G.M. Cox, Experimental Designs, 2nd edn. (New York, Wiley,, 1992)
M. Kim, S.H. Yoon, E. Choi, B. Gil, LWT-Food Sci. Technol. 41(4), 701 (2008)
Z. Movasaghi, B. Yan, C. Niu, Ind. Crops Prod. 127, 237 (2019)
S. Banerjee, G.C. Sharma, R.K. Gautam, M.C. Chattopadhyaya, S.N. Upadhyay, Y.C. Sharma, J. Mol. Liq. 213, 162 (2016)
N. Neitzel, M. Eder, R. Hosseinpourpia, T. Walther, S. Adamopoulos, Mater. Today Commun. 36, 106602 (2023)
J.I. Morán, V.A. Alvarez, V.P. Cyras, A. Vázquez, Cellulose. 15, 149 (2008)
E. Ahmed, A. Zeitoun, G. Hamad, M.A. Zeitoun, A. Taha, S.A. Korma, T. Esatbeyoglu, Foods. 11(19), 3149 (2022)
Y.A. Hui, Bailey’s Industrial Oil and Fat Products, 5th edn. (John Wiley & Sons, Inc., New York, USA, A Wiley-Interscience Publication, 1996)
R.S. Farag, E.A. Mahmoud, A.M. Basuny, Int. J. Food Sci. Technol. 42(1), 107 (2007)
E. Kress-Rogers, P.N. Gilat, J.B. Rosel, Food Cont. 13, 163 (1990)
M.M. Al-Harbi, H.A. Al-Kabtani, Food Chem. 48, 395 (1993)
D. Firestone, R.F. Stier, M.M. Blumenthal, Food Technol. 45(2), 90 (1991)
R.S. Farag, A.M. El-Anany, J. Sci. Food Agric. 86(13), 2228 (2006)
Acknowledgements
The all authors of this paper appreciatively acknowledge the Research Councils of Jahrom University (Jahrom, Fars, Iran) for financially supporting of this reported research.
Funding
This study was financially supported by Jahrom University Research Council (Grant Nos. are not available).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no any conflict of interest for this submission to be declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pashangeh, S., Aminlari, L. Enhancing the quality of fried sunflower oil by oak hull ash as an efficient adsorbent. Food Measure 18, 6356–6364 (2024). https://doi.org/10.1007/s11694-024-02654-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02654-z